Abstract
Enteroaggregative Escherichia coli (EAEC) is increasingly being recognized as a cause of diarrheal disease in diverse populations. No small animal model is currently available to study this pathogen. We report here that conventional mice orally inoculated with prototype EAEC strain 042 generally became colonized, though the abundance of organisms cultured from their stool varied substantially among individual animals. In contrast, mice whose water contained 5 g/liter streptomycin consistently became colonized at high levels (ca. 108 CFU/g of stool). Neither conventional nor streptomycin-treated mice developed clinical signs or histopathologic abnormalities. Using specific mutants in competition with the wild-type strain, we evaluated the contribution of several putative EAEC virulence factors to colonization of streptomycin-treated mice. Our data suggest that the dispersin surface protein and Pic, a serine protease autotransporter secreted by EAEC and Shigella flexneri, promote colonization of the mouse. In contrast, we found no role for the aggregative adherence fimbriae, the transcriptional activator AggR, or the surface factor termed Air (enteroaggregative immunoglobulin repeat protein). To study Pic further, we constructed a single nucleotide mutation in strain 042 which altered only the Pic catalytic serine (strain 042PicS258A). Fractionation of the tissue at 24 h and 3 days demonstrated an approximate 3-log10 difference between 042 and 042PicS258A in the lumen and mucus layer and adherent to tissue. Strains 042 and 042PicS258A adhered similarly to mouse tissue ex vivo. While no growth differences were observed in a continuous-flow anaerobic intestinal simulator system, the wild-type strain exhibited a growth advantage over 042PicS258A in a culture of cecal mucus and in cecal contents in vitro; this difference was manifest only after 6 h of growth. Moreover, enhanced growth of the wild type was observed in comparison with that of the mutant in minimal medium containing mucin but not in the absence of mucin. The data suggest a novel metabolic role for the Pic mucinase in EAEC colonization.
Enteroaggregative Escherichia coli (EAEC) is an emerging agent of diarrheal illness in multiple epidemiologic settings (19). The pathogen has been associated with persistent diarrhea in children (4, 5), acute endemic diarrhea (38), persistent diarrhea in AIDS patients (13), food-borne outbreaks (20), and traveler's diarrhea (1). Pathogenesis is believed to occur in the ileum and colon, where the bacteria adhere in a thick, aggregating biofilm, mediated by aggregative adherence fimbriae (AAFs) (7, 21, 28). Following adherence, EAEC causes tissue damage and fluid secretion by elaborating one or more enterotoxins, including the Pet cytotoxin (31), EAST1 (39) and/or ShET1 (11, 12).
Adherence to human intestinal mucosa ex vivo requires the expression of AAFs (7), although additional colonization factors are likely to contribute. We have also described dispersin, a 10-kDa hydrophilic protein which attaches noncovalently to the bacterial cell surface (40). Both dispersin and AAF/II, the AAF allele expressed by strain 042, are under the control of the transcriptional activator AggR (7, 30, 40). In the absence of dispersin, AAF pili collapse onto the surface of the cells, and the bacteria display a hyperaggregative phenotype (40). We have also shown, however, that dispersin mutants are impaired in their abilities to penetrate an artificial mucus layer (40), and they may therefore be paradoxically more adherent but less adept at colonization. In addition, EAEC genome characterization has identified an adhesin called Air (enteroaggregative immunoglobulin repeat protein), which features a large number of immunoglobulin-like repeats and which may promote bacterial aggregation and colonization (41).
In addition to toxins and adhesins, EAEC secretes Pic, a protein with hemagglutinin and mucinolytic activity in vitro (17). Some epidemiologic studies suggest a role for pic or its linked genes in EAEC disease (35). Notably, pic alleles are also present in Shigella flexneri 2a (36) and in uropathogenic E. coli (UPEC) strains (16). Guyer et al. found pic to be more common among pyelonephritis isolates than fecal strains, suggesting a role in urinary tract infections (15, 34). In the CBA mouse model of ascending urinary tract infections, wild-type UPEC tended to colonize better than a pic mutant (16); however, this difference did not reach statistical significance, and further studies have not been reported.
Pic is a 116-kDa secreted autotransporter protein of the SPATE (serine protease autotransporter of enterobacteriaceae) family (18). Sequence alignments and functional comparisons (9) have shown that Pic and its alleles are most similar to the SPATE proteins Tsh from avian pathogenic E. coli (43), an identical protein termed Hbp from a human pathogenic isolate (33), and SepA from Shigella flexneri (3). Both Tsh (22) and another SPATE from enterohemorrhagic E. coli, termed EpeA (24), have been reported to be mucinolytic. These reports demonstrate the potential for this group of the SPATE proteins to contribute to pathogenesis, perhaps via mucus layer interaction and mucosal colonization.
The streptomycin-treated mouse is a well-established model for studying E. coli colonization and interactions with the mucus layer; E. coli mutants with a reduced ability to penetrate mucus (25, 27) and grow in mucus (45) have been shown to exhibit colonization defects in this model. Here, we report that EAEC isolates are able to efficiently colonize the mouse intestine only when the animal is pretreated with streptomycin. In addition, our studies suggest roles for dispersin and Pic in intestinal colonization.
MATERIALS AND METHODS
Strains and plasmids.
Isolated from a child with diarrhea in Lima, Peru, the prototype EAEC 042 strain caused diarrhea in adult volunteers (29) and was used for the generation of all isogenic strains. Strain 042 is naturally streptomycin resistant. A streptomycin-resistant derivative of the commensal E. coli strain HS (23) was generated by selection on Luria-Bertani (L) agar plates with 100 μg/ml streptomycin. Other strains and plasmids used for genetic manipulations are listed in Table 1. Strains were grown in L broth or maintained on L agar with ampicillin (100 μg/ml), kanamycin (50 μg/ml), nalidixic acid (50 μg/ml), and streptomycin (100 μg/ml) as required. All antibiotics were purchased from Sigma Chemical Co. (St. Louis, MO).
TABLE 1.
Characteristics and source of host strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| E. coli strains | ||
| 042 | Prototype EAEC strain (044:H18) lac+, Sm Tc Cm | 27 |
| 042PicS258A | 042 Ser258 to Ala | This study |
| 042PicS258A (Nalr) | Nal derivative of 042PicS258A | This study |
| 042 pic::aph3 | 042 Km insertion in pic | This study |
| 042 aap | 042 harboring pJP5603 inserted into aap, Km | 39 |
| 042 aafA 3.4.14 | 042 harboring TnphoA inserted into the aafA gene, Km | 7 |
| 042 aggR | 042 harboring pJP5603 inserted into aggR, Km | 28 |
| 042 air | 042 harboring pJP5603 inserted into air, Km | 40 |
| DH5α | F′ φ80dlacZΔM15 Δ(lacZYA-argF)U169endA1recA1hsdR17supE44thi-1gyrA96 relA1 phoA | 37 |
| SY327λpir | Δ(lacpro) argE (Am) rifnalA recA56 | 22 |
| SM10λpir | thrthi leu tonA lacY supErecA::RP4-2-Tc::Mu (Km) | 42 |
| HS | Commensal E. coli, Sm | 18 |
| Plasmids | ||
| pPic | pACYC184 derivative containing an EcoRI-ScaI genomic fragment containing pic cloned from EAEC strain 042, Tc | 14 |
| pPicS258A | pPic, Ser 258 to Ala | This study |
| pPic::aph3 | pPic (Km insertion at Ala 254) | This study |
| pCVD442 | 6.2-kb suicide vector, R6K origin, Ap | 8 |
| pSMH1 | 2-kb pPicS258A PCR product cloned into pCVD442 | This study |
| pSMH2 | 3-kb pPicaph3 PCR product cloned into pCVD442 | This study |
| pK18 | Contains aminoglycoside 3′-O-phosphotransferase (aph3) | 34 |
Ap, ampicillin resistance; Tc, tetracycline resistance; Cm, chloramphenicol resistance; Km, kanamycin resistance; Sm, streptomycin resistance; Nal, nalidixic acid resistance; lac, lactose fermentation.
Construction of isogenic mutants in strain 042.
Mutations in the genes aggR, aap, air, and aafA were constructed by single-crossover integration of suicide plasmid pJP5603, and all were described previously (7, 30, 40, 41) (Table 1).
The pic and set1AB loci are overlapping genes encoded on opposite strands; a pic mutant was constructed in which pic is inactivated via exchange of the catalytic serine with alanine, without disruption of setBA. The catalytic serine in Pic was first changed to alanine by site-directed mutagenesis in pPic, a previously described pACYC184 derivative containing a genomic fragment encoding full-length pic, cloned from strain 042 (17) to yield strain pPicS258A. Site-directed mutagenesis employed QuikChange according to the manufacturer's protocols (Stratagene, La Jolla, CA) and using 25 ng of plasmid template DNA, 125 ng of primers PicMut-F and PicMut-R (Table 2), 0.5 mM deoxynucleoside triphosphate mix, 1× buffer, and 2.5 U Pfu Turbo DNA polymerase (Stratagene). Cycling conditions were 95°C for 30 s, followed by 20 cycles of 95°C for 30 s, 55°C for 1 min, and 68°C for 20 min. Confirmation of mutations by nucleotide sequencing was performed at the University of Maryland Biopolymer Core Facility. To introduce this mutation into strain 042, an approximately 2-kb region encoding the C-terminal half of the pic gene product in strain pPicS258A was amplified with primers designated PicKmSphI-F and PicKmXbaI-R (Table 2) containing restriction sites for SphI and XbaI. The PCR product was subcloned into the SphI and XbaI sites in the suicide vector pCVD442 to generate pSMH1 and was transformed into chemically competent SY327λpir (8). Purified pSMH1 was subsequently transformed into SM10λpir (8) for mating with EAEC strain 042. Allelic exchange was performed as described previously (8). The S258A point mutation was verified by nucleic acid sequence analysis. To facilitate the recovery of strains in coinfection experiments, a nalidixic acid-resistant derivative of strain 042PicS258A was generated by being plating on L agar containing 50 μg/ml nalidixic acid.
TABLE 2.
Primers for construction of pic mutants
| Primer designation | Sequencea |
|---|---|
| PicMut-F | 5′-GGAGCCCCTGGGGATGCTGGTTCTCCTTTGTTTGC |
| PicMut-R | 5′-GCAAACAAAGGAGAACCAGCATCCCCAGGGGCTCC |
| PicNotI-F | 5′-AAGGAAAAAAGCGGCCGCGCCTGGGGATAGTGGTTCTCCTTTG |
| PicNotI-R | 5′-AAGGAAAAAAGCGGCCGCGGCTCCATAGTCAGGTAAAGGGCCG |
| KanNotI-F | 5′-AAGGAAAAAAGCGGCCGCGCTTGCAGTGGGCTTACATG |
| KanNotI-R | 5′-AAGGAAAAAAGCGGCCGCCGGTCATTTCGAACCCCAGAG |
| PicKmSphI-F | 5′-ACATCCATGCCTGGCATTAAGGCTACATACGGC |
| PicKmXbaI-R | 5′-CTAGTCTAGACTGAGCCGGATCATTCAGGCTG |
| PicKmXbaI-F | 5′-CTAGTCTAGACTGGCATTAAGGCTACATACGGC |
| PicKmXbaI-R | 5′-CTAGTCTAGACTGAGCCGGATCATTCAGGCTG |
Underlined nucleotides indicate the Ser-to-Ala mutation site.
The pic gene was also insertionally inactivated in strain 042 using a similar approach. To construct a site into which the kanamycin resistance gene would be cloned, a NotI restriction site was inserted in pPic by inverse PCR analysis. The reaction was performed as for site-directed mutagenesis, using primers PicNotI-F and PicNotI-R (Table 2). Cycling conditions were 95°C for 30 s, followed by 18 cycles of 95°C for 30 s, 60°C for 1 min, and 68°C for 25 min. The PCR product was gel purified, ligated, and transformed into DH5α. The Tn5 aminoglycoside 3′-O-phosphotransferase gene (aph3) encoding resistance to kanamycin was amplified from pK18 with primers KanNotI-F and KanNotI-R (Table 2). The 1,029-bp product of this reaction was gel purified and cloned into the NotI site in pPic to generate pPicaph3. A 3.1-kb region of pPicaph3 was then amplified such that the kanamycin resistance gene would be flanked by 1 kb of the pic sequence using the primers PicKmXbaI-F and PicKmXbaI-R (Table 2) containing restriction sites for XbaI. Allelic exchange was performed as described above.
Bacterial growth curves.
Growth of strain 042 and its mutants in various growth media was performed by subculturing overnight broth cultures into the appropriate medium in triplicate and reading the optical density at 600 nm (OD600) at various time points. Data represent the means of the three points. Growth in minimal medium was performed in M9 medium with 1% glucose; 0.05% glucose was employed during experiments in which mucin was added. The mucin used was bovine submaxillary mucin preparation obtained from Sigma Chemical Co., St. Louis, MO.
Mouse colonization.
The streptomycin-treated mouse model was adapted from previous reports (26, 32). Eleven- to 12-week-old female BALB/c mice (Charles River Laboratories, Wilmington, MA) were provided with drinking water ad libitum containing 5 g/liter streptomycin from 24 to 48 h prior to inoculation and for the duration of the experiment. Stool samples were documented to be free of streptomycin-resistant E. coli at the time of inoculation. Inoculation strains were grown overnight in L broth, diluted 1:500, and incubated to late exponential phase (approximately 6 h). Bacteria were pelleted by centrifugation, washed once with sterile phosphate-buffered saline (PBS; pH 7.4), and resuspended in PBS to a final concentration of 5 × 103 CFU/ml. For coinfections, a 1:1 mixed suspension of strain 042 and the mutant was prepared at a final total concentration of 1 × 104 CFU/ml. Prior to infection with bacterial strains, mice were given 0.2 ml half-saturated sodium bicarbonate solution orogastrically to neutralize gastric acid. Approximately 15 min later, 0.2 ml of the inoculum was administered orogastrically. Bacterial inocula were quantified by plate counts. Fresh fecal pellets were collected at the indicated time points for up to 15 days postinfection. Feces were weighed, diluted, and homogenized in sterile PBS; serial dilutions were plated on MacConkey agar (Sigma) with antibiotics. Antibiotic concentrations used in these media were 100 μg/ml streptomycin for strain 042, 100 μg/ml streptomycin and 10 μg/ml nalidixic acid for strain 042PicS258A, or 100 μg/ml streptomycin and 25 μg/ml kanamycin for all other mutants. The limit of detection was approximately 103 CFU/g feces. For cultures in which colony counts were less than the lower limit of detection, statistical analysis was performed with values set at the limit of detection.
For enumeration of bacteria in intestinal compartments, a 2- to 3-cm section of the ileum that was approximately 1 cm away from the ileo-cecal valve, an ∼2-cm length of the colon, and the entire cecum were excised. Lumenal contents were expelled and weighed, and serial dilutions were made in PBS for plating to MacConkey agar; bacterial counts were expressed as CFU/gram of intestinal content. Intestinal segments were opened and rinsed thoroughly in PBS to remove fecal material, homogenized in 1 ml PBS with a Handi-Shear tissue homogenizer (VirTis, Gardiner, NY), and diluted in PBS for plating on MacConkey agar. For quantitation of bacteria colonizing the mucus layer, tissue was washed with PBS and then the mucus layer was removed by being scraped with a rubber spatula. Mucus was diluted in 200 μl PBS, and serial dilutions were plated on MacConkey agar. After being scraped, the tissue was homogenized and bacterial counts were determined as described above.
Growth in cecal lumenal contents and in cecal mucus.
The experiments defining growth in mucus and cecal contents in vitro were performed as previously described (46). Twenty CD-1 5-week-old male mice (Charles River Laboratories, Wilmington, MA) were given pine shaving bedding, sterile water, and mouse and rat diet (Harlan Teklad, Madison, WI) and acclimatized to the facilities for approximately 1 week. Mice were given 5 g/liter streptomycin sulfate in their drinking water, provided ad libitum, for 1 day. Ten mice were asphyxiated using CO2, and their ceca were excised; contents were collected into a 15-ml tube kept on ice. The cecal tissue was then rinsed in a petri dish filled with ice-cold HEPES-Hanks' buffer (HHB; 8 g NaCl, 0.4 g KCl, 0.14 g CaCl2·2H2O, 0.20 g MgSO4·7H2O, 0.05 g Na2HPO4, 0.35 g KH2PO4, 2.6 g HEPES per liter). Ceca were blotted dry on paper towels, and mucus was scraped off of the tissue into a petri dish using a soft rubber policeman; mucus from these 10 mice was pooled for later use.
Overnight L broth bacterial cultures were grown at 37°C in a shaking water bath. One milliliter of cells from E. coli strains 042 and 042PicS258A was pelleted at 10,000 rpm for 1.5 min. The pellet was rinsed with cold HHB and resuspended in 1 ml HHB. The washed bacteria were mixed together and inoculated into the pooled mucus sample or the pooled cecal contents to produce a final inoculum concentration of 104 CFU/ml. Following inoculation, the mucus or cecal cultures were incubated overnight at 37°C in a shaking water bath. At each time point, 50 μl was removed, diluted 10-fold in HHB, and plated on MacConkey agar plates containing 100 μg/ml streptomycin sulfate or 100 μg/ml streptomycin sulfate and 50 μg/ml nalidixic acid. Plates were incubated at 37°C overnight. From the cultures in mucus, samples were taken at time zero and at 2, 4, 6, 8, and 24 h. From the cultures in cecal contents, samples were taken at time zero and at 24 h.
For longer incubation experiments, E. coli 042 and 042PicS258A were grown to late logarithmic phase and added to mucus at a final concentration of 1 × 105 CFU/ml. Sampling from cultures was performed at 0, 6, 12, and 24 h and daily, thereafter, for 6 days. Fresh streptomycin was added to the culture each day to a final concentration of 50 μg/ml.
Adherence to mouse colonic tissue.
This protocol was adapted from the method described by El Asmar et al. (10). Eleven- to 12-week-old female BALB/c mice were given 5 g/liter streptomycin in their drinking water for 48 h prior to collection of colonic tissue. Mice were anesthetized with isoflurane (Abbott, Chicago, IL) and sacrificed by cervical dislocation. The entire colon was excised and rinsed thoroughly in sterile PBS, pH 7.4. Tissue segments ∼0.5 cm in length were cut and placed mucosal side up onto polycarbonate filters cut from a Snapwell tissue culture chamber (Corning Costar). The tissue and filter were sandwiched between two plastic rings with a 3-mm-diameter central hole and placed back into the Snapwell chamber. Tissues were equilibrated for 30 min at 37°C in an atmosphere of 5% CO2 in prewarmed Dulbecco's modified Eagle medium containing 4.5 g/liter glucose, l-glutamine, 110 mg/liter sodium pyruvate, and pyridoxine hydrochloride. Five milliliters of Dulbecco's modified Eagle medium was added to the serosal side and 200 μl to the mucosal side of the tissue in the chamber.
Bacterial strains were grown to late logarithmic phase, washed, and standardized as described for previous studies. Following the tissue equilibration period, 5 μl of 1 × 108 CFU/ml of each strain was added to the mucosal side of each of three wells. Cocultures were also performed in triplicate using 5 μl of a mixed culture of strains 042 and 042PicS258A or 042 and 042 pic::aph3 with each strain at 1 × 108 CFU/ml. Snapwell chambers were incubated at 37°C for 3 h. After the incubation period, chambers were washed five times with PBS to remove nonadherent bacteria. Tissue was removed and homogenized in 200 μl PBS in Eppendorf tubes with plastic pestles using a pellet pestle motor (Kontes). Homogenates were diluted and plated on MacConkey media as described above. Adherent bacteria were quantified as CFU/tissue segment because the area of tissue available for bacterial adherence was identical for each well.
Growth competition assay in a continuous-flow fermentor.
EAEC strains 042 and 042PicS258A were grown simultaneously in a continuous-flow anaerobic culture either with or without introduction of a human fecal sample as previously described (37) The fecal sample comprised 60 g of fresh stool from a healthy human subject. After addition of the fecal sample, the system was stabilized for at least 24 h at pH 6.5. Following the stabilization period, the system was inoculated with 50 ml of a mixed culture of strains 042 and 042PicS258A to a final concentration of 107 CFU/ml each. The continuous-flow culture was sampled at 6, 12, and 24 h and once a day for 5 days postinoculation. Samples were diluted and plated on MacConkey media with appropriate antibiotics. Culture plates were incubated, and strains were quantified as described for previous experiments.
Statistical analysis.
Analyses were performed on log-transformed data for all colony counts, employing software available at the Universiteit van Amsterdam website (www.fon.hum.uva.nl/). The paired t test was used to compare means of log-transformed data from different sites of the mouse intestine. For the adherence assay involving multiple comparisons, statistical analysis was performed with a one-way analysis of variance, followed by Bonferroni error correction, using the Analyze-it add-in for Microsoft Excel database software. For in vitro growth experiments, means of log10 CFU per milliliter were compared by using the paired Student's t test (two-tailed) using Excel database software.
RESULTS
Colonization of BALB/c mice with EAEC.
We infected 11- to 12-week-old BALB/c mice with high doses of EAEC after neutralization of gastric acidity. We found that EAEC strain 042 colonized inconsistently and was often eliminated within 3 days of inoculation. Excretion levels ranged from 0 to 105 CFU/g of feces, typically higher during the first day after inoculation. All mice remained clinically well with normal intestinal histopathology.
The streptomycin-treated mouse model has been used to study intestinal colonization with E. coli and Salmonella spp. (32, 45), and the model has recently been shown to be useful for inducing colitis with Salmonella strains (44). The prototype strain 042 is naturally resistant to streptomycin. Mice were given water containing 5 g/liter streptomycin ad libitum for 24 to 48 h prior to inoculation and for the duration of the experiment. Strain 042 colonized streptomycin-treated mice consistently, at levels of approximately 108 to 109 CFU/g of stool. Mice remained colonized as long as streptomycin was provided in the water, though they commonly cleared colonization when streptomycin was removed. All mice remained well. Histopathologic examination of infected mice was normal.
Effects of EAEC adherence factors on mouse gastrointestinal (GI) colonization.
Since streptomycin treatment resulted in high levels of colonization, we sought to determine whether the model could be used to assess the contribution of putative EAEC colonization factors. We tested mutants in the AAF/II-encoding genes, the global regulator AggR, the dispersin coat protein, and a recently discovered agglutinin called Air for the ability to colonize streptomycin-treated mice.
An inoculum of 1 × 103 CFU of each strain was introduced individually to groups of four to eight mice, and fecal colonization was quantified for up to 12 days. All mutants were able to colonize streptomycin-treated mice when fed individually at levels not significantly different from that of strain 042 (data not shown).
Competition between the mutants and the wild-type strain was then employed as a more sensitive readout of colonization efficiency. Accordingly, mice were infected simultaneously with 1 × 103 CFU each of strain 042 and one of the isogenic mutants. Isogenic strains with mutations in aafA, air, and surprisingly aggR cocolonized at levels similar to that of the wild-type parent. However, strain 042 aap, which contains a mutation in the gene encoding the dispersin protein coat and which displays a hyperaggregative phenotype in vitro, was consistently deficient in colonization compared to the wild-type parent (Fig. 1).
FIG. 1.
Coinfection of streptomycin-treated mice with E. coli strain 042 and the dispersin mutant 042 aap. Mice were infected simultaneously with 2 × 103 CFU of each strain. The bacteria were individually quantitated in fresh fecal pellets on days 1, 3, and 4. Graph represents a typical experiment with six mice in each group; bars represent the mean ratio of wild type to mutant.
Pic contributes to colonization.
In addition to testing mutants in adherence-related factors, we also addressed the potential role of the Pic mucinase in intestinal colonization of the streptomycin-treated mouse. For these initial studies, we utilized a mutant in which the aph3 gene was inserted into pic. As with the adherence factors, strain 042 pic::aph3 showed no defect in colonization of the streptomycin-treated mouse when tested independently.
In competition experiments, however, strain 042 pic::aph3 was outcompeted by the wild type (Fig. 2a). Colonization of the wild-type parent strain reached ∼1 × 108 CFU/g feces within the first 2 days after inoculation and was generally maintained for the duration of the experiment. With the pic mutant, however, most of the mice colonized initially to a level of ∼1 × 107 to 1 × 108 CFU/g feces over the first several days. However, colonization was not maintained thereafter, declining around day 7 and reaching a level of ∼1 × 104 to 1 × 105 CFU/g after day 10. By the last 5 days of the experiment, there existed a 2- to 3-log10 difference in colonization between strains 042 and 042 pic::aph3.
FIG. 2.
Coinfection of streptomycin-treated mice with E. coli strain 042 and pic mutants. (a) Eight female BALB/c mice were infected simultaneously with 1 × 103 CFU of EAEC strain 042 (▪) or strain 042 pic::aph3 (○). Data points are log10 CFU/g of fresh fecal sample collected from each mouse on days 1, 2, and 3 and approximately every other day for 15 days. The line shows the median log10 CFU/g feces. A P value of ≤0.0156 was recorded for the difference between strains 042 and 042 pic::aph3 on days 2 to 15 (except day 7) by the Wilcoxon matched-pairs signed-ranks test. (b) Nine female BALB/c mice were infected simultaneously with 1 × 103 CFU of EAEC strain 042 (▪) and strain 042PIC258A (○). A P value of ≤0.0039 was recorded for the difference between strains 042 and 042PicS258A in the matched-pairs signed-ranks test.
Since Pic is encoded in overlapping configuration with genes encoding the ShET1 enterotoxin, the defect of our mutant could have been due to disruption of ShET1 and/or Pic production. Attempts to complement the mutant in trans were unsuccessful, as the complemented strain did not grow as well as the mutant in vitro. We therefore constructed a pic mutant that would disrupt protease function but allow production of a noncatalytic Pic protein and an unmodified ShET1; the construction employed the allelic exchange of a single nucleotide to replace the Pic catalytic serine with alanine, retaining the native amino acid sequence on the opposite strand. This mutant was designated 042PicS258A.
In competition experiments with the wild-type strain, 042PicS258A was also impaired in colonization. The parent colonized at a level of 1 × 108 to 1 × 109 CFU/g feces by 40 h postinfection; this was maintained for 15 days on streptomycin (Fig. 2b). Strain 042PicS258A, however, was not able to maintain a high level of colonization. While colonization in some mice was as high as 1 × 107 CFU/g feces for the first few days after inoculation, bacterial counts of the mutant gradually declined toward the lower limit of detection (∼1 × 103 CFU/g). For several mice, detectable colonization with the mutant was never established. At all time points beyond day 2, a significant difference (3 to 5 log10) in fecal colonization was observed between strains 042 and 042PicS258A. Thus, two independently constructed mutations in pic demonstrated significant and consistent defects in colonization of the mouse GI tract compared with their parent strains.
Sites of colonization by strains 042 and 042PicS258A.
We sought to characterize further the mechanism of Pic's contribution to colonization. We performed competition experiments in the streptomycin-treated mouse model, quantitated the inoculated strains in the intestinal lumen, within the colonic mucous layer, and associated with the tissue underlying the mucous layer.
Five mice were infected simultaneously with 1 × 103 CFU of strains 042 and 042PicS258A and sacrificed at 24 h and at 3 days postinfection. These time points were chosen to evaluate the distribution of strains very early in colonization and when the mutant was found in highest numbers in the feces (day 3). At 24 h postinoculation, we found higher numbers of strain 042 than 042PicS258A in the lumina of both the cecum and colon; few cells of either bacterium were found in the ileum. At this early time point, no strain 042PicS258A cells and comparatively low numbers of strain 042 (∼1 × 104 in two mice) were detected adherent to tissues of the cecum and colon. Whereas strain 042 was more adherent at the 24-h time point, the low level of colonization limited our ability to draw statistically significant inferences.
By 3 days postinfection, EAEC bacteria were present in the ileum, cecum, and colon, but as on day 1, colonization of both strains was higher in the cecum and colon than in the ileum (Table 3). For the cecum and colon, the median CFU/g for strain 042 was approximately 3 log10 greater than the pic mutant. This ratio of wild-type to mutant bacteria was consistently observed in the tissues and lumenal contents of the cecum and colon.
TABLE 3.
Competitive colonization on day 3 postinfectiona
| Location of colonization | Bacterial counts of indicated strain in luminal contents (log10 CFU/g)
|
P valueb | Bacterial counts of indicated strain in homogenized tissue (log10 CFU/g)
|
P valueb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 042
|
042PicS258A
|
042
|
042PicS258A
|
|||||||
| Mean | Median | Mean | Median | Mean | Median | Mean | Median | |||
| Ileum | 4.5 ± 2.1 | 3.0 | ≤3.0 ± 0.0 | ≤3.0 | 1.0 | 3.7 ± 0.9 | 3.2 | ≤3.0 ± 0.0 | ≤3.0 | 1.0 |
| Cecum | 8.6 ± 0.5 | 8.5 | 5.5 ± 0.7 | 5.59 | 0.004 | 6.1 ± 1.1 | 5.7 | 3.1 ± 0.2 | 3.1 | 0.004 |
| Colon with mucus layer | 7.9 ± 1.1 | 8.1 | 4.7 ± 0.7 | 4.8 | 0.005 | 6.2 ± 0.9 | 6.3 | 3.4 ± 0.5 | ≤3.0 | 0.001 |
| Colonic mucus layer | 5.5 ± 0.9 | 5.4 | ≤3.0 ± 0.0 | ≤3.0 | 0.01 | 5.5 ± 0.9 | 5.5 | 3.1 ± 0.1 | ≤3.0 | 0.003 |
Five mice were given 1 × 103 CFU of strains 042 and 042PicS258A simultaneously. Numbers in the table are the means ± 1 standard deviation and median log10 CFU/g tissue or per milliliter of contents at 3 days postinfection. Limit of detection was approximately 1 × 103 CFU/g. If no organisms were detected, mean and median are recorded as ≤3.0 log10 CFU/g.
P values for the difference between the mean colonization of strains 042 and 042PicS258A for each site were determined by using the paired t test of log-transformed data. If none was detected, the P value is based on 3 log10 CFU/g.
To further address the potential roles of penetration and adherence, the mucous layer was scraped from a section of colon in each of the five mice, and the wild type and pic mutant were enumerated in the mucus and underlying colonic tissue. At 24 h, no strain 042PicS258A cells were detected and only rarely were strain 042 cells detected (in one mouse) adherent to tissues and in the mucous layer. In contrast, on day 3, there was an approximately 2-log10 difference between the median CFU/g of the two strains in both the scraped mucus and underlying colon tissue (Table 3).
Thus, the higher proportion of the wild type versus the mutant was consistently observed in the lumen, mucus layer, and underlying tissue. That difference was present in the lumen by 24 h postinfection and in the lumen, mucous layer, and underlying tissue by 3 days postinfection.
Adherence to mouse colonic tissue by strain 042 and mutants.
We next evaluated the relative abilities of the wild type and mutant to adhere to fresh colonic tissue excised from a streptomycin-treated mouse in an ex vivo format, using the previously described Snapwell system (10), in which tissue is mounted between two plastic disks and bathed in cell culture medium with separation of mucosal and serosal surfaces into distinct compartments. EAEC strains were more adherent to the mouse colonic mucosa than commensal E. coli HS; however, there was no difference in the ability of strain 042, 042PicS258A, or 042 pic::aph3 to adhere to the tissue when cultured individually (data not shown). Similarly, coculture of strain 042 with either mutant strain did not show any differences in relative adherence.
In vitro growth characteristics of pic mutants.
Either individually or in coculture, the pic mutants grew at rates similar to that of their wild-type parent in L broth cultures. Both mutants retained the ability to form a biofilm on polystyrene (42) and displayed motilities similar to that of the parent strain (data not shown).
We addressed whether the colonization deficiency of the pic mutants could be due to differences in anaerobic growth. To test this hypothesis, we employed the continuous-flow anaerobic culture system established in our laboratory to simulate the microenvironment of the human colon (37). The growth medium for these experiments has been established for EAEC strain 042 (37). An advantage of this system is that the growth characteristics of a test strain can be assessed in the presence or absence of other fecal bacteria.
The culture system was equilibrated with a human fecal sample and then inoculated with a mixed culture of 042 and 042PicS258A to a concentration of ∼1 × 107 CFU/ml of each strain. Growth of each strain was monitored for 5 days. Colony counts gradually declined for 48 h but then stabilized at ∼1 × 105 to 1 × 106 CFU/ml. No significant difference in growth between the wild type and the pic mutant was observed (Fig. 3a). We then repeated these experiments but without the addition of the fecal inoculum. Without competition from other bacteria, EAEC increased in numbers for the first 12 h and then declined to a steady-state level of ∼6.5 log10 CFU/ml (Fig. 3b). Again, no significant difference between the wild type and mutant was seen. The competitive advantage of strain 042 in vivo was therefore not reproduced with the intestinal simulator, suggesting that the advantage conferred by the Pic protein was due to neither enhanced fitness for anaerobic growth nor improved competition with typical human commensal flora.
FIG. 3.
Growth of strain 042 (▪) and strain 042PicS258A (○) in a mixed culture in a continuous-flow anaerobic culture system with human fecal bacteria (a) or without addition of a human fecal sample (b). Growth is expressed as means ± 1 standard deviation log10 CFU/ml of two samples removed from the system at the indicated time points.
Growth in cecal mucus and lumenal contents.
As a mucinase, Pic could hypothetically enhance fitness via improved use of nutrients derived from some lumenal component, perhaps mucin itself. We therefore evaluated growth in mucus and cecal lumenal contents harvested from the intestines of mice. Lumenal contents and mucus were extracted separately and inoculated with 1 × 104 CFU of strains 042 and 042PicS258A and then cultured aerobically and sampled over time.
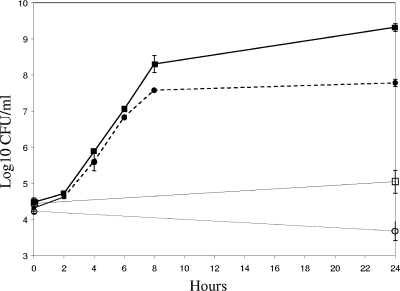
As shown in Fig. 4, the strains grew similarly for the first 6 h of cocultivation, with similar generation times of 33 to 40 min. However, at the 8-h time point and subsequently, growth of strain 042PicS258A slowed, with a generation time increasing to 53 min at 6 to 8 h; in contrast, strain 042 continued to grow in mucus for the duration of the experiment, and the 6- to 8-h generation time was approximately 32 min. Growth in cecal contents for both strains was slow, yet numbers increased for strain 042 over the 24 h of the experiment and declined slightly for strain 042PicS258A. This experiment was repeated using longer incubation times; the difference in growth between the two strains in mucus was reproducible and remained significant up to 48 h (data not shown).
FIG. 4.
Relative growth in cecal mucus and cecal contents. Tubes containing cecal mucus (filled symbols) or cecal contents (open symbols) were inoculated simultaneously with a mixture of 104 CFU of strain 042 (squares; solid lines) and 104 CFU of strain 042PicS258A (circles; dashed lines) and incubated at 37°C with aeration. At the times indicated, tubes were sampled to determine viable counts. Bars representing the standard error of the mean log10 CFU/ml for duplicate experiments are presented for each time point.
The fact that strains 042 and 042PicS258A grew similarly in mucus for several hours, after which time strain 042 exhibited superior growth, suggested that Pic was providing some metabolic advantage to the bacterium, presumably after the exhaustion of a more readily available nutrient. An alternative hypothesis was that mucin was inhibiting the growth of the bacterium, with this inhibition being relieved by Pic. To test these alternative hypotheses, we characterized growth curves of strain 042 versus strain 042PicS258A in M9 minimal medium containing 0.05% glucose to which bovine submaxillary mucin was added during logarithmic phase (after 5 h of growth). As shown in Fig. 5, strains 042 and 042PicS258A entered stationary growth phase at similar times. However, the wild type then resumed slow growth for several additional hours, whereas the Pic mutant remained at a uniform OD600.
FIG. 5.

Pic enhances growth of strain 042 in the presence of mucin. Thirteen-hour growth curves of wild-type strain 042 and strain 042PicS258A (042S) are shown in M9 minimal medium with 0.05% glucose, with and without the addition of mucin after 5 h of incubation (arrow). Results are typical of repeated experiments; measurements are performed in triplicate with an error of <0.003 U for all points.
DISCUSSION
Our studies suggest that the streptomycin-treated mouse model has some advantages and some limitations for the study of EAEC colonization factors. The AAF/II fimbriae of strain 042 are essential for adherence to the human intestine ex vivo but were not shown to confer a colonization advantage in the mouse. This is in spite of our observation that AAF/II fimbriae enhance adherence to the mouse intestinal mucosa in vitro. Why adherence does not translate into increased colonization is not apparent. Interestingly, however, the dispersin protein required for normal morphology of the AAF adhesins and which modulates EAEC aggregation was found to be required for efficient colonization. We have demonstrated previously that EAEC bacteria lacking dispersin are highly aggregated and move more slowly than bacteria of strain 042 through a viscous column of purified mucin (40). The effect of the dispersin mutant in the mouse was apparently dependent on expression of the AAF adhesin, as the aggR mutant, unable to make either dispersin or AAF, no longer exhibited any colonization disadvantage. These experiments do not exclude a role for an as-yet-unidentified AggR-dependent factor. Further characterization of the contribution of dispersin will be published elsewhere.
Our studies did not reveal a role for the newly discovered chromosomally encoded aggregin, Air, which is under the control of the HilA-like regulator EilA (41). Like absence of dispersin, the presence of Air promotes aggregation of bacteria. It is interesting, therefore, that our data suggest no influence of Air on colonization of the mouse. It is possible that the natural role of Air could be in EAEC adherence outside of the host, or perhaps the role is complex and relevant at later stages in the colonization process.
Given the occurrence of the Pic mucinase in EAEC, Shigella spp., and UPEC strains, we were particularly interested in its possible contribution to colonization. A number of studies have demonstrated two stages of colonization for E. coli in the streptomycin-treated mouse model (26, 46). When fed alone to mice, low numbers of bacteria (1 × 105) will increase to between 1 × 108 and 1 × 109 CFU/g feces within 1 to 3 days postinfection; this is postulated to be due to limited competition for nutrients from other aerobic species. After this initiation phase, however, counts of E. coli will diminish to approximately 1 × 107 to 1 × 108 CFU/g feces, where they will remain indefinitely; this second stage has been termed the maintenance stage (6). The maintenance stage is thought to represent stable establishment of a metabolic niche within the intestine, in which the bacteria metabolize some carbon sources better than the incumbent microflora. When fed individually, both strain 042 and two different pic mutants were able to colonize to high numbers and remained stable for the 12 days of the experiment, exhibiting normal initiation and maintenance phases. However, when fed to mice simultaneously, the wild type maintained its level of colonization at 1 × 107 to 1 × 108 CFU/g feces, whereas the pic mutants initiated infection in some mice but failed to maintain it, gradually declining to between 1 × 103 and 1 × 105 CFU/g feces. This reveals the relative superiority of the wild type for maintaining colonization of the GI niche and suggests a metabolic advantage of the wild type.
We had initially speculated that the mechanism of Pic's effect would involve penetration of the mucus layer. However, the superiority of the parent over its pic mutant was apparent at all sites and environments of the GI tract, including at the lumen and the mucus layer, as well as at the level of the epithelial surface. Two bacterial metalloproteases have been reported to have mucinase activity and also to contribute to bacterial adherence. StcE, in enterohemorrhagic E. coli O157:H7, promotes intimate adherence to HEp-2 cells (14), and Hap of Vibrio cholerae acts as a detachase, freeing adherent bacteria (2). However, we did not find evidence to suggest that the role of Pic included adherence. Nor did our data suggest increased fitness in aerobic or anaerobic environments, even in the presence of fecal bacteria.
In aggregate, our data are most consistent with a metabolic role for Pic, dependent on its mucinase activity and presumably by allowing the use of mucus as a growth substrate. Using the streptomycin-treated mouse model, E. coli has been shown to utilize gluconate in mucus as a major carbon source (45). Notably, we found that EAEC strain 042 and its pic mutant grew equally well in cecal mucus in vitro for approximately the first 6 h, indicating that both strains utilize gluconate or another nutrient equally during this time period. However, after 6 to 8 h, growth of the mutant slowed considerably, whereas strain 042 continued rapid growth for at least 48 additional hours. This model, therefore, recapitulated the in vivo effect. In standard growth media, including M9 minimal medium containing glucose, strain 042 and an isogenic mutant lacking only the Pic catalytic serine grew equally well. However, when mucin was added to the medium, strain 042 demonstrated more robust growth, beginning after the initiation of stationary phase. The similar growth rates early in the experiment suggest that glucose may be a preferred substrate for both the wild type and the pic mutant but that the use of mucus as a substrate occurs when other preferred substrates are limiting; limitation may prevail either because preferred substrates are not present at that intestinal site or because they are more efficiently utilized by competing microflora.
Our data therefore strongly support the hypothesis that the contribution of Pic to intestinal colonization is nutritional. The fact that both the wild type and the Pic mutant grow well initially in mouse mucus preparations prior to the deviation of the growth curves supports this interpretation of the data. Whether the postulated advantage of Pic occurs via the release of glycans from intestinal mucus and/or from degradation of the mucin protein backbone cannot be determined from our data, and it is the subject of ongoing studies in our laboratories. Moreover, the presence of Pic in Shigella spp. and UPEC strains and the presence of mucinolytic Pic homologs in other pathogens suggest that our observations may have implications for pathogens other than EAEC.
Acknowledgments
We gratefully acknowledge Alessio Fasano and Klara Margaretten for assistance with the adherence experiments, Araceli Santiago for assistance with the mouse infection technique, and Alfredo Torres for helpful suggestions regarding the allelic exchange protocol.
This work was supported by U.S. PHS grants AI33096, AI43615, and AI03615 to J.P.N. and AI48945 to P.S.C.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 6 April 2009.
REFERENCES
- 1.Adachi, J. A., Z. D. Jiang, J. J. Mathewson, M. P. Verenkar, S. Thompson, F. Martinez-Sandoval, R. Steffen, C. D. Ericsson, and H. L. DuPont. 2001. Enteroaggregative Escherichia coli as a major etiologic agent in traveler's diarrhea in 3 regions of the world. Clin. Infect. Dis. 321706-1709. [DOI] [PubMed] [Google Scholar]
- 2.Bénitez, J. A., R. G. Spelbrink, A. Silva, T. E. Phillips, C. M. Stanley, M. Boesman-Finkelstein, and R. A. Finkelstein. 1997. Adherence of Vibrio cholerae to cultured differentiated human intestinal cells: an in vitro colonization model. Infect. Immun. 653474-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjelloun-Touimi, Z., P. J. Sansonetti, and C. Parsot. 1995. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol. Microbiol. 17123-135. [DOI] [PubMed] [Google Scholar]
- 4.Bhan, M. K., V. Khoshoo, H. Sommerfelt, P. Raj, S. Sazawal, and R. Srivastava. 1989. Enteroaggregative Escherichia coli and Salmonella associated with nondysenteric persistent diarrhea. Pediatr. Infect. Dis. J. 8499-502. [DOI] [PubMed] [Google Scholar]
- 5.Bhan, M. K., P. Raj, M. M. Levine, J. B. Kaper, N. Bhandari, R. Srivastava, R. Kumar, and S. Sazawal. 1989. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J. Infect. Dis. 1591061-1064. [DOI] [PubMed] [Google Scholar]
- 6.Chang, D. E., D. J. Smalley, D. L. Tucker, M. P. Leatham, W. E. Norris, S. J. Stevenson, A. B. Anderson, J. E. Grissom, D. C. Laux, P. S. Cohen, and T. Conway. 2004. Carbon nutrition of Escherichia coli in the mouse intestine. Proc. Natl. Acad. Sci. USA 1017427-7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czeczulin, J. R., S. Balepur, S. Hicks, A. Phillips, R. Hall, M. H. Kothary, F. Navarro-Garcia, and J. P. Nataro. 1997. Aggregative adherence fimbria II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infect. Immun. 654135-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 594310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutta, P. R., R. Cappello, F. Navarro-Garcia, and J. P. Nataro. 2002. Functional comparison of serine protease autotransporters of Enterobacteriaceae. Infect. Immun. 707105-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Asmar R., P. Panigrahi, P. Bamford, I. Berti, T. Not, G. V. Coppa, C. Catassi, and A. Fasano. 2002. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 1231607-1615. [DOI] [PubMed] [Google Scholar]
- 11.Fasano, A., F. R. Noriega, F. M. Liao, W. Wang, and M. M. Levine. 1997. Effect of Shigella enterotoxin 1 (ShET1) on rabbit intestine in vitro and in vivo. Gut 40505-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fasano, A., F. R. Noriega, D. R. Maneval, Jr., S. Chanasongcram, R. Russell, S. Guandalini, and M. M. Levine. 1995. Shigella enterotoxin 1: an enterotoxin of Shigella flexneri 2a active in rabbit small intestine in vivo and in vitro. J. Clin. Investig. 952853-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Germani, Y., P. Minssart, M. Vohito, S. Yassibanda, P. Glaziou, D. Hocquet, P. Berthelemy, and J. Morvan. 1998. Etiologies of acute, persistent, and dysenteric diarrheas in adults in Bangui, Central African Republic, in relation to human immunodeficiency virus serostatus. Am. J. Trop. Med. Hyg. 591008-1014. [DOI] [PubMed] [Google Scholar]
- 14.Grys, T. E., M. B. Siegel, W. W. Lathem, and R. A. Welch. 2005. The StcE protease contributes to intimate adherence of enterohemorrhagic Escherichia coli O157:H7 to host cells. Infect. Immun. 731295-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guyer, D. M., J. S. Kao, and H. L. Mobley. 1998. Genomic analysis of a pathogenicity island in uropathogenic Escherichia coli CFT073: distribution of homologous sequences among isolates from patients with pyelonephritis, cystitis, and catheter-associated bacteriuria and from fecal samples. Infect. Immun. 664411-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heimer, S. R., D. A. Rasko, C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 2004. Autotransporter genes pic and tsh are associated with Escherichia coli strains that cause acute pyelonephritis and are expressed during urinary tract infection. Infect. Immun. 72593-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson, I. R., J. Czeczulin, C. Eslava, F. Noriega, and J. P. Nataro. 1999. Characterization of Pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect. Immun. 675587-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson, I. R., and J. P. Nataro. 2001. Virulence functions of autotransporter proteins. Infect. Immun. 691231-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, D. B., J. P. Nataro, H. L. DuPont, P. P. Kamat, A. D. Mhatre, P. C. Okhuysen, and T. Chiang. 2006. Enteroaggregative Escherichia coli is a cause of acute diarrheal illness: a meta-analysis. Clin. Infect. Dis. 43556-563. [DOI] [PubMed] [Google Scholar]
- 20.Itoh, Y., I. Nagano, M. Kunishima, and T. Ezaki. 1997. Laboratory investigation of enteroaggregative Escherichia coli O untypeable:H10 associated with a massive outbreak of gastrointestinal illness. J. Clin. Microbiol. 352546-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knutton, S., R. K. Shaw, M. K. Bhan, H. R. Smith, M. M. McConnell, T. Cheasty, P. H. Williams, and T. J. Baldwin. 1992. Ability of enteroaggregative Escherichia coli strains to adhere in vitro to human intestinal mucosa. Infect. Immun. 602083-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi, R. K., L. C. Gaziri, E. J. Venancio, and M. C. Vidotto. 2007. Detection of Tsh protein mucinolytic activity by SDS-PAGE. J. Microbiol. Methods 68654-655. [DOI] [PubMed] [Google Scholar]
- 23.Levine, M. M., C. Ferreccio, V. Prado, M. Cayazzo, P. Abrego, J. Martinez, L. Maggi, M. M. Baldini, W. Martin, D. Maneval, B. Kay, L. Guers, H. Lior, S. S. Wasserman, and J. P. Nataro. 1993. Epidemiologic studies of Escherichia coli diarrheal infections in a low socioeconomic level peri-urban community in Santiago, Chile. Am. J. Epidemiol. 138849-869. [DOI] [PubMed] [Google Scholar]
- 24.Leyton, D. L., J. Sloan, R. E. Hill, S. Doughty, and E. L. Hartland. 2003. Transfer region of pO113 from enterohemorrhagic Escherichia coli: similarity with R64 and identification of a novel plasmid-encoded autotransporter, EpeA. Infect. Immun. 716307-6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCormick, B. A., P. Klemm, K. A. Krogfelt, R. L. Burghoff, L. Pallesen, D. C. Laux, and P. S. Cohen. 1993. Escherichia coli F-18 phase locked ‘on’ for expression of type 1 fimbriae is a poor colonizer of the streptomycin-treated mouse large intestine. Microb. Pathog. 1433-43. [DOI] [PubMed] [Google Scholar]
- 26.Miranda, R. L., T. Conway, M. P. Leatham, D. E. Chang, W. E. Norris, J. H. Allen, S. J. Stevenson, D. C. Laux, and P. S. Cohen. 2004. Glycolytic and gluconeogenic growth of Escherichia coli O157:H7 (EDL933) and E. coli K-12 (MG1655) in the mouse intestine. Infect. Immun. 721666-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Møller, A. K., M. P. Leatham, T. Conway, P. J. Nuijten, L. A. de Haan, K. A. Krogfelt, and P. S. Cohen. 2003. An Escherichia coli MG1655 lipopolysaccharide deep-rough core mutant grows and survives in mouse cecal mucus but fails to colonize the mouse large intestine. Infect. Immun. 712142-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nataro, J. P., Y. Deng, D. R. Maneval, A. L. German, W. C. Martin, and M. M. Levine. 1992. Aggregative adherence fimbriae I of enteroaggregative Escherichia coli mediate adherence to HEp-2 cells and hemagglutination of human erythrocytes. Infect. Immun. 602297-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nataro, J. P., D. Yikang, S. Cookson, A. Cravioto, S. J. Savarino, L. D. Guers, M. M. Levine, and C. O. Tacket. 1995. Heterogeneity of enteroaggregative Escherichia coli virulence demonstrated in volunteers. J. Infect. Dis. 171465-468. [DOI] [PubMed] [Google Scholar]
- 30.Nataro, J. P., D. Yikang, D. Yingkang, and K. Walker. 1994. AggR, a transcriptional activator of aggregative adherence fimbria I expression in enteroaggregative Escherichia coli. J. Bacteriol. 1764691-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navarro-García, F., A. Canizalez-Roman, J. Luna, C. Sears, and J. P. Nataro. 2001. Plasmid-encoded toxin of enteroaggregative Escherichia coli is internalized by epithelial cells. Infect. Immun. 691053-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nevola, J. J., D. C. Laux, and P. S. Cohen. 1987. In vivo colonization of the mouse large intestine and in vitro penetration of intestinal mucus by an avirulent smooth strain of Salmonella typhimurium and its lipopolysaccharide-deficient mutant. Infect. Immun. 552884-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otto, B. R., S. J. van Dooren, J. H. Nuijens, J. Luirink, and B. Oudega. 1998. Characterization of a hemoglobin protease secreted by the pathogenic Escherichia coli strain EB1. J. Exp. Med. 1881091-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parham, N. J., U. Srinivasan, M. Desvaux, B. Foxman, C. F. Marrs, and I. R. Henderson. 2004. PicU, a second serine protease autotransporter of uropathogenic Escherichia coli. FEMS Microbiol. Lett. 23073-83. [DOI] [PubMed] [Google Scholar]
- 35.Piva, I. C., A. L. Pereira, L. R. Ferraz, R. S. Silva, A. C. Vieira, J. E. Blanco, M. Blanco, J. Blanco, and L. G. Giugliano. 2003. Virulence markers of enteroaggregative Escherichia coli isolated from children and adults with diarrhea in Brasilia, Brazil. J. Clin. Microbiol. 411827-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajakumar, K., C. Sasakawa, and B. Adler. 1997. Use of a novel approach, termed island probing, identifies the Shigella flexneri she pathogenicity island which encodes a homolog of the immunoglobulin A protease-like family of proteins. Infect. Immun. 654606-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz-Perez, F., J. Sheikh, S. Davis, E. C. Boedeker, and J. P. Nataro. 2004. Use of a continuous-flow anaerobic culture to characterize enteric virulence gene expression. Infect. Immun. 723793-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarantuya, J., J. Nishi, N. Wakimoto, S. Erdene, J. P. Nataro, J. Sheikh, M. Iwashita, K. Manago, K. Tokuda, M. Yoshinaga, K. Miyata, and Y. Kawano. 2004. Typical enteroaggregative Escherichia coli is the most prevalent pathotype among E. coli strains causing diarrhea in Mongolian children. J. Clin. Microbiol. 42133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savarino, S., A. Fasano, D. Robertson, and M. Levine. 1991. Enteroaggregative Escherichia coli elaborate a heat-stable enterotoxin demonstrable in an in vitro intestinal model. J. Clin. Investig. 871450-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheikh, J., J. R. Czeczulin, S. Harrington, S. Hicks, I. R. Henderson, B. C. Le, P. Gounon, A. Phillips, and J. P. Nataro. 2002. A novel dispersin protein in enteroaggregative Escherichia coli. J. Clin. Investig. 1101329-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheikh, J., E. G. Dudley, B. Sui, B. Tamboura, A. Suleman, and J. P. Nataro. 2006. EilA, a HilA-like regulator in enteroaggregative Escherichia coli. Mol. Microbiol. 61338-350. [DOI] [PubMed] [Google Scholar]
- 42.Sheikh, J., S. Hicks, M. Dall'Agnol, A. D. Phillips, and J. P. Nataro. 2001. Roles for Fis and YafK in biofilm formation by enteroaggregative Escherichia coli. Mol. Microbiol. 41983-997. [DOI] [PubMed] [Google Scholar]
- 43.Stathopoulos, C., D. L. Provence, and R. Curtiss III. 1999. Characterization of the avian pathogenic Escherichia coli hemagglutinin Tsh, a member of the immunoglobulin A protease-type family of autotransporters. Infect. Immun. 67772-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stecher, B., G. Paesold, M. Barthel, M. Kremer, J. Jantsch, T. Stallmach, M. Heikenwalder, and W. D. Hardt. 2006. Chronic Salmonella enterica serovar Typhimurium-induced colitis and cholangitis in streptomycin-pretreated Nramp1+/+ mice. Infect. Immun. 745047-5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sweeney, N. J., D. C. Laux, and P. S. Cohen. 1996. Escherichia coli F-18 and E. coli K-12 eda mutants do not colonize the streptomycin-treated mouse large intestine. Infect. Immun. 643504-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wadolkowski, E. A., D. C. Laux, and P. S. Cohen. 1988. Colonization of the streptomycin-treated mouse large intestine by a human fecal Escherichia coli strain: role of growth in mucus. Infect. Immun. 561030-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]