Abstract
Pigs infected with Ascaris suum or controls were given 100 μg (low-dose) or 1,000 μg (high-dose) all-trans retinoic acid (ATRA)/kg body weight in corn oil or corn oil alone per os on days after inoculation (DAI) −1, +1, and +3 with infective eggs. Treatment with ATRA increased interleukin 4 (IL4) and IL12p70 in plasma of infected pigs at 7 DAI and augmented bronchoalveolar lavage (BAL) eosinophilia observed at 7 and 14 DAI. To explore potential molecular mechanisms underlying these observations, a quantitative real-time reverse transcription (RT)-PCR array was used to examine mRNA expression in tissue. Ascaris-infected pigs had increased levels of liver mRNA for T-helper-2 (Th2)-associated cytokines, mast cell markers, and T regulatory (Treg) cells, while infected pigs given ATRA had higher IL4, IL13, CCL11, CCL26, CCL17, CCL22, and TPSB1 expression. Gene expression for Th1-associated markers (IFNG, IL12B, and TBX21), the CXCR3 ligand (CXCL9), IL1B, and the putative Treg marker TNFRSF18 was also increased. Expression of IL4, IL13, IL1B, IL6, CCL11, and CCL26 was increased in the lungs of infected pigs treated with ATRA. To determine a putative cellular source of eosinophil chemoattractants, alveolar macrophages were treated with IL4 and/or ATRA in vitro. IL4 induced CCL11, CCL17, CCL22, and CCL26 mRNA, and ATRA increased the basal and IL4-stimulated expression of CCL17 and CCL22. Thus, ATRA augments a diverse Th1-, Th2-, Treg-, and inflammation-associated response in swine infected with A. suum, and the increased BAL eosinophilia may be related to enhanced induction of eosinophil chemokine activity by alveolar macrophages.
Ascaris lumbricoides is an extracellular gastrointestinal nematode parasite that affects up to 1/4 of the world's population, including an estimated 4 million people in the United States, a number that is increasing with immigration from areas of high exposure (52). A closely related species, Ascaris suum, infects >50% of pigs raised for food production worldwide and can be transmitted to humans (53). Pigs infected with A. suum exhibit characteristic immediate-type hypersensitivity in the lungs as the larvae migrate through the alveolar spaces. Migrating larvae produce focal liver lesions and eosinophilic pneumonitis in both humans and swine (40b). Ascaris suum antigens have been used to model localized allergic hypersensitivity and asthma in several different mammalian species, including pigs, because they elicit allergic symptoms similar to those manifested in humans infected with A. lumbricoides (45). The porcine model of asthma very closely approximates the typical response of the human asthmatic airway to inhaled allergens (43, 44). Ascaris-induced immunoglobulin E (IgE) production, localized eosinophilia, and increased ex vivo T-helper-2 (Th2) cytokine production indicated that immunity to Ascaris in pigs and humans is dominated by a Th2 response (2, 11, 15). It is well established that helminth infections coexist with malnutrition (25), and nutrient deficiencies and supplementation affect the immune responses to helminths (8, 21, 25, 34, 36). Conversely, helminth infection negatively affects nutrient status (23, 25).
Vitamin A (VA) and VA-like retinoids modify Th1-, Th2-, and T regulatory (Treg)-associated immune responses in rodents and humans, but a definitive mechanism(s) of action is lacking. VA via all-trans retinoic acid (ATRA) binding to RAR-α was shown to affect the development of T-cell subsets, and a RAR-α-selective retinoid (13c) inhibited gamma interferon (IFN-γ) from antigen-stimulated mouse T cells (22, 33b). Some studies showed that systemic administration of RAR-α-selective retinoids inhibited Th1-associated immune responses, such as delayed-type hypersensitivity (DTH) (33b, 55a), the progression of experimental arthritis (33a), and skin allograft rejection (41a). The situation in vivo is likely to be more complex, since VA-deficient rodents had diminished DTH and antiviral responses (1, 41b) while exogenous administration of VA or RA increased DTH reactions and augmented Th1-related immune responses to virus (42b, 46a). In addition, the morbidity and mortality associated with malaria and measles increased with VA deficiency (39a, 41b), and VA-supplemented children infected with enteropathogenic Escherichia coli had reduced fecal protein levels of IFN-γ (26a, 26b).
In mice, VA deficiency reduced pulmonary Th2 immune responses to ovalbumin (40d), while supplemental VA (40a, 40b) or ATRA (30a) exacerbated Th2 responses to ovalbumin. We have recently demonstrated that ATRA via RAR-α mediates activation and early Th2 differentiation in human T cells (9, 11b). Experimental VA deficiency generally impairs Th2-associated immunity to helminth parasites and leads to decreased expulsion of Trichinella spiralis (8) and higher parasite burdens in schistosome-infected rats (34). Conversely, VA supplementation reduced Trichinella pseudospiralis levels in infected mice (13b), and VA-supplemented children had reduced reinfection rates with A. lumbricoides and increased fecal IL4 protein (26b, 35a).
There are no clear effects of VA and retinoids on other Th2-related effector responses. Retinoic acid variably affected production of IgE (5, 47, 54) and generally inhibited mast cell (1a, 18, 19, 24), basophil, and eosinophil (12, 26, 35, 55) growth and function. Furthermore, ATRA inhibited IL4-induced eotaxin-1 (CCL11) production in a human bronchial epithelial cell line (46), and VA inhibited Sephadex-induced eotaxin production in the lungs of rats (48); however, it increased IL4- and IL13-induced production of CCL11 and eotaxin 3 (CCL26) in primary bronchial epithelial cells. Recently Grenningloh et al. (16) demonstrated inhibition of experimentally induced allergic lung inflammation, including eosinophilia, in mice treated with an antagonist of the retinoic acid X receptor.
Within the last 2 years, a large body of evidence has accumulated regarding the role of ATRA in modulating Treg activity. We previously observed an expansion of the CD8+ CD28− set of Treg cells during VA deficiency in rats (11a), and Stephensen et al. described expansion of an IL10-secreting CD4+ T-cell subset in VA-deficient mice (42a). ATRA and RAR-α agonists potently stimulated the in vitro development of mouse CD4+ CD25+ FoxP3+ Treg cells in the presence of transforming growth factor beta 1 (TGF-β1) and IL2 (13a, 23a, 40c). In addition, ATRA stimulated human T-cell differentiation into Foxp3+ cells without additional cytokines (23a).
Parasite infection generally evokes powerful activation of humoral, cellular, and regulatory responses at multiple tissue sites and is a useful tool for examining the interaction between diet and immune function. Pigs inoculated with A. suum express localized gene expression patterns for multiple markers of inflammation that are associated with physiological and immunological responses to migrating larvae in the liver, lungs, and intestines that express both Th1- and Th2-derived components (10). Therefore, pigs infected with A. suum and treated with ATRA represent a convenient natural infection model for evaluation of the hypothesis that supplemental ATRA can modulate a multifaceted immune and inflammatory response.
MATERIALS AND METHODS
Animal infection model.
Eight- to 14-week-old Poland China × Landrace × Yorkshire pigs were obtained from the experimental farm at the Beltsville Agricultural Research Center. They were housed in stalls with a nonabsorptive concrete floor surface, two pigs per pen, and had access to water and feed ad libitum. The diet was a corn-soybean formulation containing 16% crude protein and vitamins and minerals that exceeded National Research Council guidelines (10). In the first experiment, 36 pigs (2 time points, 6 treatments, 3 animals per group) either were used as controls or were infected with A. suum (10). The inoculation dose of 20,000 infective eggs was based on a test infection that yielded between 1,500 and 2,000 migrating larvae in the lungs and small intestines of test pigs at 7 and 14 days after inoculation (DAI), respectively (10). ATRA in corn oil or corn oil alone was administered per os by oral gavage at −1, +1, and +3 DAI. Pigs were given 100 (low dose [LD]) or 1,000 (high dose [HD]) μg/kg of body weight ATRA in corn oil; control pigs were given an equivalent amount of corn oil. The maximal dose of ATRA used (0.1 mg/kg) was chosen because it is physiologically relevant, is far below what is generally used (1 to 50 mg/kg) for in vivo rodent studies, and is consistent with the treatment doses administered to humans (4, 28, 41). Pigs were sacrificed 7 and 14 DAI by an overdose of sodium pentobarbital and exsanguination using procedures approved by the Beltsville Animal Care and Use Committee as protocol no. 03-410. A second experiment used only LD ATRA with a similar design; a total of 40 pigs (2 time points, 4 treatments, 5 pigs per group) were sacrificed at 7 and 14 DAI.
Tissue preparation.
Whole blood was obtained by venipuncture in an EDTA Vacutainer (BD, Franklin Lakes, NJ). A complete differential whole-blood count and blood chemistry profile were determined for each pig pre- and postinfection using an automated clinical analyzer (HemaVet 3700 hematology analyzer; CDC Technologies). Comparative profiles of clinical parameters measured are shown in Table S1 in the supplemental material. Bronchoalveolar lavage (BAL) cells were obtained from the lungs of pigs. The large right lobe of the excised lung was gravity filled with 500 ml of phosphate-buffered saline (PBS), followed by massage for 30 s and draining of the cell suspension into 50-ml polypropylene tubes. Anatomically defined portions of the liver and lung were excised with scalpels and forceps and cut into 3-mm3 sections; all samples were immediately flash frozen and stored at −80°C until they were processed for RNA isolation (42).
Cloning and identification of porcine CCL11, CCL17, CCL24, CCL26, CHIT1, CHI3L2, FOXP3, IL9, and RETNLB.
All primers were designed using the Primer Express software package (Applied Biosystems). Genes or partial genes were amplified by 45 cycles of PCR. Primers and products under 100 bp were removed from amplified material using the Qiaquick PCR purification kit (Qiagen). The presence of a single band of expected size was determined using the Agilent Bioanalyzer 2100 and a DNA 1000 Labchip kit (Agilent Technologies, Palo Alto, CA). These products were then amplified for sequencing using a BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems). After purification by Performa spin columns (Edge Biosystems, Gaithersburg, MD), amplicons were spun dry, resuspended in Hi-Di formamide (Applied Biosystems), denatured at 95°C for 2 min, and sequenced on a 3100 Genetic Analyzer 16 capillary array (Applied Biosystems) at the Environmental Microbial Safety Laboratory, ANRI, USDA, Beltsville, MD. A consensus sequence for each gene target was assembled from four independent sequence reads and then compared to the human reference sequences. For CCL26, the human sequence was BLAST searched against the nonredundant GenBank database, yielding a porcine genomic DNA clone, accession no. AC095024. The sequences were assembled to the predicted human gene structure using the software program ClustalW (http://www.ebi.ac.uk/clustalw/). The resulting assembly was used to design primers to porcine CCL26, yielding a 510-bp sequence containing the entire predicted coding region. The open reading frame of porcine CCL26 is predicted to encode a 94-amino-acid protein that shares high amino acid identity and similarity with canine (75%, 89%), bovine (72%, 85%), and human (65%, 77%) CCL26 but only limited amino acid identity and similarity to rat (44%, 65%) and mouse (41%, 58%) eotaxin-3-like proteins. For CCL11, bovine and human cross-reactive primers generated a partial sequence (159 bp). For CCL17, canine and human cross-reactive primers generated a partial sequence (179 bp). Rat, mouse, and human cross-reactive primers were used to clone the full-length sequence (1,518 bp) for FOXP3. For CHIT1, CHI3L2, IL9, and RETNLB, human reference sequences for each gene were BLAST searched against GenBank trace archives containing porcine genomic DNA. Sequences were assembled to the predicted human gene structure using the program ClustalW. PCR primers were designed to the predicted sequences and were used to generate partial sequences corresponding to the CHIT1 (186 bp), CHI3L2 (390 bp), IL9 (164 bp), and RETNLB (220 bp) open reading frames from a mixed-tissue cDNA library.
BAL cell preparation and culture.
The BAL cells were washed 2× with and resuspended in RPMI 1640 medium (Gibco/Invitrogen, Gaithersburg, MD) with 5% heat-inactivated fetal bovine serum (HyClone, Logan, UT), and cell counts and viability were determined after Trypan blue staining. A porcine alveolar macrophage line, 3D4/21 (ATCC, Manassas, VA), was also tested. Cells were suspended at 2.5 × 106 cells/ml of RPMI 1640 medium containing 5% heat-inactivated fetal bovine serum, and 4 ml was added per well to a six-well plate and then pretreated for 24 h with ethanol as a control, 100 nM ATRA, or 100 nM of the RAR-α agonist, Am580. This was followed by treatment with 5 ng/ml porcine IL4 (Biosource Invitrogen, Carlsbad, CA) or human IL13 (Biosource Invitrogen) for 24 h. Cells were washed 2× with PBS without calcium or magnesium and homogenized in 1 ml of Trizol (Invitrogen, Carlsbad, CA).
Flow cytometry.
BAL cells were stained with specific monoclonal antibodies against porcine macrophages (CD203a/SWC9) (clone 18-5, provided by Y. B. Kim, Department of Microbiology and Immunology, The Chicago Medical School), porcine granulocytes (CD172a/SWC3) (clone 74-22-15, provided by Joan Lunney, ANRI, USDA), and IgG1 and IgG2b isotype controls (Serotec, Raleigh, NC). An isotype-specific (Fab′2) antibody labeled with fluorescein isothiocyanate or R-phycoerythrin (Southern Biotechnology, Birmingham, AL) was used as a secondary detecting antibody. After 15 min on ice, cells were washed and resuspended in PBS-1% formaldehyde (Polysciences, Warrington, PA). Independent gates for monocyte/macrophage and granular cells were used for data collection from at least 10,000 events. The percentage of bound secondary antibody was quantified using the Becton Dickinson FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) and used to measure the relative percentage of positive SWC9 cells (macrophages) and positive SWC3 cells (granulocytes) using the CellQuest software program (Becton Dickinson).
ELISA measurements.
Levels of IL1β, IL2, IL4, IL6, IL8, IL10, IL12p70, IFN-γ, and tumor necrosis factor alpha (TNF-α) in plasma were measured by using the SearchLight porcine cytokine array enzyme-linked immunosorbent assay (ELISA) (Pierce Biotechnology, Rockford, IL) according to the manufacturer's instructions.
RNA extraction, cDNA synthesis, and real-time PCR analysis.
Frozen 3-mm3 tissue sections were rapidly homogenized in Trizol. RNA was extracted from homogenized samples according to the manufacturer's instructions. RNA integrity, quantity, and genomic DNA contamination were assessed using the Agilent Bioanalyzer 2100 and RNA 6000 Labchip kit (Agilent Technologies). RNA was treated with DNase in the presence of RNase inhibitor and quantitatively remeasured and cDNA synthesized using Superscript reverse transcriptase and oligo(dT) (42). All probes and primers were designed by using the Primer Express software (Applied Biosystems, Foster City, CA) and sequences obtained from GenBank or The Institute for Genome Research porcine expressed sequence tag database. Primers and high-performance liquid chromatography-purified, 5′,6-carboxy-4,7,2′,7′-tetrachlorofluorescein-, 3′ Black Hole Quencher-1-labeled fluorescent probes were synthesized (Biosource, Camarillo, CA). Real-time reverse transcription (RT)-PCR was performed using a commercially available kit (ABgene USA, Rochester, NY) using 75 ng/well of cDNA in 25 μl on an ABI 7700 PRISM 7900 sequence detector system (Applied Biosystems, Foster City, CA) or using 45 ng/well of cDNA in 15 μl on an ABI 7900 sequence detector system (Applied Biosystems). A total of 170 or 80 genes were analyzed by real-time RT-PCR in liver or lung tissue, respectively. The selected genes were associated with the development of allergy or asthma, chemokines, response to helminth infection, and/or development of Th1, Th2, or Treg cells. The sequences of the assays and functional annotations are found in the supplemental material (see Table S2) and in our online database (http://www.ars.usda.gov/Services/docs.htm?docid=6065). Data for gene expression in the liver were adjusted for the housekeeping gene RPL32, while data for the lung and alveolar macrophage experiments were adjusted for the housekeeping gene PPIA. Housekeeping gene-adjusted data were analyzed using the ΔΔCT method (27), using control pigs or untreated cells as the comparison group.
Statistics.
All statistical analysis was performed using the Statview 5.0 software program for Macintosh (Abacus Concepts, Berkeley, CA). Data were analyzed for equality of variance using Fisher's F test. If the variance was heterogeneous, the appropriate transformation of the data was performed. Plasma cytokine, hematological, and clinical chemistry values, eosinophil numbers, and tissue mRNA expression (ΔΔCT values) were evaluated by one-way analysis of variance (ANOVA). Fisher's least-squares difference posthoc test was applied to assess differences between treatment groups and tissues. Simple regression analysis was conducted using housekeeping-gene-adjusted CT values of CCL11 or CCL26 as the independent variable and the BAL eosinophil percentage as the dependent variable at 14 DAI. For all analysis, P values of <0.05 were significant and P values between 0.05 and 0.1 were considered marginally significant (49).
Nucleotide sequence accession numbers.
Sequences for CCL11 (accession no. DQ640828), CCL17 (accession no. DQ640828), CCL26 (accession no. NM_001078665), FOXP3 (accession no. NM_001128438), CHIT1 (accession no. EF090909), CHI3L2 (accession no. EF090908), IL9 (accession no. EF055899), and RETNLB (accession no. EF090907) were deposited in GenBank.
RESULTS
ATRA increased Th1- and Th2-associated plasma cytokine levels.
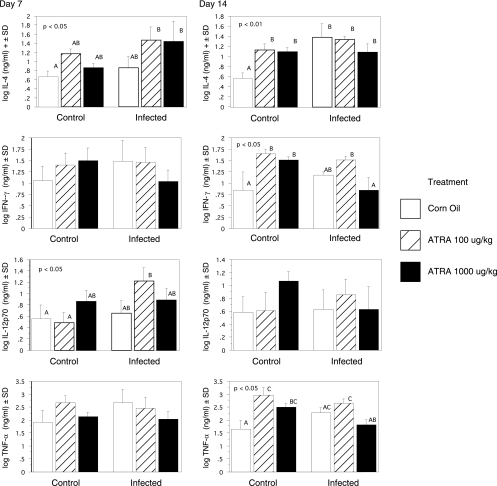
Plasma IL4 levels were significantly elevated for the infected pigs fed LD and HD ATRA at 7 DAI relative to those for control pigs (Fig. 1). At 14 DAI, all pigs fed ATRA or infected pigs had significantly elevated IL4 compared to that for control untreated pigs. IL12p70 levels were not significantly affected by infection or ATRA treatment alone at 7 DAI but were significantly (P < 0.05) increased in infected pigs given LD ATRA. Plasma IFN-γ and TNF-α levels were not affected by infection or ATRA treatment at 7 DAI. At 14 DAI, ATRA alone significantly (P < 0.05) increased IFN-γ and TNF-α. Infection with A. suum increased IFN-γ and TNF-α (P < 0.05) in pigs given LD ATRA.
FIG. 1.
Treatment of pigs with ATRA increased plasma levels of IL4 and IL12p70 after inoculation with A. suum. The IFN-γ, IL4, IL12p70, and TNF-α proteins were measured by ELISA in plasma taken from pigs 7 or 14 DAI with A. suum eggs. The results are expressed as the log of the mean in pg/ml ± SD from three pigs per group and were evaluated by one-way ANOVA. Treatment groups annotated with unique letters are statistically different at P values of <0.05. Plasma IL4 levels were significantly (P < 0.05) elevated for infected pigs fed 100 μg/kg and 1,000 μg/kg ATRA at 7 DAI relative to those for control pigs as determined by ANOVA. At 14 DAI, all pigs fed ATRA or infected pigs had significantly elevated IL4 compared to the level for control untreated pigs. There was also a significant (P < 0.05) increase in IL12p70 for infected pigs given 100 μg/kg ATRA. At 14 DAI, ATRA alone significantly (P < 0.05) increased IFN-γ and TNF-α. Infection with A. suum increased IFN-γ and TNF-α only (P < 0.05) for pigs given LD ATRA.
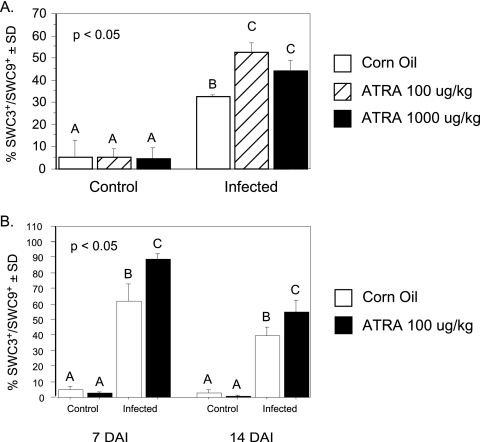
ATRA increased infection-induced lung eosinophilia.
There was a significant (P < 0.05) induction of SWC3+ SWC9− cells in BAL specimens from pigs infected with A. suum at 14 DAI (Fig. 2A). LD and HD ATRA significantly (P < 0.05) augmented this increase, although the percentage of eosinophils was lower for the HD-ATRA pigs than for the LD-ATRA pigs. LD ATRA was also effective at 7 DAI (Fig. 2B) in a replicate experiment. The SWC3 antigen (SIRP-1a/CD172a) is also expressed by neutrophils, but Wright-Giemsa staining of fixed BAL cells confirmed that the granulocytes in the BAL preparation from all infected pigs were largely eosinophils, with <2% being neutrophils and lymphocytes (data not shown).
FIG. 2.
Treatment of pigs with ATRA increased SWC3+ eosinophils in lung tissue after inoculation with A. suum. BAL cells were harvested at 14 DAI. SWC3+ SWC9− cells were enumerated by flow cytometry. Treatment groups annotated with unique letters are statistically different as determined by one-way ANOVA at P values of <0.05. Infection induced a significant increase in SWC3+ SWC9− cells in BAL specimens from pigs infected with A. suum. LD and HD ATRA significantly augmented this increase (A). Infection also induced a significant accumulation of SWC3+ SWC9− cells in BAL specimens from pigs infected with A. suum at 7 DAI in a replicate study, and LD ATRA significantly augmented this increase (B).
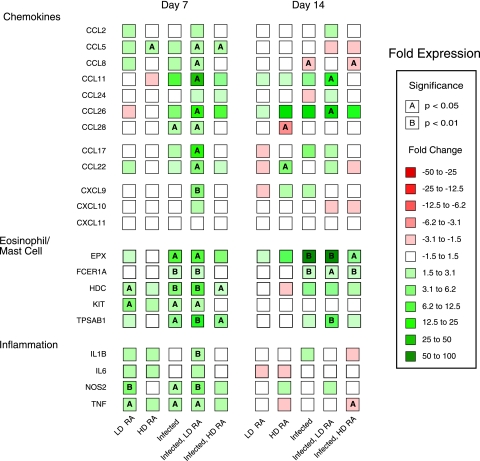
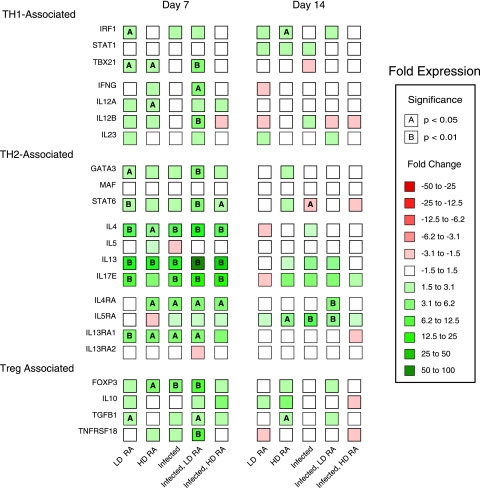
ATRA increased infection-induced expression of genes associated with inflammation, Th1, Th2, and Treg cells in liver and lung tissue.
Infection and ATRA induced the expression of a very large number of the genes in the liver, with key groups of genes highlighted in Fig. 3 and 4 (see Tables S3 [day 7] and S4 [day 14] in the supplemental material for complete details regarding other genes measured in liver specimens). Infection and ATRA altered the expression of several Th1-associated genes in the liver. The expression of the Th1-associated genes, IFNG and IL12B, was not significantly affected by infection or ATRA treatment alone at 7 DAI but increased significantly (P < 0.05 and P < 0.005, respectively) in infected pigs given LD ATRA (Fig. 4). At 7 DAI, IL12A was significantly upregulated by HD ATRA alone but was only marginally (P = 0.07) upregulated by LD ATRA for infected pigs. These relationships were not observed at 14 DAI. The data for IL12B mRNA in liver specimens paralleled that for IL12p70 protein found in plasma.
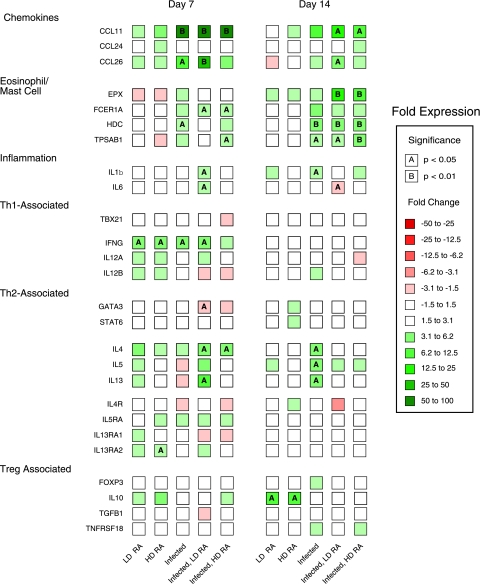
FIG. 3.
Expression of chemokines, eosinophil/mast cell, and inflammatory genes in the livers of pigs infected with A. suum and treated with ATRA. Real-time PCR detection of mRNA expression (CT values) for CCR3 and CCR4 ligands and selected genes associated with eosinophils, mast cells, and inflammation in the liver at 7 and 14 days after inoculation was evaluated by one-way ANOVA. n-fold changes relative to results for control pigs are designated by color differences, defined in the figure insert, and statistical significance by A (P < 0.05) or B (P < 0.01).
FIG. 4.
Expression of Th1, Th2, and Treg genes in the liver tissue of pigs infected with A. suum and treated with ATRA. Real-time PCR detection of mRNA expression (CT values) for selected genes associated with Th1 or Th2 cells or Tregs in the livers of pigs infected with A. suum and treated with two different doses of ATRA is shown. Tissue mRNA expression (CT values) at 7 or 14 days after inoculation was evaluated by one-way ANOVA. n-fold changes relative to results for control pigs are designated by color differences, shown in the figure insert, and statistical significance by A (P < 0.05) or B (P < 0.01).
The expression of Th2-associated genes in the liver was greatly influenced by infection and ATRA. At 7 DAI, samples from uninfected pigs given LD ATRA showed increased liver mRNA expression of IL4 (3.6-fold; P < 0.01) and IL13 (14.3-fold, P < 0.005), while those from infected pigs showed significantly (P < 0.05) higher expression of IL4 (4-fold [P < 0.005]) and IL13 (20-fold [P < 0.005]), and infected pigs given LD ATRA had higher levels of IL4 (8-fold versus the control level [P < 0.0001] and 2-fold that for infected pigs [P < 0.05]) and IL13 (61-fold versus the control level [P < 0.0001] and 3-fold that for infected animals [P = 0.06]) (Fig. 4). In samples from pigs given HD ATRA, IL4 and IL13 levels were significantly (P < 0.05) higher than those for control pigs but nearly identical to those for infected pigs. IL5 and IL9 levels were unaffected by infection or ATRA at either time point. Both GATA3 and STAT6 expression was significantly induced (P < 0.05 and P < 0.001, respectively) for control pigs and infected pigs given LD-ATRA at 7 DAI, and STAT6 expression was also significantly (P < 0.05) enhanced due to infection and treatment with HD ATRA. At 14 DAI, STAT6 expression was significantly downregulated for infected pigs versus that for controls (P < 0.05). The expression of MAF did not change at either time.
In addition to Th1- and Th2-associated genes, infection and/or ATRA induced Treg-associated gene expression in liver tissue. The expression of FOXP3 was significantly induced (4.1-fold [P < 0.05]) by HD ATRA, by Ascaris infection (4.6-fold [P < 0.01]), and by infection along with LD-ATRA treatment (9.7-fold [P < 0.001]) (Fig. 4). The degree of FOXP3 induction by Ascaris infection and LD-ATRA treatment was additive. However, significantly increased TNFRSF18/GITR expression was found only in infected and LD-ATRA-treated pigs (9.6-fold over control level [P < 0.01] and 4.8-fold over level for infected pigs [P < 0.05]). IL10 expression was unaffected by infection or either dose of ATRA at both time points. TGFB1 expression was marginally increased by infection (1.8-fold [P = 0.06]); this difference reached full statistical significance (P < 0.05) only in LD-ATRA-treated pigs with and without infection. The expression of these markers was not statistically different at 14 DAI except for an increase in TGFB1 expression in HD-ATRA-treated pigs (P < 0.05).
Infection and ATRA induced the expression of CCR3- and CCR4-associated chemokines in liver tissue. Expression of CCL11 was increased (P < 0.05) sevenfold, and that of CCL26 (EOT3) was increased fourfold for the infected pigs relative to that for control pigs at 7 DAI (Fig. 3). The expression of CCL11 (5-fold over that for infected pigs [P = 0.09]) and that of CCL26 (8.6-fold over that for infected pigs [P < 0.01]) were higher for pigs given LD ATRA. The expression of CCL11 for infected pigs given HD ATRA was similar to that for infected pigs. Expression of CCL26 for infected pigs given HD ATRA was significantly (P < 0.05) different from that for control pigs but only slightly higher than that for infected pigs. At 14 DAI, CCL11 expression was increased for infected pigs, but this difference was not statistically significant. It was significantly (P < 0.01) upregulated 11-fold for infected pigs given LD ATRA compared to the level for uninfected control pigs. CCL26 was upregulated to a similar degree for infected pigs given corn oil and LD ATRA but was lower for infected pigs given HD ATRA. The expression of CCL24 was not significantly affected by infection or ATRA.
CCL5 (RANTES) exhibited a significant (P < 0.05) two- to threefold induction in samples from pigs give LD ATRA and infected pigs given LD and HD ATRA. CCL28 (MEC) was significantly (P < 0.05) upregulated twofold in samples from infected pigs and infected pigs given LD ATRA. The expression of the CCR4 ligands CCL17 (TARC) and CCL22 (MDC) increased significantly (P < 0.05) only for the infected pigs given LD ATRA compared to levels for uninfected pigs. Treatment of infected pigs with LD ATRA selectively induced CCR3 and CCR4 ligands, since the expression of other CCL chemokines, CCL2 (MCP-1), CCL3 (MIP-1A), CCL4 (MIP-1B), CCL16 (LEC), CCL19 (MIP-3B), CCL21 (SLC), and CCL23 (MPIF-1), did not change (Fig. 3) (see Tables S3 and S4 in the supplemental material). Furthermore, the expression of the CXCR3 ligands, CXCL10 and CXCL11, did not differ between treatment groups at any time. In contrast, CXCL9 (MIG), another CXCR3 ligand, was significantly upregulated at 7 DAI by LD ATRA in infected pigs (P < 0.005). No changes in any CXC, CXCR, or CX3CR chemokines were observed at 14 DAI (see Table S4 in the supplemental material).
Mast cell- and eosinophil-associated gene expression in liver was induced by infection and ATRA. Infected pigs had significantly higher expression levels of the mast cell-associated markers, CMA1 (P < 0.05), FCER1A (P < 0.005), HDC (P < 0.01), and TPSB1 (P < 0.05) at 7 DAI, and infected pigs given LD ATRA had marginally higher levels of TPSB1 (threefold) than infected pigs (P = 0.1) (Fig. 3). The levels of FCER1A and HDC were slightly elevated and that of CMA1 was lower for this group than for infected pigs. In contrast, HD-ATRA-treated pigs had levels of TPSB1 similar to those for infected pigs. At 14 DAI, the expression of TPSB1 was higher for all infected pigs; however, it was significantly different (6.9-fold [P < 0.005]) only for infected pigs treated with LD ATRA compared to results for control uninfected pigs.
The expression of the eosinophil-associated-marker, EPX, was significantly (P < 0.05) upregulated in infected pigs (10-fold) and infected pigs given LD ATRA (9-fold); however, expression in pigs given HD ATRA increased only 4-fold and was not significant (Fig. 3). Similarly, at 14 DAI, expression of EPX was significantly (P < 0.0005) upregulated in infected pigs (30-fold) and infected pigs given LD ATRA (26-fold); however, expression in pigs given HD ATRA increased 5-fold (P = 0.08). Finally, the expression of the proinflammatory cytokine IL1B was significantly (P < 0.005) increased in the liver tissue of infected pigs treated with LD ATRA at 7 DAI (Fig. 3).
The expression of Th1- and Th2-associated genes in lung tissue was greatly influenced by infection and ATRA. The expression of key groups of genes is highlighted in Fig. 5 (see Tables S5 [day 7] and S6 [day 14] in the supplemental material for details on other genes measured). The expression of IFNG was upregulated by LD ATRA, HD ATRA, infection alone, and treatment of infected pigs with LD ATRA at 7 DAI (Fig. 5) (see Table S5 in the supplemental material). There was no significant change in expression of other Th1-associated genes at 7 or 14 DAI. The expression of IL4 and IL13 was each significantly (P < 0.005 and P < 0.0005, respectively) upregulated (four- and eightfold) by LD ATRA for infected pigs at 7 DAI. IL4 was also upregulated threefold for infected pigs given HD ATRA (P < 0.05). IL5 was upregulated for infected pigs given LD ATRA (P = 0.07). At 14 DAI, the expression of IL4, IL5, and IL13 was significantly upregulated only for corn oil-treated infected pigs. GATA3 expression was significantly induced (P < 0.05) only for infected pigs given LD ATRA at 7 DAI.
FIG. 5.
Expression of selected CCR3 ligands and selected genes associated with eosinophils, mast cells, inflammation, and Th1-, Th2-, or Treg cells in the lung of pigs infected with A. suum and treated with two different doses of ATRA. Tissue mRNA expression (CT values) at 7 or 14 days after inoculation were evaluated by one-way ANOVA. n-fold changes from results for control pigs are designated by color differences, shown in the figure insert, and statistical significance by A (P < 0.05) or B (P < 0.01).
The expression of a select group of chemokines and inflammation-associated markers was induced by infection and ATRA in lung tissue. At 7 DAI, CCL11 was highly upregulated (40- to 55-fold) in the lungs of all infected pigs regardless of the ATRA dose (Fig. 5). In contrast, CCL26 was upregulated 10-fold (P < 0.05) for infected pigs and 32-fold (P < 0.005) for infected pigs given LD ATRA; the increase for infected pigs given HD ATRA was not significant. At 14 DAI, CCL11 expression was upregulated approximately 8-fold for infected pigs (P = 0.06) and infected pigs treated with HD ATRA (P < 0.05); however, it was 14-fold higher than that for controls for pigs treated with LD ATRA (P < 0.05). CCL26 expression was upregulated approximately twofold for infected pigs and infected pigs treated with HD ATRA (both not significant); however, it was expressed at fourfold above the control level for pigs treated with LD ATRA (P < 0.05). Due to limited amounts of RNA isolated from lung tissue compared to that from liver tissue in this experiment, CCL17 and CCL22 expression was not examined. At 7 DAI, expression of the inflammation-associated genes IL1B and IL6 was significantly (P < 0.005 and P < 0.0005, respectively) upregulated threefold by LD ATRA for infected pigs.
Regulation of CCL11, CCL17, and CCL22 and CCL26 expression by IL4 and ATRA in alveolar macrophages.
We speculated that alveolar macrophages activated by Ascaris-induced endogenous IL4 and IL13 production in the lung could interact with exogenous ATRA to produce chemokines that contributed to eosinophil infiltration of BAL during Ascaris infection. The hypothesis was tested using both explanted primary alveolar macrophages isolated from untreated control pigs and an alveolar macrophage cell line, 3D4/21. Cells were pretreated for 24 h with 100 nM ATRA and then with IL4 to approximate the sequence of exposure in the in vivo model. ATRA increased basal and IL4-induced CCL17 and CCL22 expression (Table 1) in both cell populations. We obtained similar results with the RAR-α agonist Am580 (data not shown).
TABLE 1.
Synergistic induction of CCR4-binding chemokine mRNAs by IL4 and ATRAa
| Chemokine gene | Primary MΦ treatment groupb | mRNA expression
|
D4/21 cell treatment group | mRNA expression
|
||
|---|---|---|---|---|---|---|
| ΔCT (mean ± SD) | Fold change vs. control level | ΔCT (mean ± SD) | Fold change vs. control level | |||
| CCL11 | Control | 27.1 ± 1.2a | 1.0 | Control | 22.1 ± 0.3a | 1.0 |
| ATRA | 26.8 ± 1.0a | 1.3 | ATRA | 21.7 ± 0.1a | 1.4 | |
| IL4 | 14.4 ± 0.6b | 7,131 | IL4 | 10.8 ± 0.9b | 2,352 | |
| IL4/ATRA | 13.1 ± 0.6b | 17,560 | IL4/ATRA | 10.5 ± 0.1b | 3,104 | |
| CCL26 | Control | 23.6 ± 1.3a | 1.0 | Control | 21.7 ± 0.9a | 1.0 |
| ATRA | 22.8 ± 1.3a | 2.5 | ATRA | 21.6 ± 0.1a | 1.0 | |
| IL4 | 15.6 ± 2.0b | 247 | IL4 | 12.2 ± 0.9b | 676 | |
| IL4/ATRA | 13.6 ± 2.5b | 1,024 | IL4/ATRA | 12.2 ± 0.1b | 724 | |
| CCL17 | Control | 10.5 ± 1.4a | 1.0 | Control | 20.9 ± 0.3a | 1.0 |
| ATRA | 8.8 ± 0.7b | 3.1 | ATRA | 19.5 ± 0.6b | 2.7 | |
| IL4 | 8.9 ± 0.8b | 3.0 | IL4 | 16.9 ± 0.6c | 16.0 | |
| IL4/ATRA | 7.5 ± 0.6c | 8.0 | IL4/ATRA | 14.9 ± 0.5d | 69.0 | |
| CCL22 | Control | 7.7 ± 0.9a | 1.0 | Control | 16.0 ± 0.3a | 1.0 |
| ATRA | 6.1 ± 0.6b | 3.2 | ATRA | 14.8 ± 0.7b | 2.3 | |
| IL4 | 7.1 ± 0.8a | 1.6 | IL4 | 14.9 ± 0.8b | 2.1 | |
| IL4/ATRA | 5.5 ± 0.5c | 4.6 | IL4/ATRA | 13.5 ± 0.4c | 5.7 | |
Lung-adherent macrophages isolated from BAL cells of uninfected pigs or the macrophage cell line D4/21 were treated with or without ethyl alcohol or 10−6 M ATRA for 18 h and then treated with 5.0 ng/ml of porcine IL4 for 24 h. Data were normalized to the housekeeping gene PPIA. Groups were compared by one-way ANOVA.
Explanted primary alveolar macrophages isolated from untreated control pigs.
DISCUSSION
The role of retinoids or VA in the functional development and recruitment of eosinophils is confounded by data from a variety of both in vitro (30, 34, 39) and in vivo (8, 17, 53) studies. Our studies demonstrated that oral ATRA supplementation in the presence of a strong natural inducer of eosinophilia enhanced eosinophilic infiltration into the lungs commensurate with increased cytokines and chemokine ligands and receptor gene expression detected in lung parenchymal tissue.
We previously showed that pigs infected with A. suum developed a Th2-associated response characterized by increased IL4 and IL13 mRNA levels in multiple tissues (10) and that ATRA increased expression of IL4 and IL13 in human T cells activated with anti-CD3 in vitro (9). Our current study showed that feeding ATRA to pigs stimulated expression of IL4 and IL13 mRNA in the liver, enhanced the A. suum-induced increase in mRNA of these two cytokines in liver and lung, and increased circulating levels of the IL4 protein. This observation is consistent with increased fecal IL4 in children who were infected with A. lumbricoides and supplemented with VA (39). The elevation in IL4 and IL13 mRNA in the liver tissue of uninfected ATRA-treated pigs may represent enhanced responses to background stimulation by food antigens and microbial products derived from the intestine.
CCR3 is a promiscuous chemokine receptor that has 11 reported ligands, including the CCR3 exclusive ligands CCL11, CCL24, and CCL26 (40). These three chemokines are potent eosinophil chemoattractants. Pigs treated with LD ATRA had significantly increased mRNA for CCL11 and CCL26 in liver tissue at 7 DAI and in lungs at 14 DAI, times when eosinophilic lesions due to migrating larvae are prominent. In fact, regression analysis revealed a highly significant relationship between CCL11 (P = 0.002; r2 = 0.61) and CCL26 (P = 0.0015; r2 = 0.48) expression in the lungs of A. suum-infected pigs and the percentage of eosinophils in the BAL specimens by flow cytometry. Only LD ATRA, however, induced CCL26 to a level above that found in infected pigs in liver and lung tissue at 7 and 14 DAI. It is likely that ATRA increased these chemokines through expression of increased levels of IL4 and IL13, because IL4 and IL13 induced the expression of CCL26 in a porcine epithelial cell line (H. Dawson, unpublished) and CCL11 and CCL26 in explanted porcine alveolar macrophages and a porcine alveolar macrophage cell line. We have also observed increased expression of CCL11 and CCL26 in alveolar macrophages isolated from pigs infected with A. suum (G. Solano-Aguilar et al., unpublished).
The CCR4 ligands, CCL17 and CCL22, were upregulated in the liver by LD ATRA in A suum-infected pigs at 7 DAI. CCL17 and CCL22 are induced by IL4 and IL13 in various cell types and are associated with Th2 cells and eosinophilic infiltration into the human lung (30, 38). We observed that IL4 and IL13 induced the expression of CCL17 and CCL22 in porcine alveolar macrophages. We have also observed increased expression of CCL17 in macrophages isolated from pigs infected with A. suum (Solano-Aguilar et al., unpublished) and in the eosinophilic conjunctiva of Trichuris suis-infected pigs immunized and challenged with ragweed in the eye (J. F. Urban et al., unpublished) suggesting that nematode infection generally activates these chemokines in pigs.
The localized amplification of Th1-, Th2-, Treg-, and inflammation-associated hepatic and pulmonary immune responses in Ascaris-infected swine was observed by examination of the mRNA levels at the whole-tissue level so potential cell-derived mechanisms could not be directly explored. We did, however, observe increased production of the CCR4 ligands CCL17 and CCL22 when macrophages were pretreated with ATRA and increased expression of the CCR3 ligands CCL11 and CCL26 due to treatment with IL4. A comprehensive evaluation of nutritional regulation of alternatively activated macrophage development has not been described. Data presented in this article indicated that ATRA, likely acting through RAR-α, caused alveolar macrophages to assume an alternatively activated macrophage-like phenotype in vitro. This likely contributed to the recruitment of CCR3- and CCR4-expressing cells, such as Th2 cells, eosinophils, and Treg cells. Given that ATRA also increased the synthesis of IL4 and IL13 from Th2 cells (9), these data suggested that a localized positive amplification circuit involving T cells, macrophages, and ATRA exists at the site of Th2-dependent responses.
Among all of the known eosinophil chemoattractants, CCL26 or eotaxin 3 is most consistently associated with late-phase tissue eosinophilia (6, 39). Given the prominent role that eosinophil migration into pulmonary tissue plays in asthma, allergy, and nematode parasite infection, the pig can serve as a useful model for studying allergic diseases mediated by CCL26 in humans, since rodents lack a direct functional homologue of CCL26 (31). Furthermore, these data suggest that VA status can contribute to enhanced expression of allergic disease in the lungs.
STAT6 is a critical transcription factor involved in Th2-associated responses (17). STAT6 knockout mice are susceptible to nematode infections (51) and resistant to experimentally induced asthma (32). Increased mRNA expression of STAT6 is observed in the bronchial epithelium of patients with severe asthma (33). In addition, a variant in the regulatory elements of the STAT6 gene is associated with susceptibility to asthma and resistance to A. lumbricoides (14, 37). STAT6 expression was significantly induced by LD ATRA in control pigs and increased by both doses of ATRA in the livers of infected pigs. The expression of most STAT6-dependent genes, CCL11, CCL17, CCL22, CCL26, IL4, and IL13, paralleled STAT6 expression in the liver. The liver cell type(s) that exhibits increased STAT6 expression was not identified in this study; however, increased STAT6 expression is a characteristic of ATRA-treated human T cells (9).
The absence of significant ATRA induction of IL5 mRNA in the liver and lungs of A. suum-infected pigs was surprising given that ATRA enhanced IL5 production during polyclonal activation of human (9) and mouse (22) T cells and in the spleen of tetanus toxoid-immunized neonatal mice (29). The increase in IL5, however, is more tissue restricted in A. suum-infected pigs than is the case for IL4 and IL13 (10), and pulmonary eosinophilia at 7 and 14 DAI appeared to be independent of changes in IL5 (13).
It is notable that the mast-cell activation marker TPSB1 (7) was increased in parallel with IL4 and IL13. Perhaps ATRA primed or stimulated mast cells to be more responsive to parasite-derived products. Unlike the case for most inbred mouse strains maintained in closed facilities, there are appreciable numbers of mucosal mast cells in uninfected pigs maintained in confined pens without exposure to helminth infection that could account for ATRA-induced mast cell markers (50). In addition, intestinal mucosal mast cells increased following exposure to A. suum and released histamine after exposure to parasite-derived antigens via antibody-dependent cross-linking of surface receptors (3). Other investigators have shown that topically applied ATRA increased the number of tryptase-positive mast cells in the skin but had no effect on tryptase- and chymase-double-positive mast cells (19).
The increased expression of Th1-associated markers, IFNG, IL12B, and TBX21, at 7 DAI accompanied higher expression of the Th2-associated genes in liver tissue of ATRA-treated pigs. These data contrast with ATRA-induced downregulation of TBX21 and 9-cis-RA-induced downregulation in human T cells and reduced Tbx21, Ifng, and Il12b mRNA in the spleens of mice treated with a viral RNA mimetic, poly(I:C), and ATRA (28). These differences, however, may be a function of systems that differ in the nature of the stimulation, the host species, and the experimental context.
We observed an increased expression of the Treg-associated mRNAs TNFRSF18/GITR and FOXP3 (20) in the livers of LD-ATRA-treated pigs but no change in TGFB1 and IL10. In humans but not in rodents, TNFRSF18/GITR and FOXP3 are expressed by activated T cells (56). We have observed an increase in FOXP3 mRNA and protein in activated porcine T cells (H. Dawson, unpublished), suggesting another useful experimental parallel between pigs and humans. Both TNFRSF18/GITR and FOXP3 were significantly increased in the lungs of pigs treated with LD ATRA and infected with A. suum at 7 but not 14 DAI. It has not been demonstrated that these changes are due to T cells in the lungs, but there is rapid tissue remodeling in both the liver and lungs following exit of larvae from the tissues that may be associated with increased Treg activity to reduce inflammation. The increased expression of the inflammation-related genes IL1B and IL6 in the lungs of infected pigs treated with LD ATRA, however, is indicative of the early proinflammatory response to migrating larvae that is enhanced by treatment with ATRA.
In conclusion, we observed an increased expression of markers for Th1, Th2, regulatory T cells, and inflammation in liver and lungs of Ascaris-infected pigs treated with ATRA and an increase in BAL eosinophilia. These changes indicated a robust immune response appropriate to controlling pathogens that require both arms of the immune system and regulatory signals that limit inflammation as migrating larvae leave the tissue site. The changes in immune function observed in this study are physiologically relevant, since the most active dose of ATRA used (0.1 mg/kg) is below that generally used (1 to 50 mg/kg) for in vivo studies with rodents and more consistent with the treatment doses administered to VA-deficient humans in developing areas of the world (4, 28, 41). Furthermore, two different doses of ATRA elicited differential effects on several distinct immune parameters. Taken together, these data could help explain the often confounding and contradictory responses observed in inbred rodent strains supplemented with VA and other retinoids compared to responses seen in humans.
Supplementary Material
Acknowledgments
We thank Pat Boyd, Celine Chen, and Joan Lunney, APDL, ANRI, BARC, for assistance in cloning and sequencing the IL22 and FOXP3 genes; Monica Santin-Duran and Christina Hahn, EMSL, ANRI, BARC, for assistance in sequencing the remaining pig genes; and William Hare, Veterinary Services Laboratory, ANRI, BARC, for determination of the differential whole-blood count and blood chemistry profiles.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 30 March 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Ahmed, F., D. B. Jones, and A. A. Jackson. 1991. Effect of vitamin A deficiency on the immune response to epizootic diarrhoea of infant mice (EDIM) rotavirus infection in mice. Br. J. Nutr. 65475-485. [DOI] [PubMed] [Google Scholar]
- 1a.Alexandrakis, M. G., D. S. Kyriakou, D. Seretakis, W. Boucher, R. Letourneau, D. Kempuraj, and T. C. Theoharides. 2003. Inhibitory effect of retinoic acid on proliferation, maturation and tryptase level in human leukemic mast cells (HMC-1). Int. J. Immunopathol. Pharmacol. 1643-47. [DOI] [PubMed] [Google Scholar]
- 2.Artis, D., and R. K. Grencis. 2001. T helper responses during intestinal nematode infection: induction, regulation and effector function, p. 331-371. In M. W. Kennedy and W. Harnett (ed.), Parasitic nematodes: molecular biology, biochemistry and immunology. CAB International, Wallingford, Oxon, United Kingdom.
- 3.Ashraf, M., J. F. Urban, Jr., T. D. Lee, and C. M. Lee. 1988. Characterization of isolated porcine intestinal mucosal mast cells following infection with Ascaris suum. Vet. Parasitol. 29143-158. [DOI] [PubMed] [Google Scholar]
- 4.Austenaa, L. M., H. Carlsen, A. Ertesvag, G. Alexander, H. K. Blomhoff, and R. Blomhoff. 2004. Vitamin A status significantly alters nuclear factor- kappaB activity assessed by in vivo imaging. FASEB J. 181255-1257. [DOI] [PubMed] [Google Scholar]
- 5.Barnett, J. B. 1982. Immunopotentation of the IgE antibody response by 13-cis-retinoic acid. Int. Arch. Allergy Appl. Immunol. 67287-290. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard, C., N. Wang, K. F. Stringer, A. Mishra, P. C. Fulkerson, J. P. Abonia, S. C. Jameson, C. Kirby, M. R. Konikoff, M. H. Collins, M. B. Cohen, R. Akers, S. P. Hogan, A. H. Assa'ad, P. E. Putnam, B. J. Aronow, and M. E. Rothenberg. 2006. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J. Clin. Investig. 116536-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bochner, B. S., and W. W. Busse. 2005. Allergy and asthma. J. Allergy Clin. Immunol. 115953-959. [DOI] [PubMed] [Google Scholar]
- 8.Carman, J. A., L. Pond, F. Nashold, L. Wassom, and C. E. Hayes. 1992. Immunity to Trichinella spiralis infection in vitamin A-deficient mice. J. Exp. Med. 175111-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson, H., G. Collins, R. Pyle, M. Key, A. Weeraratna, V. Deep-Dixit, C. N. Nadal, and D. D. Taub. 2006. Direct and indirect effects of retinoic acid on human Th2 cytokine and chemokine expression in T lymphocytes. BMC Immunol. 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson, H. D., E. Beshah, S. Nishi, G. Solano-Aguilar, M. Morimoto, A. Zhao, K. B. Madden, T. K. Ledbetter, J. P. Dubey, T. Shea-Donohue, J. K. Lunney, and J. F. Urban, Jr. 2005. Localized multigene expression patterns support an evolving Th1/Th2-like paradigm in response to infections with Toxoplasma gondii and Ascaris suum. Infect. Immun. 731116-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson, H. D., A. R. Royaee, S. Nishi, D. Kuhar, W. M. Schnitzlein, F. Zuckermann, J. Urban, Jr., and J. K. Lunney. 2004. Identification of key immune mediators regulating T helper 1 responses in swine. Vet. Immunol. Immunopathol. 100105-111. [DOI] [PubMed] [Google Scholar]
- 11a.Dawson, H. D., and A. C. Ross. 1999. Chronic marginal vitamin A status affects the distribution and function of T cells and natural T cells in aging Lewis rats. J. Nutr. 1291782-1790. [DOI] [PubMed] [Google Scholar]
- 11b.Dawson, H., G. Collins, R. Pyle, M. Key, and D. D. Taub. 2008. The retinoic acid receptor-a mediates human T-cell activation and Th2 cytokine production. BMC Immunol. 911-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denburg, J. A., R. Sehmi, and J. Upham. 2001. Regulation of IL-5 receptor on eosinophil progenitors in allergic inflammation: role of retinoic acid. Int. Arch. Allergy Immunol. 124246-248. [DOI] [PubMed] [Google Scholar]
- 13.Domachowske, J. B., C. A. Bonville, A. J. Easton, and H. F. Rosenberg. 2002. Pulmonary eosinophilia in mice devoid of interleukin-5. J. Leukoc. Biol. 71966-972. [PubMed] [Google Scholar]
- 13a.Elias, K. M., A. Laurence, T. S. Davidson, G. Stephens, Y. Kanno, E. M. Shevach, and J. J. O'Shea. 2008. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood 1111013-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13b.Figallova, V., and J. Prokopic. 1998. Effect of vitamins on Trichinella spiralis Owen, 1835 infection in mice. Folia Parasitol. (Praha) 35157-163. [PubMed] [Google Scholar]
- 13c.Fukasawa, H., T. Iijima, H. Kagechika, Y. Hashimoto, and K. Shudo. 1993. Expression of the ligand-binding domain-containing region of retinoic acid receptors alpha, beta and gamma in Escherichia coli and evaluation of ligand-binding selectivity. Biol. Pharm. Bull. 16343-348. [DOI] [PubMed] [Google Scholar]
- 14.Gao, P. S., N. M. Heller, W. Walker, C. H. Chen, M. Moller, B. Plunkett, M. H. Roberts, R. P. Schleimer, J. M. Hopkin, and S. K. Huang. 2004. Variation in dinucleotide (GT) repeat sequence in the first exon of the STAT6 gene is associated with atopic asthma and differentially regulates the promoter activity in vitro. J. Med. Genet. 41535-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geiger, S. M., C. L. Massara, J. Bethony, P. T. Soboslay, O. S. Carvalho, and R. Correa-Oliveira. 2002. Cellular responses and cytokine profiles in Ascaris lumbricoides and Trichuris trichiura infected patients. Parasite Immunol. 24499-509. [DOI] [PubMed] [Google Scholar]
- 16.Grenningloh, R., A. Gho, P. di Lucia, M. Klaus, W. Bollag, I. C. Ho, F. Sinigaglia, and P. Panina-Bordignon. 2006. Cutting edge: inhibition of the retinoid X receptor (RXR) blocks T helper 2 differentiation and prevents allergic lung inflammation. J. Immunol. 1765161-5166. [DOI] [PubMed] [Google Scholar]
- 17.Hebenstreit, D., G. Wirnsberger, J. Horejs-Hoeck, and A. Duschl. 2006. Signaling mechanisms, interaction partners, and target genes of STAT6. Cytokine Growth Factor Rev. 17173-188. [DOI] [PubMed] [Google Scholar]
- 18.Hirasawa, N., H. Kagechika, K. Shudo, and K. Ohuchi. 2001. Inhibition by retinoids of antigen-induced IL-4 production in rat mast cell line RBL-2H3. Life Sci. 681287-1294. [DOI] [PubMed] [Google Scholar]
- 19.Hjertson, M., P. K. Kivinen, L. Dimberg, K. Nilsson, I. T. Harvima, and G. Nilsson. 2003. Retinoic acid inhibits in vitro development of mast cells but has no marked effect on mature human skin tryptase- and chymase-positive mast cells. J. Investig. Dermatol. 120239-245. [DOI] [PubMed] [Google Scholar]
- 20.Hori, S., and S. Sakaguchi. 2004. Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes Infect. 6745-751. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda, K., S. Matsuo, K. Asano, and K.-I. Okamoto. 1994. Modulation of cytokine secretion by mesentaeric lymph node cells from vitamin A-deficient mice during Hymenolepis nana infection. In Vivo 81015-1018. [PubMed] [Google Scholar]
- 22.Iwata, M., Y. Eshima, and H. Kagechika. 2003. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int. Immunol. 151017-1025. [DOI] [PubMed] [Google Scholar]
- 23.Kaestel, P., F. J. Lewis, A. L. Willingham, H. O. Bogh, L. Eriksen, K. F. Michaelsen, B. Sandstrom, C. E. Hoy, and H. Friis. 1999. Schistosoma japonicum infection and serum and tissue concentrations of retinol and zinc in pigs. Ann. Trop. Med. Parasitol. 93489-499. [PubMed] [Google Scholar]
- 23a.Kang, S. G., H. W. Lim, O. M. Andrisani, H. E. Broxmeyer, and C. H. Kim. 2007. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J. Immunol. 1793724-3733. [DOI] [PubMed] [Google Scholar]
- 24.Kinoshita, T., K. Koike, H. H. Mwamtemi, S. Ito, S. Ishida, Y. Nakazawa, Y. Kurokawa, K. Sakashita, T. Higuchi, K. Takeuchi, N. Sawai, M. Shiohara, T. Kamijo, S. Kawa, T. Yamashita, and A. Komiyama. 2000. Retinoic acid is a negative regulator for the differentiation of cord blood-derived human mast cell progenitors. Blood 952821-2828. [PubMed] [Google Scholar]
- 25.Koski, K. G., and M. E. Scott. 2001. Gastrointestinal nematodes, nutrition and immunity: breaking the negative spiral. Annu. Rev. Nutr. 21297-321. [DOI] [PubMed] [Google Scholar]
- 26.Leber, B. F., and J. A. Denburg. 1997. Retinoic acid modulation of induced basophil differentiation. Allergy 521201-1206. [DOI] [PubMed] [Google Scholar]
- 27.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 27a.Long, K. Z., T. Estrada-Garcia, J. L. Rosado, J. Ignacio Santos, M. Haas, M. Firestone, J. Bhagwat, C. Young, H. L. DuPont, E. Hertzmark, and N. N. Nanthakumar. 2006. The effect of vitamin A supplementation on the intestinal immune response in Mexican children is modified by pathogen infections and diarrhea. J. Nutr. 1361365-1370. [DOI] [PubMed] [Google Scholar]
- 27b.Long, K. Z., J. I. Santos, T. Estrada Garcia, M. Haas, M. Firestone, J. Bhagwat, H. L. Dupont, E. Hertzmark, J. L. Rosado, and N. N. Nanthakumar. 2006. Vitamin A supplementation reduces the monocyte chemoattractant protein-1 intestinal immune response of Mexican children. J. Nutr. 1362600-2605. [DOI] [PubMed] [Google Scholar]
- 28.Ma, Y., Q. Chen, and A. C. Ross. 2005. Retinoic acid and polyriboinosinic:polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations. J. Immunol. 1747961-7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma, Y., and A. C. Ross. 2005. The anti-tetanus immune response of neonatal mice is augmented by retinoic acid combined with polyriboinosinic:polyribocytidylic acid. Proc. Natl. Acad. Sci. USA 10213556-13561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manabe, K., Y. Nishioka, J. Kishi, M. Inayama, Y. Aono, Y. Nakamura, F. Ogushi, H. Bando, K. Tani, and S. Sone. 2005. Elevation of macrophage-derived chemokine in eosinophilic pneumonia: a role of alveolar macrophages. J. Med. Investig. 5285-92. [DOI] [PubMed] [Google Scholar]
- 30a.Maret, M., C. Ruffie, B. Periquet, A. M. Campo, M. Menevret, A. Phelep, K. Dziewiszek, A. Druilhe, and M. Pretolani. 2007. Liposomal retinoic acids modulate asthma manifestations in mice. J. Nutr. 1372730-2736. [DOI] [PubMed] [Google Scholar]
- 31.Mestas, J., and C. C. Hughes. 2004. Of mice and not men: differences between mouse and human immunology. J. Immunol. 1722731-2738. [DOI] [PubMed] [Google Scholar]
- 32.Miyata, S., T. Matsuyama, T. Kodama, Y. Nishioka, K. Kuribayashi, K. Takeda, S. Akira, and M. Sugita. 1999. STAT6 deficiency in a mouse model of allergen-induced airways inflammation abolishes eosinophilia but induces infiltration of CD8+ T cells. Clin. Exp. Allergy 29114-123. [DOI] [PubMed] [Google Scholar]
- 33.Mullings, R. E., S. J. Wilson, S. M. Puddicombe, J. L. Lordan, F. Bucchieri, R. Djukanovic, P. H. Howarth, S. Harper, S. T. Holgate, and D. E. Davies. 2001. Signal transducer and activator of transcription 6 (STAT-6) expression and function in asthmatic bronchial epithelium. J. Allergy Clin. Immunol. 108832-838. [DOI] [PubMed] [Google Scholar]
- 33a.Nagai, H., S. Matsuura, K. Bouda, Y. Takaoka, T. Wang, S. Niwa, and K. Shudo. 1999. Effect of Am-80, a synthetic derivative of retinoid, on experimental arthritis in mice. Pharmacology 58101-112. [DOI] [PubMed] [Google Scholar]
- 33b.Niwa, S., T. Ochi, Y. Hirano, T. Wang, N. Inagaki, K. Shudo, and H. Nagai. 2000. Effect of Am-80, a retinoid derivative, on 2,4-dinitrofluorobenzene- induced contact dermatitis in mice. Pharmacology 60208-214. [DOI] [PubMed] [Google Scholar]
- 34.Parent, G., R. Rousseaux-Prevost, Y. Carlier, and A. Capron. 1984. Influence of vitamin A on the immune response of Schistosoma mansoni-infected rats. Trans. R. Soc. Trop. Med. Hyg. 78380-383. [DOI] [PubMed] [Google Scholar]
- 35.Paul, C. C., S. Mahrer, M. Tolbert, B. L. Elbert, I. Wong, S. J. Ackerman, and M. A. Baumann. 1995. Changing the differentiation program of hematopoietic cells: retinoic acid-induced shift of eosinophil-committed cells to neutrophils. Blood 863737-3744. [PubMed] [Google Scholar]
- 35a.Payne, L. G., K. G. Koski, E. Ortega-Barria, and M. E. Scott. 2007. Benefit of vitamin A supplementation on ascaris reinfection is less evident in stunted children. J. Nutr. 1371455-1459. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen, S., I. Saeed, H. Friis, and K. F. Michaelsen. 2001. Effect of iron deficiency on Trichuris suis and Ascaris suum infections in pigs. Parasitology 122589-598. [DOI] [PubMed] [Google Scholar]
- 37.Peisong, G., A. Yamasaki, X. Q. Mao, T. Enomoto, Z. Feng, F. Gloria-Bottini, E. Bottini, T. Shirakawa, D. Sun, and J. M. Hopkin. 2004. An asthma-associated genetic variant of STAT6 predicts low burden of ascaris worm infestation. Genes Immun. 558-62. [DOI] [PubMed] [Google Scholar]
- 38.Pilette, C., J. N. Francis, S. J. Till, and S. R. Durham. 2004. CCR4 ligands are up-regulated in the airways of atopic asthmatics after segmental allergen challenge. Eur. Respir. J. 23876-884. [DOI] [PubMed] [Google Scholar]
- 39.Ravensberg, A. J., F. L. Ricciardolo, A. van Schadewijk, K. F. Rabe, P. J. Sterk, P. S. Hiemstra, and T. Mauad. 2005. Eotaxin-2 and eotaxin-3 expression is associated with persistent eosinophilic bronchial inflammation in patients with asthma after allergen challenge. J. Allergy Clin. Immunol. 115779-785. [DOI] [PubMed] [Google Scholar]
- 39a.Ross, A. C., and C. B. Stephensen. 1996. Vitamin A and retinoids in antiviral responses. FASEB J. 10979-985. [PubMed] [Google Scholar]
- 40.Rothenberg, M. E., and S. P. Hogan. 2006. The eosinophil. Annu. Rev. Immunol. 24147-174. [DOI] [PubMed] [Google Scholar]
- 40a.Ruhl, R., A. Hanel, A. L. Garcia, A. Dahten, U. Herz, F. J. Schweigert, and M. Worm. 2007. Role of vitamin A elimination or supplementation diets during postnatal development on the allergic sensitisation in mice. Mol. Nutr. Food Res. 511173-1181. [DOI] [PubMed] [Google Scholar]
- 40b.Sakakibara, A., K. Baba, S. Niwa, T. Yagi, H. Wakayama, K. Yoshida, T. Kobayashi, T. Yokoi, K. Hara, M. Itoh, and E. Kimura. 2002. Visceral larva migrans due to Ascaris suum which presented with eosinophilic pneumonia and multiple intra-hepatic lesions with severe eosinophil infiltration—outbreak in a Japanese area other than Kyushu. Intern. Med. 41574-579. [DOI] [PubMed] [Google Scholar]
- 40c.Schambach, F., M. Schupp, M. A. Lazar, and S. L. Reiner. 2007. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur. J. Immunol. 372396-2399. [DOI] [PubMed] [Google Scholar]
- 40d.Schuster, G. U., N. J. Kenyon, and C. B. Stephensen. 2008. Vitamin A deficiency decreases and high dietary vitamin A increases disease severity in the mouse model of asthma. J. Immunol. 1801834-1842. [DOI] [PubMed] [Google Scholar]
- 41.Seguin-Devaux, C., Y. Devaux, V. Latger-Cannard, S. Grosjean, C. Rochette-Egly, F. Zannad, C. Meistelman, P. M. Mertes, and D. Longrois. 2002. Enhancement of the inducible NO synthase activation by retinoic acid is mimicked by RARalpha agonist in vivo. Am. J. Physiol. Endocrinol. Metab. 283E525-E535. [DOI] [PubMed] [Google Scholar]
- 41a.Seino, K., T. Yamauchi, A. Ishibashi, N. Tokuhara, S. Kobayashi, K. Fukunaga, H. Taniguchi, Y. Takada, K. Yuzawa, M. Otsuka, T. Todoroki, and K. Fukao. 2000. Prolongation of mouse skin allograft survival by novel agonists selective for retionic acid receptor-alpha. Transplant Proc. 32257-258. [DOI] [PubMed] [Google Scholar]
- 41b.Smith, S. M., N. S. Levy, and C. E. Hayes. 1987. Impaired immunity in vitamin A-deficient mice. J. Nutr. 117857-865. [DOI] [PubMed] [Google Scholar]
- 42.Solano-Aguilar, G. I., D. Zarlenga, E. Beshah, K. Vengroski, L. Gasbarre, D. Junker, M. Cochran, C. Weston, D. Valencia, C. Chiang, H. Dawson, J. F. Urban, and J. K. Lunney. 2002. Limited effect of recombinant porcine interleukin-12 on porcine lymphocytes due to a low level of IL-12 beta2 receptor. Vet. Immunol. Immunopathol. 89133-148. [DOI] [PubMed] [Google Scholar]
- 42a.Stephensen, C. B., X. Jiang, and T. Freytag. 2004. Vitamin A deficiency increases the in vivo development of IL-10-positive Th2 cells and decreases development of Th1 cells in mice. J. Nutr. 1342660-2666. [DOI] [PubMed] [Google Scholar]
- 42b.Stevenson, M. M., and E. M. Riley. 2004. Innate immunity to malaria. Nat Rev Immunol 4169-180. [DOI] [PubMed] [Google Scholar]
- 43.Sylvin, H., M. Kumlin, and K. Alving. 2003. Cysteinyl leukotrienes in porcine acute allergic airway reactions: bronchial and vascular effects. Inflamm. Res. 52185-190. [DOI] [PubMed] [Google Scholar]
- 44.Sylvin, H., I. van der Ploeg, and K. Alving. 2001. The effect of a bradykinin B2 receptor antagonist, NPC-567, on allergen-induced airway responses in a porcine model. Inflamm. Res. 50453-459. [DOI] [PubMed] [Google Scholar]
- 45.Sylvin, H., E. Weitzberg, and K. Alving. 2002. Endothelin-induced vascular and bronchial effects in pig airways: role in acute allergic responses. J. Appl. Physiol. 931608-1615. [DOI] [PubMed] [Google Scholar]
- 46.Takamura, K., Y. Nasuhara, M. Kobayashi, T. Betsuyaku, Y. Tanino, I. Kinoshita, E. Yamaguchi, S. Matsukura, R. P. Schleimer, and M. Nishimura. 2004. Retinoic acid inhibits interleukin-4-induced eotaxin production in a human bronchial epithelial cell line. Am. J. Physiol. Lung Cell. Mol. Physiol. 286L777-L785. [DOI] [PubMed] [Google Scholar]
- 46a.Tetteh, J. K., M. M. Addae, N. Ishiwada, S. M. Yempewu, S. Yamaguchi, D. Ofori-Adjei, H. Kamiya, Y. Komada, and B. D. Akanmori. 2003. Plasma levels of Th1 and Th2 cytokines in Ghanaian children with vaccine-modified measles. Eur. Cytokine Netw. 14109-113. [PubMed] [Google Scholar]
- 47.Tokuyama, H., and Y. Tokuyama. 1999. The regulatory effects of all-trans-retinoic acid on isotype switching: retinoic acid induces IgA switch rearrangement in cooperation with IL-5 and inhibits IgG1 switching. Cell Immunol. 19241-47. [DOI] [PubMed] [Google Scholar]
- 48.Torii, A., M. Miyake, M. Morishita, K. Ito, S. Torii, and T. Sakamoto. 2004. Vitamin A reduces lung granulomatous inflammation with eosinophilic and neutrophilic infiltration in Sephadex-treated rats. Eur. J. Pharmacol. 497335-342. [DOI] [PubMed] [Google Scholar]
- 49.Tukey, J. W. 1991. The philosophy of multiple comparisons. Stat. Sci. 6100-116. [Google Scholar]
- 50.Urban, J. F., Jr., H. Alizadeh, and R. D. Romanowski. 1988. Ascaris suum: development of intestinal immunity to infective second-stage larvae in swine. Exp. Parasitol. 6666-77. [DOI] [PubMed] [Google Scholar]
- 51.Urban, J. F., Jr., N. Noben-Trauth, D. D. Donaldson, K. B. Madden, S. C. Morris, M. Collins, and F. D. Finkelman. 1998. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8255-264. [DOI] [PubMed] [Google Scholar]
- 52.Valentine, C. C., R. J. Hoffner, and S. O. Henderson. 2001. Three common presentations of ascariasis infection in an urban emergency department. J. Emerg. Med. 20135-139. [DOI] [PubMed] [Google Scholar]
- 53.Wagner, B., and L. Polley. 1997. Ascaris suum prevalence and intensity: an abattoir survey of market hogs in Saskatchewan. Vet. Parasitol. 73309-313. [DOI] [PubMed] [Google Scholar]
- 54.Worm, M., U. Herz, J. M. Krah, H. Renz, and B. M. Henz. 2001. Effects of retinoids on in vitro and in vivo IgE production. Int. Arch. Allergy Immunol. 124233-236. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi, Y., H. Nishio, T. Kasahara, S. J. Ackerman, H. Koyanagi, and T. Suda. 1998. Models of lineage switching in hematopoietic development: a new myeloid-committed eosinophil cell line (YJ) demonstrates trilineage potential. Leukemia 121430-1439. [DOI] [PubMed] [Google Scholar]
- 55a.Yoshimura, H., K. Kikuchi, S. Hibi, K. Tagami, T. Satoh, T. Yamauchi, A. Ishibahi, K. Tai, T. Hida, N. Tokuhara, and M. Nagai. 2000. Discovery of novel and potent retinoic acid receptor alpha agonists: syntheses and evaluation of benzofuranyl-pyrrole and benzothiophenyl-pyrrole derivatives. J. Med. Chem. 432929-2937. [DOI] [PubMed] [Google Scholar]
- 56.Ziegler, S. F. 2006. FOXP3: of mice and men. Annu. Rev. Immunol. 24209-226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.