Abstract
Campylobacter jejuni colonization of chickens is presumably dependent upon multiple surface-exposed proteins termed adhesins. Putative C. jejuni adhesins include CadF, CapA, JlpA, major outer membrane protein, PEB1, Cj1279c, and Cj1349c. We examined the genetic relatedness of 97 C. jejuni isolates recovered from human, poultry, bovine, porcine, ovine, and canine sources by multilocus sequence typing (MLST) and examined their profile of putative adhesin-encoding genes by dot blot hybridization. To assess the individual contribution of each protein in bacterium-host cell adherence, the C. jejuni genes encoding the putative adhesins were disrupted by insertional mutagenesis. The phenotype of each mutant was judged by performing in vitro cell adherence assays with chicken LMH hepatocellular carcinoma epithelial cells and in vivo colonization assays with broiler chicks. MLST analysis indicated that the C. jejuni isolates utilized in this study were genetically diverse. Dot blot hybridization revealed that the C. jejuni genes encoding the putative adhesins, with the exception of capA, were conserved among the isolates. The C. jejuni CadF, CapA, Cj1279c, and Cj1349c proteins were found to play a significant role in the bacterium's in vitro adherence to chicken epithelial cells, while CadF, PEB1, and Cj1279c were determined to play a significant role in the bacterium's in vivo colonization of broiler chicks. Collectively, the data indicate that Cj1279c is a novel adhesin. Because Cj1279c harbors fibronectin type III domains, we designated the protein FlpA, for fibronectin-like protein A.
Campylobacter jejuni is a gram-negative, spiral, microaerophilic bacterium, which is motile via a bipolar or unipolar flagellum. This organism is one of the leading bacterial causes of diarrhea in the United States and accounts for 5 to 14% of diarrhea worldwide (1). Experimental C. jejuni infections in humans have revealed that as few as 800 bacteria can cause human illness (4). Campylobacteriosis (C. jejuni-mediated gastroenteritis) generally occurs 2 to 5 days after ingestion of the bacterium and is a self-limiting infection. This disease is characterized by fever, nausea, malaise, abdominal pain, and loose-to-watery stools, which may contain blood and/or fecal leukocytes (5). C. jejuni infections can result in several serious sequelae, including Guillain-Barré syndrome, an acute autoimmune disease affecting the peripheral nervous system (35).
C. jejuni infection frequently occurs through the ingestion of C. jejuni in undercooked chicken or from the consumption of food products cross-contaminated with raw poultry. This infection is linked to poultry due to the fact that by 2 to 3 weeks of age most commercial chickens become commensally colonized by as many as 108 CFU of C. jejuni per g of cecal contents (33). Not surprisingly, Campylobacter organisms are frequently recovered from processed broiler carcasses (34). Bacterial adherence to host epithelial cells appears to be crucial for C. jejuni colonization of chickens, as it may prevent host-mediated mechanical forces such as peristalsis from clearing the bacterium. Previous work has revealed that one C. jejuni adhesin, termed CadF (Campylobacter adhesion to fibronectin [Fn]), is required to colonize Leghorn chickens (36).
Researchers have identified a number of C. jejuni proteins that bind to cultured epithelial cells. These adhesive proteins include CadF, CapA, JlpA, major outer membrane protein (MOMP), and PEB1. Konkel et al. (18) identified CadF, which is a 37-kDa Fn-binding protein. CadF facilitates C. jejuni adherence to Fn, which is a ubiquitous ∼250-kDa glycoprotein found in the extracellular matrix and regions of cell-to-cell contact. A C. jejuni cadF mutant shows a 50% reduction in adhesion to human INT 407 intestinal cells compared to a C. jejuni wild-type isolate (28). Ashgar et al. (3) identified a putative autotransporter in silico, which was termed CapA, for Campylobacter adhesion protein A. C. jejuni capA is a contingency gene, in which expression of the functional protein is dependent upon frameshifts within a homopolymeric nucleotide tract located near the 5′ end of the capA coding region. A C. jejuni capA mutant demonstrated a reduction in adherence to human Caco-2 colorectal adenocarcinoma epithelial cells and a decrease in colonization efficiency of chickens. Jin et al. (13) identified JlpA (jejuni lipoprotein A), which is a 43-kDa protein. Disruption of jlpA reduces C. jejuni adherence to human HEp-2 epithelial cells by 18 to 19.4% relative to the wild-type strain. Moser et al. (29) reported that MOMP, encoded by porA, bound to INT 407 cells. The C. jejuni 43-kDa MOMP is also known to allow passage of hydrophilic molecules across the outer membrane and to provide structural stability to the outer membrane (6, 11). C. jejuni porA mutants have yet to be characterized, as mutations in porA are presumably lethal due to the critical structural and transport functions of the MOMP. Kervella et al. (15) characterized PEB1, a C. jejuni 28-kDa putative adhesin. Disruption of peb1A reduces C. jejuni adherence to human HeLa epithelial cells by 50- to 100-fold; such mutants also failed to colonize the intestinal tract of mice (32). In addition to the previously studied proteins listed above, Cj1279c and Cj1349c have been reported to contain Fn type III domains and to act as an Fn and fibrinogen-binding protein, respectively (http://www.microbesonline.org/; VIMSS identification no. 47155 and 47224, respectively). Due to the well-established role of CadF in C. jejuni cell adherence and chicken colonization, Cj1279c and Cj1349c were included in this study.
C. jejuni binding to host cells and colonization are a multifactorial process dependent on motility, chemotaxis, and the synthesis of multiple adhesins (20). The goal of this study was to evaluate the contribution of the CadF, CapA, JlpA, PEB1, MOMP, Cj1279c, and Cj1349c proteins in C. jejuni-host cell interactions. Dot blot assays were used to assess the differences in the content of the putative adhesin-encoding genes among the C. jejuni isolates. To assess the roles of the putative adhesins in promoting host cell binding and host colonization, we generated C. jejuni adhesin mutants via insertional mutagenesis and performed in vitro adherence and in vivo chicken colonization assays. To our knowledge, this is the first time the roles of these proteins have been compared in a single genetic background and the first time that the functional role of many of these proteins has been examined in chickens (i.e., the natural host). We report that some of the C. jejuni proteins play a significant role in chicken colonization, while other proteins are not essential for colonization.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Ninety-seven C. jejuni isolates were obtained from human clinical cases and poultry, bovine, porcine (swine), ovine, and canine sources (see Table S1 in the supplemental material). All human isolates were obtained from individuals with clinical signs of campylobacteriosis. C. jejuni F38011 was isolated from an individual with bloody diarrhea. In total, we used 43 human strains (F38011, 81-176, 81116, M129, H1, H2, H4 to H7, H9 to H24, H26 to H32, and H34 to H43), 41 poultry strains (RM1221, Turkey, S1, S2B, USDA02-833L, A2a, A5a, A18a, D34a, G11a, Iowa 2, Iowa 4 to Iowa 9, Iowa 11 to Iowa 13, Iowa 15, Iowa 21 to Iowa 26, Iowa 33 to Iowa 36, Iowa 39, Iowa 42, Iowa 44, Iowa 77 to Iowa 81, and Iowa 83), 5 bovine strains (C913, C973, C1086, C1129, and C1144), 5 porcine strains (93-55, 93-58, 93-338, 93-343, and 92-1578), 2 ovine strains (ov48 and ov112), and 1 canine strain (can1979858). C. jejuni isolates were cultured at 37°C under microaerobic conditions (5% O2, 10% CO2, 85% N2) on Mueller-Hinton (MH) agar plates supplemented with 5% citrated bovine blood (MH-blood agar plates). C. jejuni strains were subcultured to a fresh plate every 48 h. The C. jejuni F38011 cadF (kanamycin resistant [Kanr]), capA (tetracycline resistant [Tetr]), jlpA (Kanr), peb1A (Kanr), Cj1278c (Tetr), Cj1279c (Kanr), and Cj1349c (Kanr) mutants were generated as outlined below. When appropriate, the growth media were supplemented with antibiotics at 50 μg/ml for Kan (Sigma, St. Louis, MO) and 2.0 μg/ml for Tet (Sigma).
Motility assay.
Motility was determined using MH medium supplemented with 0.4% Select agar (Invitrogen, Carlsbad, CA). Briefly, 10 μl of each bacterial suspension in MH broth was added to the surface of the agar and the plates were incubated at 37°C under microaerobic conditions. Motility was determined by measuring the diameter of the bacterial migration zone after 48 h of incubation.
MLST.
For multilocus sequence typing (MLST), genomic DNA was isolated from the C. jejuni isolates using phenol chloroform extractions. Briefly, bacteria were cultured on MH-blood agar plates and harvested in 5 ml of phosphate-buffered saline (PBS). After incubation for 1 h at 37°C with 500 μl 10% sodium dodecyl sulfate (SDS) and 5 μl proteinase K (20 mg/ml), three phenol and isoamyl chloroform extractions (24 parts chloroform and 1 part isoamyl alcohol) were performed with the aqueous layer retained each time. An equal volume of cold isopropanol and 250 μl of 2.5 M sodium acetate were added to the aqueous layer, prior to incubation at −20°C for 5 min. The DNA was pelleted by centrifugation at 11,600 × g for 15 min. The pellet was washed with 70% ethanol, spun at 11,600 × g for 15 min, resuspended in sterile water, and RNase treated at 37°C for 1 h. DNA purity, using an optical density at 260 nm (OD260)/OD280 ratio, and concentration were determined using a NanoDrop 1000 (Thermo Fisher Scientific, Wilmington, DE).
C. jejuni housekeeping genes coding for aspartase A (aspA), glutamine synthetase (glnA), citrate synthase (gltA), serine hydroxymethyl transferase (glyA), phosphoglucomutase (pgm), transketolase (tkt), and the ATP synthase alpha subunit (uncA) were amplified and sequenced using primers described elsewhere (26). PCR was performed using approximately 50 ng of genomic DNA and 1 U Taq polymerase (New England Biolabs, Beverly, MA) in a 50-μl reaction volume with 50 pmol of each primer, 1× MasterAmp PCR buffer (Epicentre, Madison, WI), 1× MasterAmp PCR enhancer (Epicentre), 2.5 mM MgCl2, and 250 μM (each) deoxynucleoside triphosphates (dNTPs). Genes were amplified using the following amplification parameters: 94°C for 30 s, 53°C for 30 s, and 72°C for 2 min (30 cycles). Amplicons were confirmed by agarose gel electrophoresis and purified on a BioRobot 8000 workstation (Qiagen, Valencia, CA). Cycle sequencing reactions were performed on a Tetrad thermocycler (Bio-Rad, Hercules, CA), using the ABI BigDye terminator cycle sequencing kit (version 3.1; Applied Biosystems, Foster City, CA) and standard protocols. Cycle sequencing extension products were purified using DyeEx 96 plates (Qiagen). DNA sequencing was performed on an ABI PRISM 3730 DNA analyzer (Applied Biosystems), using POP-7 polymer and ABI data collection and sequencing analysis software. Nucleotide sequences were aligned and analyzed using Seqman Pro (version 7.2; DNASTAR, Madison, WI). Alleles and sequence types (STs) were assigned using MLSTparser3 (Miller et al., unpublished observations).
Dot blot hybridization.
The C. jejuni putative adhesin-encoding genes examined in this study were porA, cadF, capA, jlpA, peb1A, Cj1279c, and Cj1349c. The sequence of each gene from C. jejuni NCTC 11168 was obtained from online resources (http://www.sanger.ac.uk/Projects/C_jejuni/). Gene-specific probes were generated as outlined below. An internal fragment of each gene was amplified via PCR using the primers listed in Table 1. The amplifications were performed using high-fidelity Taq DNA polymerase (Invitrogen) with C. jejuni NCTC 11168 chromosomal DNA as the template. Genes were amplified using the following parameters: 94°C for 2 min (1 cycle); 94°C for 45 s, 60°C (−1°C per cycle) for 30 s, and 70°C for 1.5 min (10 cycles); 94°C for 45 s, 50°C for 30 s, and 70°C for 1.5 min (25 cycles); and 70°C for 8 min (1 cycle). The amplified PCR fragments were ligated into the vector pCR2.1 according to the manufacturer's directions (original TA cloning kit, Invitrogen) and electroporated into Escherichia coli InvαF′. The purified plasmids were nick translated using a nick translation kit according to the manufacturer's directions (Roche Applied Science, Indianapolis, IN). One hundred nanograms of C. jejuni genomic DNA, isolated via phenol chloroform extractions as described above, was vacuum transferred to a GeneScreen membrane (Perkin-Elmer, Waltham, MA) using a Schleicher and Schuell Minifold II slot-blotter (Jencons, United Kingdom). Depurinating solution (0.25 M HCl) was added to each slot for 4 min, followed by denaturating solution (1.5 M NaOH and 0.5 NaCl) for 3 min, neutralizing solution (1.0 M Tris and 1.5 M NaCl [pH 8.0]) for 3 min, and 20× SSC (3.0 M NaCl plus 0.3 M sodium citrate) for 20 min. DNA was cross-linked to the membrane using a Gene Linker UV chamber, according to the manufacturer's instructions (Bio-Rad, Hercules, CA).
TABLE 1.
Genes targeted for mutagenesis
| Locus tag (gene designation)a | Gene product (protein designation) | No. of nucleotides/ residues | Amplified fragment(s) in nucleotides | Primer | Sequence |
|---|---|---|---|---|---|
| Cj1478c (cadF) | CadF | 960/320 | 620 | cadF-Fb | TATTTCTATGGTTTAGCAGGTGGAG |
| cadF-Rb | GCTCTACCTTCTTTAGTGTCATTGC | ||||
| Cj0628/Cj0629 | CapA | 3,436/1,145 | 1321, 1635 | capA-Fb | TGAATCGAAGTGGAAAAATAGAAG |
| (capA) | capA-Rb | CCCATTTTTGTATCTTCATAACCT | |||
| capA-SstI-Fc | ATGAGCTCAAAGTTGTTCCTAAGGGTAAAGC | ||||
| capA-SstII-Rc | ATACCGCGGAGTTTTATTCATAAATATTCCCTTTCC | ||||
| capA-SstII-Fc | ATACCGCGGGCTCAGTTTAATTATCTTTGGTAATC | ||||
| capA-XhoI-Rc | ATACTCGAGCATTTTACAAGCCCTATAAGAAGG | ||||
| Cj0983 (jlpA) | JlpA | 1,119/373 | 868 | jlpA-Fb | TCTCAGGACTCTGGAATAAAGATTG |
| jlpA-Rb | GTGTGCTATAGTCACTAACAGGGATG | ||||
| Cj0921c (peb1A) | PEB1 | 780/260 | 560 | peb1A-Fb | TCTAGGTGCTTGTGTTGCATTTAG |
| peb1A-Rb | TGTCTACAGAAAACGCATCAACTC | ||||
| Cj1279c | Cj1279c (FlpA) | 1,233/411 | 832 | Cj1279c-Fb | TCAGAAGATGGCAAGGTTATAGAAG |
| Cj1279c-Rb | GTTATTGCTATTGATTCAGCTGGAC | ||||
| Cj1349c | Cj1349c | 1,308/436 | 1115 | Cj1349c-Fb | TATTTTTGATCTTACTCGTGCAATG |
| Cj1349c-Rb | TTAAGGTATAATCGACCCAATACGA | ||||
| Cj1278c | Cj1278c | 1,179/393 | 1033, 991 | Cj1278c-BamHI-Fc | ATATAGGATCCGTATCGTTCTAGTGATGAAAATCC |
| Cj1278c-SstII-Rc | ATATACCGCGGTTTTAAAATTTGGCACTACTGAGC | ||||
| Cj1278c-SstII-Fc | ATATACCGCGGGTTTAAAATATAATTTTTCTTGAAAATTAAGC | ||||
| Cj1278c-BamHI-Rc | ATATAGGATCCTTTTCAGAAACATCATTTTTCAAACG |
The gene number is from the genome sequence from C. jejuni NCTC 11168.
Primer used to amplify a DNA fragment for the generation of a suicide vector (gene knockout) and/or the probe for dot blot hybridization.
Primer used to amplify the DNA fragments for construction of the vectors to generate the mutants (i.e., capA and Cj1278c) via a double-crossover event. The two fragments were cloned into pBSK-Kan2 and disrupted by insertion of a Tetr cassette.
Each membrane was blocked for 15 min at room temperature with 100 μl denatured salmon sperm DNA in hybridization solution that had been warmed to 50°C. The hybridization solution was composed of 5 ml formamide, 2 ml 5× P buffer (1.0% bovine serum albumin, 1.0% polyvinylpyrrolidone, 1.0% Ficoll, 0.5% sodium pyrophosphate, 5.0% SDS, 250 mM Tris [pH 7.5]), 2 ml 50% dextran sulfate, and 0.58 g NaCl. The radioactively labeled probe was denatured by heating for 15 min at 95°C, chilled on ice for 15 min, and added to the hybridization solution. The membrane was incubated with the hybridization solution at 35°C in a hybridization incubator (Robbins Scientific, Hudson, NH) overnight. Membranes were washed twice with 2× SSC at 25°C for 10 min and twice with a solution of 2× SSC and 1% SDS at 35°C for 20 min. Autoradiography was performed with Kodak BioMax MR film at −80°C for approximately 2 h.
Generation of C. jejuni cadF, jlpA, peb1A, Cj1279c, and Cj1349c suicide vectors.
The PCR amplicons used as probes for the dot blot hybridizations were removed from the pCR2.1 multiple cloning site and ligated into pBSK-Kan2. The pBSK-Kan2 vector is identical to pBlueScript (Invitrogen), except that the original kanamycin cassette was replaced with one that functions in both C. jejuni and E. coli (22). The resulting pBSK-Kan2 vectors (pMEK252-pMEK256) were confirmed by DNA sequencing and were electroporated into E. coli InvαF′ electrocompetent cells.
Generation of C. jejuni capA and Cj1278c suicide vectors.
DNA regions upstream and downstream of the C. jejuni capA and Cj1278c genes were amplified by PCR using Taq DNA polymerase (Invitrogen) and the primers listed in Table 1. C. jejuni NCTC 11168 chromosomal DNA was used for the amplification of DNA regions flanking capA, while C. jejuni F38011 chromosomal DNA was used for the regions flanking Cj1278c. The reaction conditions were 94°C for 2 min (1 cycle); 94°C for 45 s, 63°C (−1°C per cycle) for 30 s, and 70°C for 4 min (8 cycles); 94°C for 45 s, 50°C for 30 s, and 70°C for 4 min (25 cycles); and 70°C for 8 min (1 cycle). The two flanking regions were cloned individually in pCR2.1. Thereafter, one fragment was cloned into the pCR2.1 vector harboring the other fragment, and a Tetr cassette was inserted between the two flanking regions. The resulting fragment was then moved into the multiple cloning site of pBSK-Kan2. The mutation construct was verified by DNA sequencing.
Generation of C. jejuni F38011 mutants.
C. jejuni F38011 was grown overnight in MH broth with shaking at 37°C under microaerobic conditions to a final OD540 of 1.0. Two hundred milliliters of culture was centrifuged at 6,000 × g for 5 min to pellet the cells. The cells were washed once in sterile water and once in 10% glycerol and resuspended in 350 μl of 10% glycerol. Approximately 2 μg of a CsCl-concentrated suicide vector was mixed with 50 μl of the electrocompetent C. jejuni and pulsed at 2.50 kV. The cells were immediately suspended in 200 μl of MH broth and plated on MH-blood agar plates. After overnight incubation at 37°C in a microaerobic environment, one-half of the growth was streaked onto MH-blood plates containing the appropriate antibiotic (50 μg/ml Kan or 2 μg/ml Tet). After 48 h of incubation, the isolated colonies were screened by PCR using gene-specific primers. Each C. jejuni mutant was confirmed using gene-specific primers, and in the case of the C. jejuni capA and Cj1278c mutants by sequencing the DNA flanking regions. The motility of each C. jejuni mutant was assessed prior to use.
Tissue culture.
Chicken LMH hepatocellular carcinoma cells (ATCC CRL-2117) were obtained from the American Type Culture Collection (Manassas, VA). Stock cultures of LMH cells were grown in flasks coated with 0.1% gelatin in Waymouth's MB 752/1 medium supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, UT). Cells were maintained at 37°C in a humidified 5% CO2 incubator.
C. jejuni-LMH binding assay.
LMH cells were seeded to a cell density of 3.0 × 105 cells/ml and incubated for 24 h at 37°C in a humidified, 5% CO2 incubator. The cells were rinsed once with minimal essential medium (Invitrogen) supplemented with 1% fetal bovine serum and inoculated with approximately 3.0 × 107 CFU bacteria. Each plate was then subjected to centrifugation at 600 × g for 5 min to promote bacterium-host cell contact and incubated at 37°C for 30 min. To quantitate cell adherence, the C. jejuni-inoculated cells were rinsed three times with PBS and lysed with a solution of 0.1% (vol/vol) Triton X-100 (Calbiochem, La Jolla, CA) in PBS. Ten-fold serial dilutions of the samples were made and plated on MH-blood agar plates to determine the number of adherent bacteria. The reported values represent the mean counts ± standard deviation from triplicate wells.
Chicken colonization experiments.
All of the experiments and procedures described below were performed in compliance with protocols approved by the Institutional Animal Care and Use Committee (IACUC protocol no. 3248) at Washington State University. A total of 80 1-week-old chicks were obtained, divided into eight groups, and placed into isolation chambers (Horsfall Bauer isolators) on wire mesh. Water and a commercial chick starter feed were provided ad libitum. Each isolator was equipped with two removable metal trays. Fecal matter was collected and autoclaved before disposal. The chicks were inoculated with C. jejuni by oral gavage with 0.5 ml of a bacterial suspension (∼107 bacteria); the C. jejuni F38011 strain was cultured in Bolton's broth at 42°C for 16 h under microaerobic conditions prior to inoculation of the birds. One group of 10 chicks was kept as the uninoculated control group. The remaining groups of chicks were inoculated with the following C. jejuni F38011 strains: (i) the wild-type strain, (ii) cadF mutant, (iii) capA mutant, (iv) jlpA mutant, (v) peb1A mutant, (vi) Cj1279c mutant, and (vii) Cj1349c mutant. After the chicks were inoculated, the remaining bacterial suspensions were serially diluted and plated on Campy-Cefex agar (30) to confirm the CFU of each inoculum.
The chicks were euthanized and necropsied at 7 days postinoculation (dpi). The cecum was dissected from each chick, weighed, diluted 1:10 (wt/vol) in Bolton's broth medium, and thoroughly stomached. For enumeration, serial 10-fold dilutions of the cecal contents were made and plated onto Campy-Cefex agar plates. The plates were incubated in a microaerobic environment at 37°C, and the CFU were counted after 72 h of incubation. PCR was performed with C. jejuni cadF- and capA-specific primers to confirm that the counted colonies were C. jejuni (Table 1).
Nucleotide sequence accession number.
Novel alleles and STs have been submitted to the PubMLST C. jejuni/C. coli database (http://pubmlst.org/campylobacter/).
RESULTS
The C. jejuni strains used in this study are genetically diverse.
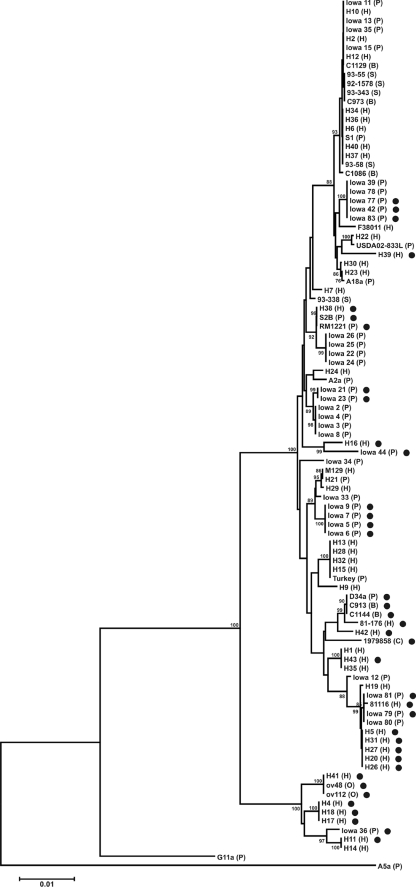
MLST is commonly used for molecular typing of C. jejuni isolates (8, 25). We collected a total of 97 isolates from humans, poultry, bovine, porcine, ovine, and canine sources and assessed their genetic relatedness by MLST (Fig. 1). The C. jejuni isolates comprised 45 STs (see Table S1 in the supplemental material). Eighty-four isolates were assigned to one of 18 clonal complexes (CCs). The complexes with the greatest number of isolates were CC21, CC48, and CC45, which were comprised of 19, 10, and 10 isolates, respectively. In total, 2 human isolates and 11 animal isolates did not belong to a CC in the MLST database. We also compared the allelic profiles, or ST, of each isolate. The most common ST was ST-21, represented by isolates H2, H10, H12, Iowa 11, Iowa 13, Iowa 15, Iowa 35, and C1129. The second most common ST was ST-50, represented by isolates H6, H34, H36, H37, H40, S1, and 93-58. Several STs were comprised of three to five isolates, whereas 27 STs were represented by a single isolate. Newly identified STs were generated with four human isolates (F38011, H11, H14, and H30) and one poultry isolate (USDA02-833L). In total, 105 alleles were identified among the seven loci, and a new pgm allele (pgm431) was reported. Based on the MLST analysis, we concluded that the C. jejuni strains used in this study were genetically diverse.
FIG. 1.
Dendrogram of the C. jejuni MLST profiles identified in this study. The MLST STs were determined as outlined in Materials and Methods. For each isolate, the allelic sequences were concatenated in the order aspA-uncA-glnA-gltA-glyA-pgm-tkt and aligned in ClustalX. The dendrogram was constructed in Mega 4 using the aligned concatenated sequences, the neighbor-joining algorithm, and the Kimura two-parameter distance estimation method. Bootstrap values of >75%, generated from 500 replicates, are shown at the nodes. The scale bar represents substitutions per site. The filled circles (•) indicate those C. jejuni strains in which the capA gene is absent. Labels in parentheses indicate isolate source: H, human; P, poultry; S, porcine (swine); B, bovine; O, ovine; and C, canine.
The adhesin-encoding genes, except capA, are conserved among C. jejuni strains.
The presence of genes encoding putative adhesins in the C. jejuni strains was determined by dot blot hybridization coupled with gene-specific probes. The essential features of these genes are listed in Table 2. Six of the seven putative adhesin-encoding genes (i.e., cadF, jlpA, peb1A, porA, Cj1279c, and Cj1349c), were detected in every C. jejuni strain tested (not shown), indicating that these genes are conserved within C. jejuni. One of the seven putative adhesin-encoding genes, capA, was not conserved among the strains assayed (Fig. 1). C. jejuni capA was absent in 17 of the 43 (40%) human isolates and from 21 of the 54 (39%) animal isolates. The presence or absence of capA often correlated to specific STs. STs 50, 48, and 21, comprising 20 isolates, all possessed capA, while STs 464, 459, 61, and 45, comprising 15 isolates, lacked capA.
TABLE 2.
C. jejuni genes encoding putative adhesins
| ORF | BLAST identification | Signal peptide cleavage sites | Genes within the putative operona | Relevant reference |
|---|---|---|---|---|
| porA/Cj1259 | MOMP, C. jejuni | Residues 22-23, SpI cleavageb | None identified | 29 |
| cadF/Cj1478c | Structural outer membrane porin OprF, Pseudomonas aeruginosa 2e-27 | Residues 26-27, SpI cleavageb | Cj1478c, Cj1477c | 18 |
| capA/Cj0628 and Cj0629 | Autotransporter β-domain, C. jejuni | None identified | None identified | 1 |
| jlpA/Cj0983 | Surface-exposed lipoprotein, C. jejuni | Residues 17-18, SpII cleavagec | None identified | 13 |
| peb1A/Cj0921c | Amino acid ABC transporter, amino acid-binding protein, Streptococcus pneumoniae 1e-52 | Residues 26-27, SpI cleavageb | Cj0922c, Cj0921c, Cj0920c, Cj0919c | 15 |
| Cj1279c | Fibronectin type III domain containing protein lipoprotein, Sulfurimonas denitrificans 1e-57 | Residues 20-21, SpII cleavagec | Cj1280c, Cj1279c, Cj1278c, Cj1277c, Cj1276c, Cj1275c, Cj1274c, Cj1273c, Cj1272c, Cj1271c, Cj1270c, Cj1269c, Cj1268c | |
| Cj1349c | Fn/fibrinogen-binding protein FBP54, Streptococcus pyogenes 1.7e-05 | None identified | Cj1350c, Cj1349c, Cj1348c, Cj1347c, Cj1346c, Cj1345c, Cj1344c, Cj1343c, Cj1342c |
Genes within the putative operons were determined using NMPDR.
Putative signal peptide cleavage site.
Putative lipoprotein signal peptide cleavage site.
CadF, CapA, Cj1279c, and Cj1349c contribute to C. jejuni adherence to chicken LMH cells.
To determine the role of the putative adhesins in promoting the binding of C. jejuni to cultured chicken epithelial cells, in vitro adherence assays were performed with C. jejuni mutants and chicken LMH hepatocellular carcinoma epithelial cells (Fig. 2). A mutation in the porA gene was not attempted, as a mutation of this gene is hypothesized to be lethal due to its critical structural and pore activity (2). All of the C. jejuni mutants (i.e., cadF, capA, jlpA, peb1A, Cj1279c, and Cj1349c) generated were motile (not shown). The LMH cell line was chosen for these experiments because it is the only chicken epithelial cell line readily available to researchers. While LMH cells are derived from the liver, previous C. jejuni adherence studies indicate similar bacterium-host cell adherence efficiency with LMH and human INT 407 epithelial cells (16, 23). Mutations in jlpA and peb1A had little effect on the ability of C. jejuni to bind to the LMH cells. In contrast, a significant reduction (P < 0.05) was observed in the binding of the C. jejuni cadF, capA, Cj1279c, and Cj1349c mutants to LMH cells compared with the C. jejuni wild-type strain. In addition, C. jejuni isolates were genetically matched (H11 and H14 to Iowa 80 and Iowa 81) based upon MLST and tested for cell adherence: the H11 and Iowa 81 isolates contained capA, and the H14 and Iowa 80 isolates did not (Fig. 1). Strains lacking capA showed a significant reduction (P < 0.05) in binding to LMH cells relative to strains in possession of the gene (not shown).
FIG. 2.
Number of C. jejuni cells bound to chicken LMH hepatocellular carcinoma epithelial cells. Binding assays were performed as outlined in Materials and Methods. Each bar represents the mean ± standard deviation number of C. jejuni cells bound to LMH cells in each well of a 24-well plate. An asterisk indicates a statistically significant difference (P < 0.05) between the C. jejuni F38011 wild-type isolate and an isogenic mutant, as determined by Student's t test.
CadF, PEB1, and Cj1279c contribute to C. jejuni colonization of broiler chickens.
To determine the relative importance of each putative adhesin in chicken colonization, we inoculated 1-week-old chicks with the defined C. jejuni mutants. Eighty chicks were divided into groups, with each group consisting of 10 chicks (Fig. 3). All chicks were euthanized at 7 dpi and the number of C. jejuni per gram of cecal material was determined. C. jejuni was not recovered from any of the uninoculated chicks. Mutations in the capA, jlpA, and Cj1349c genes had little effect on the ability of C. jejuni to colonize the chicks, as judged by comparison with the wild type. In contrast, the C. jejuni cadF, peb1A, and Cj1279c mutants demonstrated a marked impairment in their ability to colonize chicks, as only 2 of 10 chickens inoculated with the C. jejuni cadF and Cj1279c mutants were colonized. None of the 10 chicks inoculated with the C. jejuni peb1A mutant was colonized.
FIG. 3.
CadF, PEB1, and Cj1279c contribute to C. jejuni colonization of broiler chickens. Cecal samples were collected from the broiler chickens at 7 dpi. The CFU per gram of cecal content was determined as described in Materials and Methods. N indicates the number of chickens in each group of 10 that were colonized with C. jejuni (limit of detection, 103 CFU/g of cecal contents). The bar indicates the median CFU for each group, which was determined using all birds within the group. The absence of a bar indicates the number of C. jejuni cells was below the limit of detection.
Cj1279c is required for efficient cell adherence and chicken colonization.
In silico analysis of Cj1279c revealed that this gene is located within a putative operon consisting of 13 genes (http://www.microbesonline.org/). The Cj1279c gene is situated downstream of Cj1280c, which encodes a putative ribosomal pseudouridine synthase and upstream of 11 C. jejuni genes involved in various functions, including cellular division and metabolism. To alleviate the concern of a polar effect, the Cj1278c gene downstream of Cj1279c was mutated. Adherence assays performed with chicken LMH cells demonstrated that the observed phenotype of the Cj1279c mutant was not due to a polar effect, as a difference in binding was not observed with the Cj1278c mutant relative to the wild-type strain (Fig. 4). Although variations were observed from one experiment to another in the number of C. jejuni cells that bound to the chicken LMH cells (Fig. 2 and 4), these results appeared to be due to fluctuations in the multiplicity of infection. Regardless, the C. jejuni cadF and Cj1279c mutants consistently showed reductions in cell binding compared to the wild-type strain in all experiments performed. Because Cj1279c has not been previously characterized, we propose that it is a novel adhesin. As indicated above, the Cj1279c mutant demonstrates a reduction in both adherence to chicken LMH cells and the colonization of chickens. Based on these findings and the fact that Cj1279c contains Fn type III domains, we will refer to the Cj1279c gene as flpA, for fibronectin-like protein A, from this point forward.
FIG. 4.
Cj1279c (flpA) encodes an adhesin. Binding assays were performed with C. jejuni and chicken LMH hepatocellular carcinoma epithelial cells as outlined in Materials and Methods. Each bar represents the mean ± the standard deviation number of C. jejuni cells bound to LMH cells in each well of a 24-well plate. An asterisk indicates a statistically significance difference (P < 0.05) between the C. jejuni F38011 wild-type isolate and an isogenic mutant, as determined by Student's t test.
DISCUSSION
Bacterial adherence to host epithelial cells is proposed to be critical for chicken colonization, as cell attachment may prevent clearance of the bacteria via host-mediated mechanical force. The goal of this study was to assess the conservation of the putative C. jejuni adhesin-encoding genes cadF, capA, jlpA, peb1A, porA, Cj1279c (flpA), and Cj1349c and the contribution of the corresponding proteins in C. jejuni host cell interactions. In this study, we found that the cadF, jlpA, porA, peb1A, flpA, and Cj1349c genes were conserved among the isolates, whereas the presence of the capA gene was variable. We found that the C. jejuni CadF, CapA, FlpA, and Cj1349c proteins contribute to the bacterium's in vitro adherence to chicken LMH hepatocellular carcinoma epithelial cells, while CadF, PEB1, and FlpA contribute to the bacterium's in vivo colonization of broiler chicks. This is the first study to show that FlpA promotes the binding of C. jejuni to host cells and plays a role in C. jejuni colonization of chickens.
This study was performed with C. jejuni isolates collected from human, poultry, bovine, porcine, ovine, and canine sources. These isolates were genetically diverse, as judged by MLST. The isolates were found to comprise 42 unique sequence types, 4 of which had not been identified previously. The CCs identified amid the C. jejuni livestock (i.e., bovine, porcine, and ovine) isolates included two complexes, CC42 and CC61, that were determined in previous studies (7, 21) to be associated significantly with bovine and ovine isolates. Furthermore, the 11 CCs identified in the 41 poultry isolates included several poultry-associated complexes (i.e., CC45, CC257, and CC354) (9). Thus, the results from this study are consistent with previous associations of CC and source. Additionally, the CCs of the human isolates identified in this study were also found within the poultry and livestock isolates and vice versa. Therefore, no predominant food animal source of human infection was identified in this study.
As indicated above, genetic analysis of the adhesin profiles among the strains via dot blot assays demonstrated conservation of the C. jejuni cadF, jlpA, porA, peb1A, flpA, and Cj1349c genes. While the dot blot hybridization assay is stringent enough to detect the presence or absence of the well-conserved adhesin genes, it cannot detect strain-to-strain sequence variations. However, the amino acid sequences of the putative adhesins CadF, JlpA, PEB1, Cj1279c, and Cj1349 are all greater than 95% identical between C. jejuni strains, and CapA is greater than 85% identical between C. jejuni strains. The sequence of MOMP is the most variable between strains; however, MOMP is required for viability. Fouts et al. (10) demonstrated the conservation of the characterized and putative C. jejuni adhesins in the C. jejuni NCTC 11168 and RM1221 strains and three other Campylobacter species. Although capA was omitted in the study by Fouts et al. (10), Ashgar et al. (3) reported that 9 of 20 C. jejuni human clinical isolates tested in their study lacked the capA gene. In agreement with Ashgar et al. (3), we found that the capA gene was absent from 40% of the C. jejuni strains recovered from humans and was absent from 39% of the C. jejuni strains recovered from animals.
While some of the C. jejuni proteins examined in this study have been the focus of previous work, this is the first time the functional role of these proteins has been compared by generating a mutation in these genes within a single genetic background. The C. jejuni CadF, CapA, FlpA, and Cj1349c proteins were found to play a significant role in the bacterium's in vitro adherence to chicken epithelial cells, whereas JlpA and PEB1 did not appear to play a role in cell adherence. Previous work has indicated that the C. jejuni lipoprotein JlpA is located predominately in the bacterial inner membrane and can be found loosely associated with the outer membrane and in culture medium during growth. Further, JlpA interacts with the human heat shock protein 90α on the surface of human HEp-2 cells and is correlated with induction of the preinflammatory response due to the activation of NF-κB and p38 mitogen-activated protein kinase (14). Jin et al. (13) reported that disruption of jlpA reduces the adherence of C. jejuni to human HEp-2 epithelial cells by 18 to 19.4% compared with a wild-type isolate. We found that insertional mutagenesis of jlpA did not result in a reduction in binding to chicken LMH cells. In agreement with the results from the in vitro binding assays, the jlpA mutant was able to colonize broiler chickens at a level comparable to that of a wild-type isolate.
While we found that a C. jejuni peb1A mutant bound to chicken LMH cells at a level comparable to that of a wild-type isolate, the mutant did not colonize broiler chickens. Based on our in vitro data and previously published data (12, 24), PEB1 does not appear to act as an adhesin but rather plays a critical role in aspartate and glutamate transport. In support of these findings, Leon-Kempis et al. (24) found PEB1 in the periplasm and supernatant, which is typical of an ABC transporter (31), but not in the outer membrane. In addition, the investigators demonstrated that a peb1A mutant failed to transport l-glutamate, showed a large reduction in l-aspartate transport, and did not grow in minimal essential medium supplemented with l-aspartate or l-glutamate. Also relevant, Guccione et al. (12) reported that C. jejuni growth primarily depends upon four amino acids: aspartate, glutamate, serine, and proline. Interestingly, a C. jejuni aspartase mutant, which is unable to utilize aspartate, glutamate, and proline, grows poorly in a complex medium and is impaired in chicken colonization. These findings indicate that amino acid utilization in the chicken intestine is vital to the bacterium's colonization and persistence.
The CapA protein was identified as a putative autotransporter based on in silico analysis. Ashgar et al. (3) reported that a capA knockout reduced the binding of C. jejuni to human Caco-2 colorectal adenocarcinoma cells by approximately 30% and failed to colonize and persist in Rhode Island Red chickens. We found that the capA gene was not conserved among C. jejuni isolates. Indeed, the dot blot assay revealed that 15 of the C. jejuni poultry isolates utilized in this study lacked the capA gene. We also found that the C. jejuni capA mutant exhibited a 47% reduction in binding to chicken LMH epithelial cells compared with the wild-type isolate, yet was able to colonize broiler chickens as efficiently as the wild-type isolate. The reason for the discrepancy between our data and those of Ashgar et al. (3) is not known; however, different methods and species of chickens were used in the two studies. Based on our results, we conclude that the CapA protein is an adhesin but that it is not required for the colonization of broiler chickens.
CadF is a highly conserved 37-kDa outer membrane protein that binds to the extracellular matrix component Fn (17-19, 27). Previous reports have indicated that a C. jejuni cadF mutant shows a 60% reduction in binding to immobilized Fn and a 59% reduction in adherence to INT 407 cells compared to the wild-type isolate (18, 28). In addition, a cadF mutant fails to colonize the intestinal tract of Leghorn chickens (36). In this study, the C. jejuni cadF mutant demonstrated a 41% reduction in binding to chicken LMH cells and was unable to efficiently colonize broiler chickens. These findings are in agreement with previously published studies (17-19, 27, 28, 36).
Since the Fn-binding protein CadF is critical to C. jejuni host cell adherence, we hypothesized that FlpA and Cj1349c may play a role in host cell attachment. Cj1349c has been annotated as a putative Fn/fibrinogen-binding protein. The Cj1349c mutant demonstrated a 14% reduction in binding to chicken LMH cells (P < 0.05). However, we did not observe reduced colonization of broiler chicks with a Cj1349c mutant compared with the wild-type isolate. Based on the in vitro experiments, Cj1349c may act as an adhesin. However, the functional role of Cj1349c in vivo is not clear based on the chicken colonization experiments. FlpA contains Fn type III domains. Interestingly, the flpA mutant showed a 39% reduction in binding to chicken LMH epithelial cells relative to the wild-type isolate. In addition, the flpA mutant failed to efficiently colonize broiler chickens, as only 2 of 10 broiler chicks were colonized. To address the concern that a mutation in flpA may have a polar effect, a mutation was generated in Cj1278c. The Cj1278c mutant did not show a significant reduction in binding to chicken LMH cells relative to the wild-type isolate. These data suggest that FlpA is a novel C. jejuni adhesin involved in C. jejuni-host cell adherence and chicken colonization.
In summary, this work indicates that the cadF, jlpA, peb1A, porA, flpA, and Cj1349c genes are conserved among C. jejuni isolates, whereas the presence of the capA gene is variable. We report that the CadF, CapA, FlpA, and Cj1349c proteins facilitate C. jejuni adherence to chicken LMH cells. This finding is consistent with the hypothesis that more than one protein contributes to the binding of C. jejuni to host epithelial cells. We conclude that both the CadF and FlpA proteins play a significant role in C. jejuni colonization of chickens. Based on the in vivo assays, it is apparent that the CapA and Cj1349c proteins are not essential for C. jejuni to colonize chickens; however, we cannot rule out the possibility that they contribute to the process. We found that the PEB1 protein is required for C. jejuni to colonize chickens, but this finding is likely due to that fact that it is involved in amino acid transport required for viability within the host. We were unable to assess the importance of MOMP, primarily due to the fact that mutations in porA are likely to be lethal to the bacterium. Based on the results herein, we propose that FlpA (Cj1279c) is a novel C. jejuni adhesin. Additional studies are in progress to dissect the function of the FlpA protein.
Supplementary Material
Acknowledgments
We thank Travis Ruff for assistance with the dot blots, Dennis Schaberg and Kari Shoaf-Sweeney for assistance with C. jejuni inoculation of the chickens and with sample collection, Stuart Perry for animal care, Sylvia K. Weber and Fonda Wier for assistance in preparing chicken tissue samples for plating, and Emma Yee for assistance with MLST. This work made use of the Campylobacter MultiLocus Sequence Typing website (http://pubmlst.org/campylobacter/) developed by Keith Jolley at the University of Oxford. We also thank Charles Larson (School of Molecular Biosciences, Washington State University) for critical review of the manuscript.
This work was supported from funds awarded to M.E.K. from the USDA National Research Initiative's Food Safety 32.0 program (2006-35201-16553 and 2006-35201-17305).
Editor: V. J. DiRita
Footnotes
Published ahead of print on 6 April 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Altekruse, S. F., N. J. Stern, P. I. Fields, and D. L. Swerdlow. 1999. Campylobacter jejuni—an emerging foodborne pathogen. Emerg. Infect. Dis. 528-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amako, K., S. N. Wai, A. Umeda, M. Sigematsu, and A. Takade. 1996. Electron microscopy of the major outer membrane protein of Campylobacter jejuni. Microbiol. Immunol. 40749-754. [DOI] [PubMed] [Google Scholar]
- 3.Ashgar, S. S. A., N. J. Oldfield, K. G. Wooldridge, M. A. Jones, G. J. Irving, D. P. J. Turner, and D. A. A. Ala'Aldeen. 2007. CapA, an autotransporter protein of Campylobacter jejuni, mediates association with human epithelial cells and colonization of the chicken gut. J. Bacteriol. 1891856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157472-479. [DOI] [PubMed] [Google Scholar]
- 5.Blaser, M. J., I. D. Berkowitz, F. M. LaForce, J. Cravens, L. B. Reller, and W. L. Wang. 1979. Campylobacter enteritis: clinical and epidemiologic features. Ann. Intern. Med. 91179-185. [DOI] [PubMed] [Google Scholar]
- 6.Bolla, J.-M., E. Loret, M. Zalewski, and J.-M. Pages. 1995. Conformational analysis of the Campylobacter jejuni porin. J. Bacteriol. 1774266-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colles, F. M., K. Jones, R. M. Harding, and M. C. Maiden. 2003. Genetic diversity of Campylobacter jejuni isolates from farm animals and the farm environment. Appl. Environ. Microbiol. 697409-7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dingle, K. E., F. M. Colles, D. R. A. Wareing, R. Ure, A. J. Fox, F. E. Bolton, H. J. Bootsma, R. J. Willems, R. Urwin, and M. C. J. Maiden. 2001. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 3914-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dingle, K. E., F. M. Colles, R. Ure, J. A. Wagenaar, B. Duim, F. J. Bolton, A. J. Fox, D. R. Wareing, and M. C. Maiden. 2002. Molecular characterization of Campylobacter jejuni clones: a basis for epidemiologic investigation. Emerg. Infect. Dis. 8949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fouts, D. E., E. F. Mongodin, R. E. Mandrell, W. G. Miller, D. A. Rasko, J. Ravel, L. M. Brinkac, R. T. DeBoy, C. T. Parker, S. C. Daugherty, R. J. Dodson, A. S. Durkin, R. Madupu, S. A. Sullivan, J. U. Shetty, M. A. Ayodeji, A. Shvartsbeyn, M. C. Schatz, J. H. Badger, C. M. Fraser, and K. E. Nelson. 2005. Major structural differences and novel potential virulence mechanisms from the genomes of multiple Campylobacter species. PLoS Biol. 3e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goulhen, F., E. Dé, J. M. Pages, and J. M. Bolla. 2004. Functional refolding of the Campylobacter jejuni MOMP (major outer membrane protein) porin by GroEL from the same species. Biochem. J. 378851-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guccione, E., R. Leon-Kempis Mdel, B. M. Pearson, E. Hitchin, F. Mulholland, P. M. van Diemen, M. P. Stevens, and D. J. Kelly. 2008. Amino acid-dependent growth of Campylobacter jejuni: key roles for aspartase (AspA) under microaerobic and oxygen-limited conditions and identification of AspB (Cj0762), essential for growth on glutamate. Mol. Microbiol. 6977-93. [DOI] [PubMed] [Google Scholar]
- 13.Jin, S., A. Joe, J. Lynett, E. K. Hani, P. Sherman, and V. L. Chan. 2001. JlpA, a novel surface-exposed lipoprotein specific to Campylobacter jejuni, mediates adherence to host epithelial cells. Mol. Microbiol. 391225-1236. [DOI] [PubMed] [Google Scholar]
- 14.Jin, S., Y. C. Song, A. Emili, P. M. Sherman, and V. L. Chan. 2003. JlpA of Campylobacter jejuni interacts with surface-exposed heat shock protein 90α and triggers signalling pathways leading to the activation of NF-κB and p38 MAP kinase in epithelial cells. Cell. Microbiol. 5165-174. [DOI] [PubMed] [Google Scholar]
- 15.Kervella, M., J.-M. Pagès, Z. Pei, G. Grollier, M. J. Blaser, and J.-L. Fauchère. 1993. Isolation and characterization of two Campylobacter glycine-extracted proteins that bind to HeLa cell membranes. Infect. Immun. 613440-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konkel, M. E., J. E. Christensen, A. S. Dhillon, A. B. Lane, R. Hare-Sanford, D. M. Schaberg, and C. L. Larson. 2007. Campylobacter jejuni strains compete for colonization in broiler chicks. Appl. Environ. Microbiol. 732297-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konkel, M. E., J. E. Christensen, A. M. Keech, M. R. Monteville, J. D. Klena, and S. G. Garvis. 2005. Identification of a fibronectin-binding domain within the Campylobacter jejuni CadF protein. Mol. Microbiol. 571022-1035. [DOI] [PubMed] [Google Scholar]
- 18.Konkel, M. E., S. G. Garvis, S. L. Tipton, D. E. Anderson, Jr., and W. Cieplak, Jr. 1997. Identification and molecular cloning of a gene encoding a fibronectin-binding protein (CadF) from Campylobacter jejuni. Mol. Microbiol. 24953-963. [DOI] [PubMed] [Google Scholar]
- 19.Konkel, M. E., S. A. Gray, B. J. Kim, S. G. Garvis, and J. Yoon. 1999. Identification of the enteropathogens Campylobacter jejuni and Campylobacter coli based on the cadF virulence gene and its product. J. Clin. Microbiol. 37510-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konkel, M. E., M. R. Monteville, V. Rivera-Amill, and L. A. Joens. 2001. The pathogenesis of Campylobacter jejuni-mediated enteritis. Curr. Issues Intest. Microbiol. 255-71. [PubMed] [Google Scholar]
- 21.Kwan, P. S. L., M. Barrigas, F. J. Bolton, N. P. French, P. Gowland, R. Kemp, H. Leatherbarrow, M. Upton, and A. J. Fox. 2008. Molecular epidemiology of Campylobacter jejuni populations in dairy cattle, wildlife, and the environment in a farmland area. Appl. Environ. Microbiol. 745130-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labigne-Roussel, A., J. Harel, and L. Tompkins. 1987. Gene transfer from Escherichia coli to Campylobacter species: development of shuttle vectors for genetic analysis of Campylobacter jejuni. J. Bacteriol. 1695320-5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson, C. L., D. H. Shah, A. S. Dhillon, D. R. Call, S. Ahn, G. J. Haldorson, C. Davitt, and M. E. Konkel. 2008. Campylobacter jejuni invade chicken LMH cells inefficiently and stimulate differential expression of the chicken CXCLi1 and CXCLi2 cytokines. Microbiology 1543835-3847. [DOI] [PubMed] [Google Scholar]
- 24.Leon-Kempis Mdel, R., E. Guccione, F. Mulholland, M. P. Williamson, and D. J. Kelly. 2006. The Campylobacter jejuni PEB1a adhesin is an aspartate/glutamate-binding protein of an ABC transporter essential for microaerobic growth on dicarboxylic amino acids. Mol. Microbiol. 601262-1275. [DOI] [PubMed] [Google Scholar]
- 25.Levesque, S., E. Frost, R. D. Arbeit, and S. Michaud. 2008. Multilocus sequence typing of Campylobacter jejuni isolates from humans, chickens, raw milk, and environmental water in Quebec, Canada. J. Clin. Microbiol. 463404-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, W. G., S. L. W. On, G. Wang, S. Fontanoz, A. J. Lastovica, and R. E. Mandrell. 2005. Extended multilocus sequence typing system for Campylobacter coli, C. lari, C. upsaliensis, and C. helveticus. J. Clin. Microbiol. 432315-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monteville, M. R., and M. E. Konkel. 2002. Fibronectin-facilitated invasion of T84 eukaryotic cells by Campylobacter jejuni occurs preferentially at the basolateral cell surface. Infect. Immun. 706665-6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monteville, M. R., J. E. Yoon, and M. E. Konkel. 2003. Maximal adherence and invasion of INT 407 cells by Campylobacter jejuni requires the CadF outer-membrane protein and microfilament reorganization. Microbiology 149153-165. [DOI] [PubMed] [Google Scholar]
- 29.Moser, I., W. Schroeder, and J. Salnikow. 1997. Campylobacter jejuni major outer membrane protein and a 59-kDa protein are involved in binding to fibronectin and INT 407 cell membranes. FEMS Microbiol. Lett. 157233-238. [DOI] [PubMed] [Google Scholar]
- 30.Oyarzabal, O. A., K. S. Macklin, J. M. Barbaree, and R. S. Miller. 2005. Evaluation of agar plates for direct enumeration of Campylobacter spp. from poultry carcass rinses. Appl. Environ. Microbiol. 713351-3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pei, Z., and M. J. Blaser. 1993. PEB1, the major cell-binding factor of Campylobacter jejuni, is a homolog of the binding component in Gram-negative nutrient transport systems. J. Biol. Chem. 26818717-18725. [PubMed] [Google Scholar]
- 32.Pei, Z., C. Burucoa, B. Grignon, S. Baqar, X.-Z. Huang, D. J. Kopecko, A. L. Bourgeois, J.-L. Fauchere, and M. J. Blaser. 1998. Mutation in the peb1A locus of Campylobacter jejuni reduces interactions with epithelial cells and intestinal colonization of mice. Infect. Immun. 66938-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahin, O., T. Y. Morishita, and Q. Zhang. 2002. Campylobacter colonization in poultry: sources of infection and modes of transmission. Anim. Health Res. Rev. 395-105. [DOI] [PubMed] [Google Scholar]
- 34.Stern, N. J., and S. Pretanik. 2006. Counts of Campylobacter spp. on U.S. broiler carcasses. J. Food Prot. 691034-1039. [DOI] [PubMed] [Google Scholar]
- 35.Young, V. B., and L. S. Mansfield. 2005. Campylobacter infection—clinical context, p. 1-12. In J. M. Ketley and M. E. Konkel (ed.), Campylobacter: molecular and cellular biology. Horizon Bioscience, Norwich, United Kingdom.
- 36.Ziprin, R. L., C. R. Young, L. H. Stanker, M. E. Hume, and M. E. Konkel. 1999. The absence of cecal colonization of chicks by a mutant of Campylobacter jejuni not expressing bacterial fibronectin-binding protein. Avian Dis. 43586-589. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.