Abstract
Our previous studies showed that surfactant protein D (SP-D) is present in human tear fluid and that it can protect corneal epithelial cells against bacterial invasion. Here we developed a novel null-infection model to test the hypothesis that SP-D contributes to the clearance of viable Pseudomonas aeruginosa from the healthy ocular surface in vivo. Healthy corneas of Black Swiss mice were inoculated with 107 or 109 CFU of invasive (PAO1) or cytotoxic (6206) P. aeruginosa. Viable counts were performed on tear fluid collected at time points ranging from 3 to 14 h postinoculation. Healthy ocular surfaces cleared both P. aeruginosa strains efficiently, even when 109 CFU was used: e.g., <0.01% of the original inoculum was recoverable after 3 h. Preexposure of eyes to bacteria did not enhance clearance. Clearance of strain 6206 (low protease producer), but not strain PAO1 (high protease producer), was delayed in SP-D gene-targeted (SP-D−/−) knockout mice. A protease mutant of PAO1 (PAO1 lasA lasB aprA) was cleared more efficiently than wild-type PAO1, but this difference was negligible in SP-D−/− mice, which were less able to clear the protease mutant. Experiments to study mechanisms for these differences revealed that purified elastase could degrade tear fluid SP-D in vivo. Together, these data show that SP-D can contribute to the clearance of P. aeruginosa from the healthy ocular surface and that proteases can compromise that clearance. The data also suggest that SP-D degradation in vivo is a mechanism by which P. aeruginosa proteases could contribute to virulence.
Pseudomonas aeruginosa, an opportunistic pathogen, is a leading cause of bacterial keratitis. While normal, healthy human corneas remain resistant to infection, contact lens wear or corneal injury/surgery can enable susceptibility (5, 15, 26). The mechanisms by which these factors predispose to infection are not yet well understood.
A murine scarification model has been used exclusively to study the pathogenesis of P. aeruginosa corneal infection (3, 9, 30). That model involves scratching the cornea with a sterile needle prior to adding bacteria, which enables bacteria to directly access the exposed stroma. The resulting disease resembles P. aeruginosa infection in people. More recently, we used a healing model of murine corneal infection to show that 6 h after scratching, the mouse cornea remains susceptible to infection, but by 12 h, it regains resistance to infection despite loss of barrier function to fluorescein staining (18). These injury models that enable P. aeruginosa to infect the cornea have led to a wealth of information about how infection develops and resulting pathology. Yet, the mechanisms by which the normal ocular surface remains healthy under normal circumstances have not been explored in vivo. This cannot be studied using a scratch model. The corneas' ability to resist disease despite constant daily exposure to potential pathogens is remarkable, and learning about the mechanisms involved could help us to develop new therapies for disease of the eye and possibly other sites.
Results from the 12-hour healing situation suggested that defense systems other than barrier function can protect the ocular surface against infection. These defenses could involve biochemical factors constitutively expressed or upregulated in response to injury or bacterial exposure. Candidate factors could include defensin or other antimicrobial peptides, secretory immunoglobulin A, and mucin glycoproteins (11, 14, 23).
In this study we focused on surfactant protein D (SP-D), which we have previously shown is present at the ocular surface, is upregulated by P. aeruginosa or its antigens, and can protect corneal epithelial cells against invasion (27, 28). Others have shown that SP-D-deficient mice lose their capacity to recover from P. aeruginosa keratitis when the eye is made susceptible using an injury model (25). Here our aim was to explore the role of SP-D in protecting the healthy eye against bacterial colonization. Thus, we developed a novel null-infection model in which the cornea is not damaged prior to inoculation with P. aeruginosa. The objective was to allow bacteria to interact with the healthy ocular surface (intact cornea) to enable us to study host factors that normally protect the eye from developing infection when it is not susceptible and the potential role that bacterial factors might play in compromising those defenses. This new model was then used to test the hypothesis that SP-D contributes to the clearance of P. aeruginosa from the healthy ocular surface and to explore the role of P. aeruginosa proteases in promoting ocular colonization in vivo.
MATERIALS AND METHODS
Bacterial strains.
Cytotoxic strain 6206 and invasive strain PAO1 expressing green fluorescent protein (GFP) on pSMC2 plasmid (PAO1-GFP) were used for experiments comparing clearance of a cytotoxic strain and an invasive strain (see Fig. 1 and 2). PAO1-GFP was provided by Gerald B. Pier (Harvard Medical School, Boston, MA) and grown on Trypticase soy agar supplemented with carbenicillin (300 μg/ml). Wild-type PAO1 and its isogenic mutant PAO1 lasA lasB aprA (6) without GFP were used in other experiments (see Fig. 3 and 4). Inocula were prepared from overnight cultures grown on Trypticase soy agar plates at 37°C for ∼16 h before suspension in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich, St. Louis, MO) to a concentration of 109 or 1011 CFU/ml. Bacterial concentrations were confirmed by viable count.
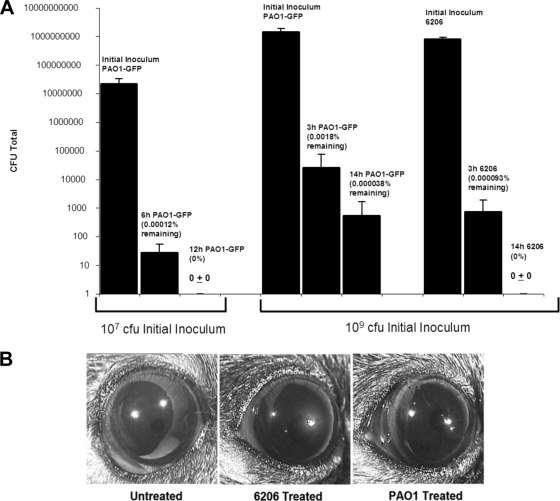
FIG. 1.
(A) Detection of invasive PAO1-GFP or cytotoxic 6206 P. aeruginosa in the tear fluid of Black Swiss mice after inoculation of healthy eyes with 107 or 109 CFU of bacteria. Data were quantified as the means ± standard deviations of viable bacteria recovered from the tear fluid at 3, 6, 12, or 14 h postinoculation and expressed as a percentage of the initial inoculum. (B) Photographs of representative eyes of Black Swiss mice stained with fluorescein to observe any superficial disruption of the corneal epithelium 14 h after inoculation with 109 CFU of 6206 or PAO1-GFP. The 6206-treated (but not PAO1-GFP-treated) ocular surface shows superficial epithelial damage.
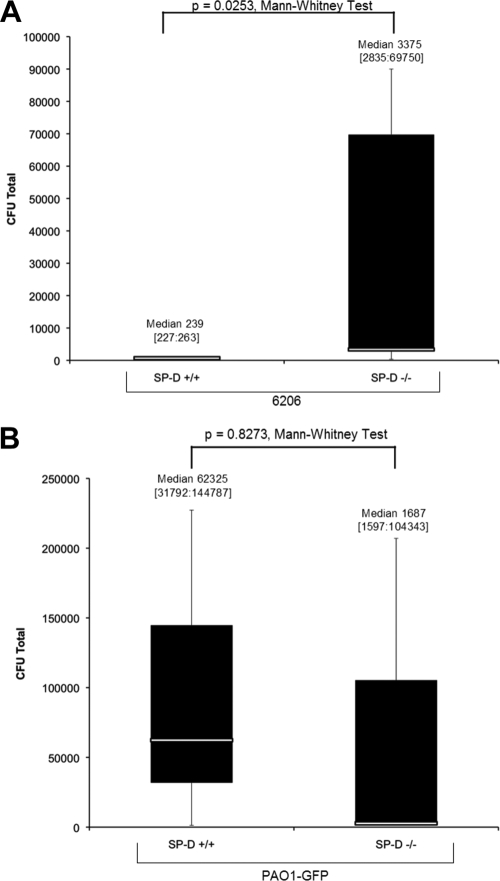
FIG. 2.
Clearance of 6206 (A) or PAO1-GFP (B) from the healthy ocular surface of wild-type versus SP-D−/− mice at 3 h postinoculation with 109 CFU bacteria. Eyes were preexposed to a similar inoculum for 16 h prior to the assay. Data are expressed as the median [lower quartile:upper quartile] number of viable bacteria recovered from the tear fluid. Data represent one of three independent experiments (n = 3 to 5 mice per group).
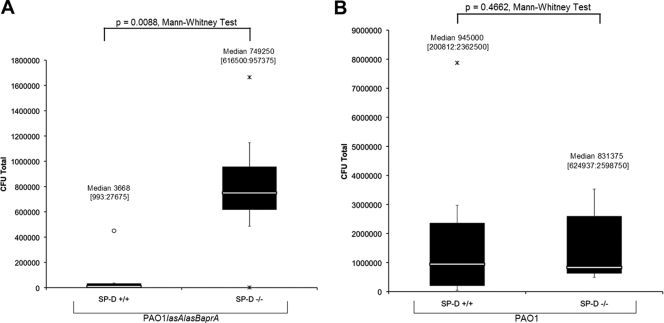
FIG. 3.
Detection of the P. aeruginosa protease mutant PAO1 lasA lasB aprA (A) or its parent PAO1 (B) in the tear fluid of wild-type or SP-D−/− mice at 3 h after inoculation with 109 CFU of bacteria. Eyes were preexposed to the same inoculum for 16 h prior to the assay. Data are expressed as the median [lower quartile:upper quartile] number of viable bacteria recovered from the tear fluid. Data represent one of three independent experiments (n = 7 to 8 mice per group).
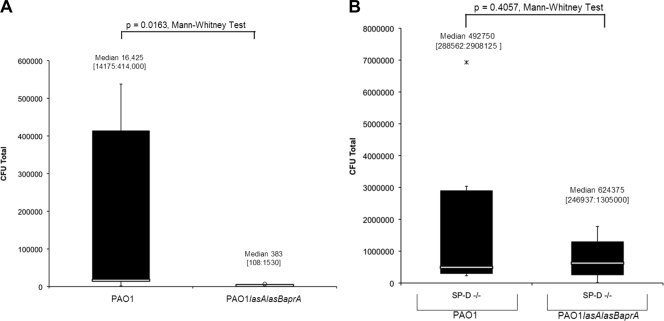
FIG. 4.
(A) Detection of viable P. aeruginosa strain PAO1 or its isogenic mutant PAO1 lasA lasB aprA in wild-type murine tear fluid 3 h after inoculation with 109 CFU of bacteria. Eyes were preexposed to the same inoculum for 16 h prior to the assay. (B) Clearance of PAO1 and that of its isogenic mutant were also compared in SP-D−/− mice. Data are expressed as the median [lower quartile:upper quartile] number of viable bacteria recovered in the tear fluid. Data represent one of three independent experiments (n = 5 to 10 mice per group).
Murine null-infection model.
Wild-type Black Swiss mice (6 to 8 weeks old) and age-matched transgenic SP-D−/− mice were used. SP-D−/− Black Swiss mice were obtained from Jeffrey Whitsett (Children's Hospital Medical Center, Cincinnati, OH) (16). Wild-type Black Swiss mice with a genetic background matching that of the SP-D−/− mice were purchased from Taconic (Seattle, WA).
After induction of anesthesia (intraperitoneal injection with 21 mg/ml ketamine, 2.4 mg/ml xylazine, and 0.3 mg/ml acepromazine), 5 μl of bacterial inoculum containing ∼107 or 109 CFU was applied to the healthy ocular surface. At 3, 6, 12, and 14 h postinoculation, tear fluid was collected from the ocular surface and the number of viable bacteria within was determined. Tear fluid was collected by capillary action using a 10-μl-volume glass capillary tube (Drummond Scientific Co., Broomall, PA) from the lateral canthus after 4 μl of phosphate-buffered saline was added to the ocular surface. In some experiments, whole eyes were enucleated after collection of tears and homogenized in 1 ml of phosphate-buffered saline with 0.25% Triton X-100 for viable counts. In other experiments, eyes were preexposed to bacteria for ∼16 h prior to inoculation in order to potentially stimulate innate defenses. Ocular health was monitored throughout the experiment to ensure the absence of disease pathology. All experiments involved between 3 and 10 animals per group and were repeated at least twice. All procedures were carried out in accordance with the protocol established by the Association for the Research in Vision and Ophthalmology and were approved by the Animal Care and Use Committee, University of California, Berkeley.
Bacterial elastolytic activity assay.
Bacterial inocula were prepared in DMEM at a concentration of 1011 CFU/ml. Inocula were centrifuged for 5 min at 14,000 × g, and 50 μl of the supernatant was added to Eppendorf tubes containing 10 mg of elastin Congo red (Elastin Products Company, Owensville, MO) in 1 ml of sodium phosphate buffer (Na2HPO4, 10 mM, pH 7.0). Known concentrations of purified elastase (Elastin Products Company, Owensville, MO; Calbiochem, Gibbstown, NJ) were included to form a standard curve. Samples were incubated at 37°C for 2 h with constant shaking before centrifugation for 5 min at 5,000 × g to remove insoluble substrate. Absorbance of the supernatants was measured as optical density at 495 nm, and elastase activity was determined by reference to the standard curve.
Measurement of elastase-mediated SP-D degradation in vivo and in vitro.
For in vivo studies, both ocular surfaces of five anesthetized wild-type Black Swiss mice were inoculated with 80 μg/ml of purified elastase in a vehicle of DMEM containing 4% (vol/vol) glycerol for 1 h. A control group of five anesthetized mice were inoculated with vehicle only (DMEM with 4% [vol/vol] glycerol). After 1 h, tear fluid was collected as described previously. The total protein concentration of each sample was measured using the DC protein assay (Bio-Rad, Hercules, CA), and equivalent amounts of each sample were resolved on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis precast Tris-HCl polyacrylamide gel (Bio-Rad, Hercules, CA) under reducing conditions (100 V, 1.5 h). SP-D was then detected by Western immunoblotting as previously described using rabbit anti-mouse SP-D antiserum diluted 1:750. For in vitro studies, recombinant SP-D or murine or human tear fluid was mixed with either DMEM (vehicle) or 20 μg/ml of purified elastase suspended in DMEM for 3 h at 37°C and SP-D was detected as described above. Human tears were collected from a healthy human volunteer using a 30-μl-volume capillary tube under a protocol approved by the Committee for the Protection of Human Subjects, University of California, Berkeley.
Statistical analysis.
The statistical significances in numbers of viable bacteria were compared between two groups nonparametrically using the Mann-Whitney U test. Accordingly, the number of bacteria recovered is expressed as the median and upper and lower quartiles. In the figures, asterisks show outliers within 3 interquartile ranges below the first quartile or above the third quartile and circles show outliers beyond 3 interquartile ranges of the first and third quartiles. Each panel of Fig. 2A to 4B is representative of independent experiments conducted on different days. In each instance, data sets were analyzed using the Brown-Forsythe test, which confirmed that there were no significant differences in the variance between the two groups compared, thereby allowing use of the Mann-Whitney U test. P < 0.05 was considered statistically significant.
RESULTS
P. aeruginosa is efficiently cleared from the healthy ocular surface.
Healthy eyes of wild-type mice cleared both strains of P. aeruginosa (6206 and PAO1-GFP) efficiently in a time-dependent manner. After inoculation with 107 CFU of invasive strain PAO1-GFP, only 0.00012% of the original inoculum was detected in the tear fluid after 6 h, and by 12 h no viable bacteria were recovered from the tears (Fig. 1A). Similar results were obtained using a larger inoculum of this strain (109 CFU), with only 0.0018% and 0.000038% of the original inoculum recovered from the tears after 3 h and 14 h, respectively. Even more efficient clearance was noted for the cytotoxic strain 6206, with only 0.000093% recovered after 3 h (∼20-fold lower than that of PAO1-GFP at the same time point), and no viable bacteria were recovered after 14 h (Fig. 1A). Similar low percentages of bacteria were recovered from ocular homogenates 14 h after inoculation using 109 CFU of either strain, showing that differences in clearance from the tear fluid did not result from bacterial relocation to other ocular sites. Fluorescein staining 14 h after inoculation showed that 6206 caused more superficial damage to the cornea than did PAO1-GFP (Fig. 1B). Nevertheless, 6206 bacteria were cleared more efficiently from the ocular surface than were PAO1-GFP bacteria and neither caused disease.
SP-D-deficient mice demonstrate delayed clearance of cytotoxic strain 6206.
SP-D-deficient mice were used to study the role of SP-D in clearance of P. aeruginosa from the healthy ocular surface. Healthy eyes of wild-type and SP-D−/− Black Swiss mice were inoculated with 109 CFU of either 6206 or PAO1-GFP for ∼16 h and were then rechallenged for 3 h with the same number of bacteria. Preexposure with bacteria was used as a potential stimulant to upregulate potential innate defense factors (17, 21, 28), although it is not known if this occurs in this model. At 3 h after inoculation with 109 CFU of 6206, significantly more bacteria (∼12-fold) were isolated from the tear fluid of the SP-D−/− mice than from that of the wild-type mice (P = 0.025, Mann-Whitney test) (Fig. 2A). However, no significant differences were found for invasive strain PAO1-GFP (Fig. 2B).
SP-D-deficient mice show delayed clearance of the protease mutant of strain PAO1.
Since the data indicated that 6206 was cleared more efficiently from the ocular surface than was PAO1-GFP and 6206 is known to express low protease activity compared to PAO1 (1, 29), we hypothesized that proteases may compromise clearance. Thus, clearance of PAO1 was compared to that of an isogenic protease mutant in both wild-type and SP-D−/− mice. The data showed that significantly more protease-mutant bacteria remained in the tear fluid of the SP-D−/− mice than in that of wild-type mice (P = 0.009, Mann-Whitney test) (Fig. 3A). No such differences were found for PAO1 (P = 0.4662, Mann-Whitney test) (Fig. 3B). In wild-type mice, significantly lower numbers of protease-mutant bacteria than protease-competent PAO1 bacteria were recovered from the tear fluid (∼43-fold, P = 0.016, Mann-Whitney test) (Fig. 4A). Differences between PAO1 and protease-mutant bacteria were not statistically significant in SP-D−/− mice (P = 0.4057, Mann-Whitney test) (Fig. 4B).
Purified P. aeruginosa elastase degrades tear fluid SP-D in vitro and in vivo.
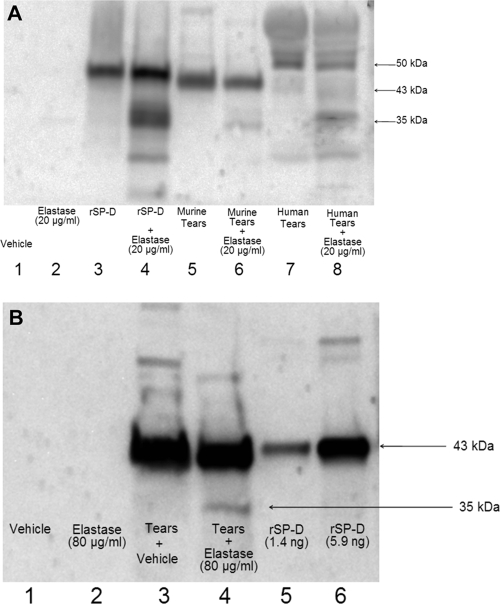
SP-D is known to be cleaved by P. aeruginosa proteases in vitro and in rat and human bronchoalveolar lavage fluid into an inactive 35-kDa form (1, 20, 22). Since the data showed that proteases delayed bacterial clearance and that SP-D expedites this clearance process in the absence of proteases, we next explored whether degradation of SP-D by P. aeruginosa proteases could occur in tear fluid, which could provide a potential mechanism for that effect. In vitro elastolytic assays with typical inocula used in this study (1011 CFU/ml) revealed that PAO1 expressed ∼70.4 μg/ml of elastase while 6206 expressed only ∼5.6 μg/ml. To avoid other inoculum-related confounding factors (i.e., toxins that might interfere with the assay), purified elastase, rather than bacteria, was used in this study. Tear fluid collected from wild-type mice was treated with 20 μg/ml of purified elastase for 3 h at 37°C. The same treatment was applied to tear fluid collected from healthy human volunteers. Control samples were treated with vehicle (DMEM). Western immunoblot analysis showed the presence of monomeric SP-D (∼43 kDa) in mouse tears and a slightly larger (∼50 kDa) form of SP-D in the human tears (Fig. 5A, lanes 5 and 7, respectively), which may relate to a form of SP-D of similar size reported previously by others in human respiratory lavage and amniotic fluid (24). A degraded SP-D fragment of ∼35 kDa was observed only in elastase-treated mouse and human tear fluid samples (Fig. 5A, lanes 6 and 8, respectively) and in elastase-treated recombinant SP-D samples (Fig. 5A, lane 4). These data showed that in the context of tear fluid, SP-D is susceptible to cleavage by P. aeruginosa elastase. To determine if tear SP-D was also degraded in vivo, purified elastase (80 μg/ml), at levels similar to those present in our experiments using bacteria, was added to the healthy ocular surface of wild-type mice for 1 h. Control eyes were inoculated with vehicle only. Western immunoblotting of collected tears showed the presence of monomeric SP-D (∼43 kDa) in control eyes (Fig. 5B, lane 3). In contrast, elastase-treated eyes showed both monomeric SP-D and a ∼35-kDa band of degraded SP-D (Fig. 5B, lane 4).
FIG. 5.
(A) Western immunoblot showing the effects of purified elastase treatment (20 μg/ml, 3 h) on murine or human tears and recombinant SP-D in vitro. Under reducing conditions, the SP-D antibody detected degraded 35-kDa product in each of the elastase-treated samples but not in vehicle (DMEM) controls (lanes 4, 6, and 8). (B) Western immunoblot of murine tear fluid collected from healthy eyes of wild-type Black Swiss mice 1 h after inoculation with purified elastase (80 μg/ml) in vivo (tears were pooled from five mice; see Materials and Methods). Under reducing conditions, monomeric SP-D (∼43 kDa) was detected in the murine tear fluid (lanes 3 and 4). The SP-D degradation product at 35 kDa was observed in eyes treated with elastase (lane 4) but not in eyes treated with vehicle (DMEM with 4% glycerol) (lane 3). The rabbit anti-mouse SP-D antibody did not react with vehicle (lane 1) or purified elastase (80 μg/ml) (lane 2) when used alone. Lanes 5 and 6 were loaded with recombinant murine SP-D at different concentrations (positive control).
DISCUSSION
In this study, we developed a novel null-infection model and used it to show that P. aeruginosa is rapidly cleared from the healthy ocular surface of mice. We found that SP-D can contribute to this process and that it can be delayed by the expression of bacterial proteases. Suggesting a connection between those two findings are the results that SP-D within tear fluid can be degraded by P. aeruginosa elastase in vitro and in vivo, that SP-D−/− animals clear wild-type and protease-mutant bacteria similarly, and that protease-mutant bacteria (or strains producing less elastase) are cleared more effectively from wild-type animals than from SP-D−/− mice.
The ocular surface is constantly exposed to a diverse array of potentially pathogenic microbes. The efficient clearance of microorganisms entering the eye is likely to be important in the maintenance of ocular health. Factors that are likely to contribute include blinking and tear exchange for physical removal of bacteria in addition to tear biochemical factors that bind, aggregate, and/or inactivate microorganisms. Known activities of SP-D include binding and aggregation of P. aeruginosa (and other microbial pathogens) (4), direct antimicrobial activity (32), and a role in limiting P. aeruginosa-induced corneal pathology in an injury model of corneal infection (25) and during infection of the respiratory tract (10). SP-D is also known to facilitate phagocytosis by macrophages and to modulate activity of phagocytes (31). It is known that there are resident dendritic cells within the cornea (13). The upregulation of corneal epithelial cell SP-D in response to bacterial antigens, which we previously reported (28), could be important in the mechanism by which SP-D contributes to clearance from the healthy ocular surface.
The data showing a relationship between protease expression and retention of P. aeruginosa at the ocular surface could involve SP-D degradation in vivo by proteases. While P. aeruginosa elastase and protease IV had already been shown to degrade purified SP-D into an inactive form (1, 20), those previous studies were done in vitro. In this study, we showed that elastase can also degrade SP-D when it is within tear fluid either in vitro or in vivo. The data also showed that differences in clearance between wild-type and protease-mutant bacteria seen in wild-type animals are no longer statistically significant in SP-D−/− mice. Taken together, these data suggest that SP-D degradation provides a possible mechanism for the delayed clearance of protease-competent bacteria compared to protease mutants. However, the relationship between bacterial proteases, SP-D expression, and ocular clearance of bacteria will require further study to determine the contribution of elastase (and other P. aeruginosa proteases) toward the in vivo degradation of SP-D and the biological significance of this finding given the continued renewal of this innate defense protein by the lacrimal apparatus and ocular surface epithelia (27, 28). In addition, proteases could also promote ocular colonization through other mechanisms. For example, P. aeruginosa proteases are known to degrade tear immunoglobulins (19), which could compromise their known ocular defense against infection (23). Whether previously demonstrated roles for elastase and other proteases in increasing bacterial adherence to the mouse cornea (12), or in invasion and penetration through epithelia (2, 6), relate to the role of proteases in colonization of the healthy cornea is yet to be determined.
Cytotoxic P. aeruginosa (6206) was found to be cleared more rapidly than the invasive strain (PAO1). Interestingly, 6206 encodes and expresses a powerful cytotoxin, ExoU (absent in PAO1), which can repress phagocyte infiltration of infected corneas in vivo (33), injure and kill corneal epithelial cells in vitro (8), and also damage the intact corneal epithelium ex vivo (7). Indeed, our data showed that at the inoculum used in this study, cytotoxic strain 6206 did damage corneal barrier function in vivo, as indicated by fluorescein staining. The rapid clearance of this cytotoxic strain (relative to PAO1), despite its capacity to cause superficial damage in vivo, may reflect its low level or lack of protease activity as previously reported (29) and confirmed in the present study.
Traditional models for studying bacterial keratitis in which disease is induced do not allow normal resistance factors to be directly examined. In this study, we developed, and demonstrated, the usefulness of a new null-infection model for this purpose. While we have shown SP-D to be involved in clearance and have shown that P. aeruginosa proteases can compromise it, there are likely to be an array of host factors that protect the eye under normal circumstances and there are also likely to be bacterial factors with the potential to compromise clearance. Further studies using this model could facilitate our understanding of the circumstances surrounding resistance and susceptibility to infection and could eventually lead to new approaches for treatment or prevention of infection of the eye and of other sites.
Acknowledgments
This work was supported by NIH research grant R01-EY11221 (S.M.J.F.) and by unrestricted gift funds from Allergan Pharmaceuticals (S.M.J.F.) and also by NIH HL24075 and HL58047 (S.H.).
S. M. J. Fleiszig and D. J. Evans are coinventors on a U.S. patent, “Methods and Composition for Treating Ocular Disease” (10/823,819).
Editor: V. J. DiRita
Footnotes
Published ahead of print on 6 April 2009.
REFERENCES
- 1.Alcorn, J. F., and J. R. Wright. 2004. Degradation of pulmonary surfactant protein D by Pseudomonas aeruginosa elastase abrogates innate immune function. J. Biol. Chem. 27930871-30879. [DOI] [PubMed] [Google Scholar]
- 2.Azghani, A. O. 1996. Pseudomonas aeruginosa and epithelial permeability: role of virulence factors elastase and exotoxin A. Am. J. Respir. Cell Mol. Biol. 15132-140. [DOI] [PubMed] [Google Scholar]
- 3.Beisel, K. W., L. D. Hazlett, and R. S. Berk. 1983. Dominant susceptibility effect on the murine corneal response to Pseudomonas aeruginosa. Proc. Soc. Exp. Biol. Med. 172488-491. [DOI] [PubMed] [Google Scholar]
- 4.Bufler, P., B. Schmidt, D. Schikor, A. Bauernfeind, E. C. Crouch, and M. Griese. 2003. Surfactant protein A and D differently regulate the immune response to nonmucoid Pseudomonas aeruginosa and its lipopolysaccharide. Am. J. Respir. Cell Mol. Biol. 28249-256. [DOI] [PubMed] [Google Scholar]
- 5.Cheng, K. H., S. L. Leung, H. W. Hoekman, W. H. Beekhuis, P. G. Mulder, A. J. Geerards, and A. Kijlstra. 1999. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet 354181-185. [DOI] [PubMed] [Google Scholar]
- 6.Cowell, B. A., S. S. Twining, J. A. Hobden, M. S. Kwong, and S. M. Fleiszig. 2003. Mutation of lasA and lasB reduces Pseudomonas aeruginosa invasion of epithelial cells. Microbiology 1492291-2299. [DOI] [PubMed] [Google Scholar]
- 7.Fleiszig, S. M., E. J. Lee, C. Wu, R. C. Andika, V. Vallas, M. Portoles, and D. W. Frank. 1998. Cytotoxic strains of Pseudomonas aeruginosa can damage the intact corneal surface in vitro. CLAO J. 2441-47. [PubMed] [Google Scholar]
- 8.Fleiszig, S. M., T. S. Zaidi, M. J. Preston, M. Grout, D. J. Evans, and G. B. Pier. 1996. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect. Immun. 642288-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerke, J. R., and M. V. Magliocco. 1971. Experimental Pseudomonas aeruginosa infection of the mouse cornea. Infect. Immun. 3209-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giannoni, E., T. Sawa, L. Allen, J. Wiener-Kronish, and S. Hawgood. 2006. Surfactant proteins A and D enhance pulmonary clearance of Pseudomonas aeruginosa. Am. J. Respir. Cell Mol. Biol. 34704-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gipson, I. K. 2007. The ocular surface: the challenge to enable and protect vision: the Friedenwald lecture. Investig. Ophthalmol. Vis. Sci. 484390-4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta, S. K., S. A. Masinick, J. A. Hobden, R. S. Berk, and L. D. Hazlett. 1996. Bacterial proteases and adherence of Pseudomonas aeruginosa to mouse cornea. Exp. Eye Res. 62641-650. [DOI] [PubMed] [Google Scholar]
- 13.Hamrah, P., and M. R. Dana. 2007. Corneal antigen-presenting cells. Chem. Immunol. Allergy 9258-70. [DOI] [PubMed] [Google Scholar]
- 14.Haynes, R. J., P. J. Tighe, and H. S. Dua. 1998. Innate defence of the eye by antimicrobial defensin peptides. Lancet 352451-452. [DOI] [PubMed] [Google Scholar]
- 15.Keay, L., K. Edwards, T. Naduvilath, K. Forde, and F. Stapleton. 2006. Factors affecting the morbidity of contact lens-related microbial keratitis: a population study. Investig. Ophthalmol. Vis. Sci. 474302-4308. [DOI] [PubMed] [Google Scholar]
- 16.Korfhagen, T. R., V. Sheftelyevich, M. S. Burhans, M. D. Bruno, G. F. Ross, S. E. Wert, M. T. Stahlman, A. H. Jobe, M. Ikegami, J. A. Whitsett, and J. H. Fisher. 1998. Surfactant protein-D regulates surfactant phospholipid homeostasis in vivo. J. Biol. Chem. 27328438-28443. [DOI] [PubMed] [Google Scholar]
- 17.Kumar, A., L. D. Hazlett, and F. S. Yu. 2008. Flagellin suppresses the inflammatory response and enhances bacterial clearance in a murine model of Pseudomonas aeruginosa keratitis. Infect. Immun. 7689-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, E. J., D. J. Evans, and S. M. Fleiszig. 2003. Role of Pseudomonas aeruginosa ExsA in penetration through corneal epithelium in a novel in vivo model. Investig. Ophthalmol. Vis. Sci. 445220-5227. [DOI] [PubMed] [Google Scholar]
- 19.Lomholt, J. A., and M. Kilian. 2008. Degradation of uniquely glycosylated secretory immunoglobulin A in tears from patients with Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci. 494939-4944. [DOI] [PubMed] [Google Scholar]
- 20.Malloy, J. L., R. A. Veldhuizen, B. A. Thibodeaux, R. J. O'Callaghan, and J. R. Wright. 2005. Pseudomonas aeruginosa protease IV degrades surfactant proteins and inhibits surfactant host defense and biophysical functions. Am. J. Physiol. Lung Cell. Mol. Physiol. 288L409-L418. [DOI] [PubMed] [Google Scholar]
- 21.Maltseva, I. A., S. M. Fleiszig, D. J. Evans, S. Kerr, S. S. Sidhu, N. A. McNamara, and C. Basbaum. 2007. Exposure of human corneal epithelial cells to contact lenses in vitro suppresses the upregulation of human beta-defensin-2 in response to antigens of Pseudomonas aeruginosa. Exp. Eye Res. 85142-153. [DOI] [PubMed] [Google Scholar]
- 22.Mariencheck, W. I., J. F. Alcorn, S. M. Palmer, and J. R. Wright. 2003. Pseudomonas aeruginosa elastase degrades surfactant proteins A and D. Am. J. Respir. Cell Mol. Biol. 28528-537. [DOI] [PubMed] [Google Scholar]
- 23.Masinick, S. A., C. P. Montgomery, P. C. Montgomery, and L. D. Hazlett. 1997. Secretory IgA inhibits Pseudomonas aeruginosa binding to cornea and protects against keratitis. Investig. Ophthalmol. Vis. Sci. 38910-918. [PubMed] [Google Scholar]
- 24.Mason, R. J., L. D. Nielsen, Y. Kuroki, E. Matsuura, J. H. Freed, and J. M. Shannon. 1998. A 50-kDa variant form of human surfactant protein D. Eur. Respir. J. 121147-1155. [DOI] [PubMed] [Google Scholar]
- 25.McCormick, C. C., J. A. Hobden, C. L. Balzli, J. M. Reed, A. R. Caballero, B. S. Denard, A. Tang, and R. J. O'Callaghan. 2007. Surfactant protein D in Pseudomonas aeruginosa keratitis. Ocul. Immunol. Inflamm. 15371-379. [DOI] [PubMed] [Google Scholar]
- 26.Morgan, P. B., N. Efron, N. A. Brennan, E. A. Hill, M. K. Raynor, and A. B. Tullo. 2005. Risk factors for the development of corneal infiltrative events associated with contact lens wear. Investig. Ophthalmol. Vis. Sci. 463136-3143. [DOI] [PubMed] [Google Scholar]
- 27.Ni, M., D. J. Evans, S. Hawgood, E. M. Anders, R. A. Sack, and S. M. Fleiszig. 2005. Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by Pseudomonas aeruginosa. Infect. Immun. 732147-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni, M., C. Tam, A. Verma, R. Ramphal, S. Hawgood, D. J. Evans, and S. M. Fleiszig. 2008. Expression of surfactant protein D in human corneal epithelial cells is upregulated by Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol. 54177-184. [DOI] [PubMed] [Google Scholar]
- 29.Nouwens, A. S., M. D. Willcox, B. J. Walsh, and S. J. Cordwell. 2002. Proteomic comparison of membrane and extracellular proteins from invasive (PAO1) and cytotoxic (6206) strains of Pseudomonas aeruginosa. Proteomics 21325-1346. [DOI] [PubMed] [Google Scholar]
- 30.Preston, M. J., S. M. Fleiszig, T. S. Zaidi, J. B. Goldberg, V. D. Shortridge, M. L. Vasil, and G. B. Pier. 1995. Rapid and sensitive method for evaluating Pseudomonas aeruginosa virulence factors during corneal infections in mice. Infect. Immun. 633497-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Restrepo, C. I., Q. Dong, J. Savov, W. I. Mariencheck, and J. R. Wright. 1999. Surfactant protein D stimulates phagocytosis of Pseudomonas aeruginosa by alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 21576-585. [DOI] [PubMed] [Google Scholar]
- 32.Wu, H., A. Kuzmenko, S. Wan, L. Schaffer, A. Weiss, J. H. Fisher, K. S. Kim, and F. X. McCormack. 2003. Surfactant proteins A and D inhibit the growth of gram-negative bacteria by increasing membrane permeability. J. Clin. Investig. 1111589-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zolfaghar, I., D. J. Evans, R. Ronaghi, and S. M. Fleiszig. 2006. Type III secretion-dependent modulation of innate immunity as one of multiple factors regulated by Pseudomonas aeruginosa RetS. Infect. Immun. 743880-3889. [DOI] [PMC free article] [PubMed] [Google Scholar]