Abstract
The saliva of hematophagous arthropods contains potent anti-inflammatory and antihemostatic activities that promote acquisition of the blood meal and enhance infection with pathogens. We have shown that polymorphonuclear leukocytes (PMN) treated with the saliva of the tick Ixodes scapularis have reduced expression of β2 integrins, impaired PMN adherence, and reduced killing of Borrelia burgdorferi, the causative agent of Lyme disease. Here we describe two Ixodes proteins that are induced upon tick feeding and expressed predominantly in the salivary glands. Using saliva harvested from ticks with reduced levels of ISL 929 and ISL 1373 through targeted RNA interference knockdown, as well as purified recombinant proteins, we show the effects of these proteins on downregulation of PMN integrins and inhibition of the production of O2− by PMN in vitro. Mice immunized with ISL 929/1373 had increased numbers of PMN at the site of tick attachment and a lower spirochete burden in the skin and joints 21 days after infection compared to control-immunized animals. Our results suggest that ISL 929 and ISL 1373 contribute to the inhibition of PMN functions shown previously with tick saliva and support important roles for these inhibitory proteins in the modulation of PMN function in vivo.
Ixodes scapularis belongs to a family of hard-shelled ticks found worldwide and is a known vector for viral and bacterial pathogens, such as those causing Lyme disease, ehrlichiosis, Rocky Mountain spotted fever, and babesiosis (2, 51, 56). Unlike other hematophagous vectors of human disease that feed rapidly, such as mosquitoes or flies, I. scapularis feeds for 3 to 10 days and delivers saliva into the host for the duration of the attachment (1). I. scapularis saliva contains a potent array of antihemostatic, anti-inflammatory, and immunomodulatory components that aid in blood feeding, inhibit the immune response, and enhance infections in vivo, including murine infection with Borrelia burgdorferi, the causative agent of Lyme disease (7, 10, 12, 15, 25, 35-38, 45, 46, 49, 54, 59, 64). The immunomodulatory components of Ixodes saliva include well-characterized antihistamines; kininases; antioxidants; anticoagulants (7, 10, 12, 24, 25, 35, 37, 59, 60); prostaglandin E2, which inhibits dendritic cell maturation (14, 48, 55); and Salp15, which inhibits CD4+ T-cell-mediated immune response in vivo and inhibits killing of spirochetes (3, 13, 21, 42, 50).
Polymorphonuclear leukocytes (PMN) are the first immune cells to arrive at the site of B. burgdorferi infection (4), and saliva inhibits critical PMN functions, such as phagocytosis and superoxide production (47). We have previously shown that one mechanism of inhibition of human PMN is through downregulation of β2 integrins, cell surface receptors that mediate adhesion and are critical for activation of the innate immune responses (23, 32, 52). Saliva-treated PMN are less adherent, bind fewer spirochetes, and show a dose-dependent downregulation of CD18, the common β-chain for leukocyte β2 integrins (32).
A recent transcriptome analysis of the salivary glands of the I. scapularis tick identified 735 clones for analysis (61), including two candidate “disintegrins,” small proteins that inhibit integrin binding and are also present in the hookworm, in snake venom, and in other arthropod vectors (19). A platelet disintegrin molecule has been described in the salivary glands of the soft tick Ornithodoros moubata (22), and rhodostomin, a disintegrin from snake venom, decreases PMN binding through integrins and reduces PMN O2− production (33, 58). Ixodes saliva demonstrates functions of disintegrins, including blocking PMN integrins (32) and reducing O2− production (47). In this study, we describe two tick salivary proteins that inhibit the functions of human PMN and modulate the course of murine infection with B. burgdorferi.
MATERIALS AND METHODS
PMN isolation.
Heparinized blood from healthy human volunteers was obtained with informed consent under the guidelines of the Human Investigations Committee of Yale University School of Medicine. PMN were isolated by dextran density sedimentation and hypotonic lysis of red blood cells, as described previously (32).
PMN flow cytometry.
PMN (106/100 μl) were resuspended in phosphate-buffered saline (PBS) containing Ca2+, Mg2+, 0.1% bovine serum albumin (BSA), and physiologic glucose (5.4 mM). The cells were untreated or preincubated for 1 h at 37°C with saliva from control- or double-stranded RNA (dsRNA)-treated ticks or with the recombinant tick proteins ISL 929 and ISL 1373. Following preincubation, the cells were stimulated with tumor necrosis factor alpha (TNF-α) (15 ng/ml; R&D Systems, Minneapolis, MN) for 30 min at 37°C. Integrins were labeled for 1 h in PBS-BSA at 4°C with specific human antibodies: fluorescein isothiocyanate-conjugated anti-CD18 and phycoerythrin-conjugated CD11b (DakoCytomation, Glostrup, Denmark). Fluorescein isothiocyanate-conjugated anti-CD15 (DakoCytomation) served as a control. We had previously shown that CD15 expression is not affected by saliva treatment (32). The cells were fixed in 0.5% paraformaldehyde and stored at 4°C until they were analyzed by fluorescence-activated cell sorting (FACS) (FACSCalibur; Becton Dickinson, Franklin Lakes, NJ).
PMN O2− production.
PMN (105/100 μl PBS with Ca2+/Mg2+, 5.4 mM glucose, and 0.1% BSA) were pretreated with saliva from control- or dsRNA-treated ticks, or recombinant ISL 929 or ISL 1373 (2 μg/106 cells), and assayed at 37°C for O2− production using the chemiluminescent Diogenes Kit (National Diagnostics, Atlanta, GA) according to the manufacturer's instructions. Production of O2− was triggered by the addition of phorbol myristate acetate (PMA) (0.2 μg/μl in dimethyl sulfoxide) to resting PMN or by the addition of 2 μg monoclonal mouse anti-human myeloperoxidase antibody to TNF-α-primed PMN (2 ng/ml; 37°C; 10 min). Measurements of Diogenes luminescence were taken for 30 min, and the cells were maintained at 37°C throughout the assay. Each measurement was collected over 3 s using a luminometer (TD-20/20; Turner Designs, Sunnyvale, CA).
Preparation of dsRNA for RNAi.
cDNAs corresponding to ISL 929 and ISL 1373 were generously provided by Ivo Fransichetti and Jose Ribeiro of the NIH Laboratory of Parasitic Diseases (61). Primers containing BglII and XhoI restriction sites were used to amplify a region homologous to both of them. The primers were 5′-GAGCTCTGTGAAGAACAATCGGAAGC-3′ and 5′-CTCGAGTTCCGCAAGGGGTACCATCC-3′. The resultant amplicons were purified and cloned into the BglII-XhoI sites of the L4440 double T7 Script II vector as described previously (36). dsRNA was designed to encompass the conserved interior regions of ISL 929 and ISL 1373 with salp 13, with which they share about 50% identity and an additional 35% similarity. dsRNA was synthesized by in vitro transcription using the Megascript RNA interference (RNAi) kit (Ambion, Austin, TX) and quantified spectrophotometrically (ND-1000; Nanodrop Technologies, Inc., Wilmington, DE).
Tick husbandry and production of tick saliva.
I. scapularis nymphs and larvae were obtained from a tick colony at the Connecticut Agricultural Experiment Station (New Haven, CT). The nymphs were fed to repletion on pathogen-free C3H/HeN mice and allowed to molt to adults. Feeding experiments with nymphs involved feeding 15 to 20 uninfected nymphs or 5 or 6 Borrelia-infected nymphs on 4- to 6-week-old female C3H mice. For feeding experiments with adult ticks, 20 pathogen-free adult female ticks were first microinjected with 0.5 μl of dsRNA prepared as described above or buffer alone (control) in the body and allowed to rest for 1 day before feeding as previously described (36). Ticks were placed on each ear of naive New Zealand White rabbits. The ears were secured with cotton socks, and a restraining collar was placed around the neck of each rabbit. Adult I. scapularis males were placed with females at a 1:1 ratio to ensure mating and feeding, and at least two rabbits were used in each experiment. The ticks were allowed to feed for 5 to 7 days until they were engorged and were then gently removed for RNA analysis, protein extraction, and saliva collection. Fed adult ticks were dissected, and individual salivary glands were resuspended in 100 μl of PBS and homogenized on ice for 1 min with a handheld homogenizer, and the protein was estimated using a BCA protein estimation kit (Pierce, IL). Saliva was collected from fed ticks following pilocarpine stimulation, and saliva from each tick was stored individually to allow the use of control saliva corresponding to positive ISL 929 and ISL 1373 expression and knockdown saliva corresponding to negative ISL 929 and ISL 1373 expression. The saliva and salivary gland homogenates were stored at −80°C until they were used (32).
RT-PCR of tick salivary glands.
I. scapularis ticks were dissected after feeding, and the salivary glands and midguts were individually suspended in TRIzol for RNA isolation according to the manufacturer's protocol (Invitrogen, CA). For temporal analysis of gene expression, at least 15 to 20 nymphal ticks were allowed to feed for 72 h on mice as described above. The midguts and salivary glands from pools of three ticks were dissected, RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions, and at least three biological replicates were examined. The isolated RNA was used to generate cDNA using the iScript reverse transcription (RT)-PCR kit (Stratagene, Cedar Creek, TX) and was analyzed by PCR for the expression of tick actin, ISL 929, and ISL 1373 (36). The primers for the tick proteins were as follows: actin 5′ primer, 5′-GAT GAC CCA GAT CAT GTT CG-3′, and 3′ primer, 5′-GCC GAT GGT GAT CAC CTG-3′; ISL 929 5′ primer, 5′-CCT AAG CTT ATG CAA CTG GCC CTC TTC ATG-3′, and 3′ primer, 5′-CCT CTC GAG CGT ACG AAG GTC ATT TGG C-3′; ISL 1373 5′ primer, 5′-AAG CTT ATG CAA CTG GCC CTC TTC CTG-3′, and 3′ primer, 5′-CTC GAG CGT ATT CGC CCT TTC AAT CC-3′. Quantitative PCR (qPCR) was performed using the iQ Syber Green Supermix (Bio-Rad, CA) on an MJ cycler (MJ Research, CA). Data were normalized to tick actin, and the results were expressed as mean ± standard error. Transcript levels were expressed as ng or pg of gene per ng of tick actin.
Expression and purification of recombinant salivary proteins.
The coding sequences of the ISL 929 (GenBank access number AF483719) and ISL 1373 (GenBank access number AF483720) genes were inserted between SpeI and XhoI into the multicloning site of the inducible secretory expression vector pMT/BiP/V5-His B of the Drosophila Expression System (Invitrogen), in frame with both the upstream signal peptide and the downstream His tag. Each expression vector was cotransfected with the pCoHygro selection vector into Drosophila S2 cells with CellFectin reagent (Invitrogen). The S2 cells were cultured in Schneider's Drosophila Medium (Invitrogen) at room temperature. Cells were selected with hygromycin B (1 mg/ml) for stable expression of tick proteins. For purification of recombinant ISL 929 and ISL 1373 proteins, stable cell lines were cultured in suspension in Express Five SFM serum-free medium (Invitrogen) and induced with 600 μM CuSO4 for 5 days. The cell supernatants were harvested, centrifuged (5,465 × g; 4°C; 20 min) to remove debris, buffer exchanged, and concentrated to 50 ml using the Amicon stirred-cell system NMWL 5000 at 35 lb/in2 N2 at 4°C (Millipore, Bedford, MA). Recombinant ISL 929 and ISL 1373 were purified using His tag affinity column chromatography with Talon beads (BD Biosciences, San Jose, CA). The eluted protein fractions were buffer exchanged into PBS containing 1× Complete EDTA Free protease inhibitor (Roche, Indianapolis, IN). The protein yield was quantified by Bradford assay, and the purity of the isolated proteins was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis. Recombinant ISL 929 and ISL 1373 were aliquoted and stored at −20°C until they were used.
Mouse immunization, tick infestation, and infection with B. burgdorferi.
C3H/HeN mice (n = 5/group) were immunized with a mixture of ISL 929 and ISL 1373 proteins (5 μg of each protein per mouse per immunization) in complete Freund's adjuvant for the initial immunization and boosted twice at 2-week intervals with 5 μg of ISL 1373 and ISL 929 or BSA in incomplete Freund's adjuvant. Sera were collected 2 weeks after the final boost and evaluated by immunoblotting (36) for the ability to react with recombinant ISL 1373 and ISL 929. End point titers were determined by enzyme-linked immunosorbent assay using serial dilutions of mouse sera and recombinant ISL 929 and ISL 1373 (1 μg/well) and detection with conjugated anti-mouse antibodies as described previously (27). The titers were determined to be >300,000 against each protein (endpoint titer at an optical density of 0.1 for the average of five mice, 1:469,042 for ISL 929 and 1:332,551 for ISL 1373); preimmune serum from each mouse served as a control.
Uninfected ticks (10 to 15/mouse) were allowed to feed on naïve control and immunized mice for up to 4 days. At harvest, the tick weights were documented and the salivary glands were dissected. In separate experiments, ISL 929/1373- and BSA-immunized mice were challenged with 5 or 6 B. burgdorferi-infected nymphs or 20 uninfected nymphs, and the ticks were allowed to feed to repletion. All of the ticks were weighed to assess the efficiency of tick engorgement. In transmission experiments, ticks were dissected in pools of three and analyzed by quantitative RT-PCR for the viable Borrelia burden in the tick as described previously (37). Skin biopsy specimens were taken at 4, 7, and 14 days postinfection, and after 21 days, the mice were sacrificed; the skin, heart, bladder, spleen, and joints were collected aseptically; and DNA was isolated using the DNAeasy Blood and Tissue kit (Qiagen, CA). The infection status of the mice was detected by culture of spirochetes from the bladder in 6 ml of Barbour-Stoenner-Kelly medium (BSK-H; Sigma Chemical Co., St. Louis, MO) for 5 days at 30°C and by assessment of the spirochete burden in harvested organs by DNA PCR using mouse actin and Borrelia flaB primers according to our standard methods (31, 34). Spirochetes were quantified in a Petroff-Hausser counter (Hausser Scientific, Horsham, PA) using a dark-field microscope (Carl Zeiss Microimaging, Inc.). The infection status was confirmed by serum reactivity against B. burgdorferi lysate by immunoblotting as described previously (31, 34).
Histopathology and semiquantitative tissue analysis.
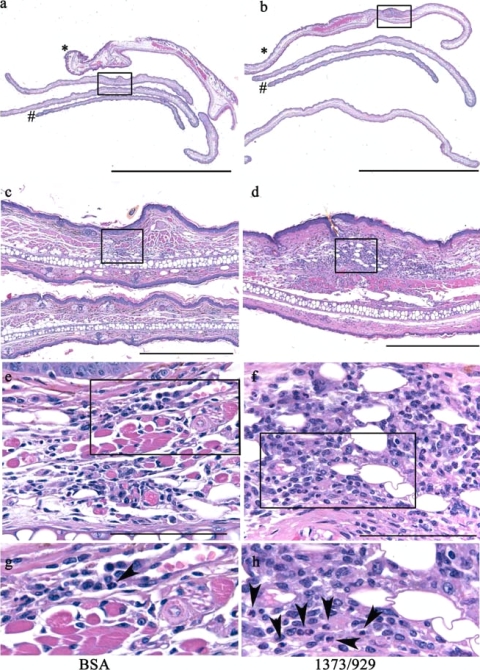
Ears were harvested at 3 days for histologic analysis of PMN recruitment at the site of the tick bite, and observers were blinded to the study conditions until after the histopathologic features were assessed. Briefly, mice were euthanized, and the external ear pinnae were excised at the base of the ear, fixed in 10% neutral buffered formalin (VWR International, Batavia, IL), and processed by routine methods. Prior to being embedded in paraffin, the ears were “bread-loafed” into four approximately equal-size strips by cutting parallel to the ear base from proximal to distal (see Fig. 3a and b). The strips were rotated 90°, embedded in paraffin (Blue RiBbon; Surgipath Medical Industries, Inc., Richmond, IL), and serially sectioned at a thickness of 5 μm. The 1st, 5th, 10th, 15th, and 20th sections of 35 total sections were stained with hematoxylin and eosin, followed by placement of coverslips. The hematoxylin- and eosin-stained slides were assessed at low and high power and scored for the presence and extent (severity) of the tissue changes using a semiquantitative criterion-based methodology adapted from our previous analysis of murine carditis (31). The entire tissue section on each slide was examined (10 to 40 microscope fields at each magnification or 60 to 90 fields per section) for each of the five sections per ear so that approximately 300 to 450 fields per ear were examined at low power. All foci of inflammation above background were examined at high power (×40) to assess the specific nature of the inflammation characterized. The sections were evaluated for pathological changes in the epidermis, dermis, subcutis, muscle, and cartilage, including inflammation, necrosis, edema, vascular congestion, and hemorrhage. The severity scores ranged from 0 to 5, with numerical values of 0 (within normal limits, absent), 1 (minimal), 2 (mild), 3 (moderate), 4 (marked), and 5 (severe). The character of the inflammation was evaluated by light microscopy for changes due to swelling, hemorrhage, or inflammatory infiltrate, and identification of the exact type of inflammatory cell in the infiltrates was based on distinct morphological differences. Digital light microscopic images were recorded using a Leica DM550B microscope (Bannockburn, IL) and an AxioCam MRC camera and AxioVision 4.4 imaging software (Zeiss).
FIG. 3.
Salivary ISL 929 and ISL 1373 modulate PMN accumulation in vivo. Uninfected ticks (10 to 15/mouse) were allowed to feed for 3 days on mice that had been immunized with BSA (left) or with ISL 929/1373 (right). (a and b) External pinnae cut at the ear base and “bread loafed” into four approximately equal strips from proximal (*) to distal (#) and rotated 90° for histopathologic analysis (scale bars = 5,000 μm). (c to h) Progressively higher magnification of the boxed areas in the panels above (scale bars, 500 μm [c and d] and 200 μm [e and f]). (g and h) Inflammation at the site of the tick bite in hematoxylin- and eosin-stained sections of ear pinnae from BSA-immunized mice showed mild inflammation with infrequent PMN (panel g, arrowhead), and sections from ISL 929/1373-immunized mice show marked inflammation with frequent PMN (panel h, arrowheads). The severity of inflammation was scored in five sections per ear; n = 3 mice/group.
Statistical analysis.
Significance was assessed as indicated in the text by paired Student's t tests (FACS, O2−, and tick weights) and the Mann-Whitney U test (PCR) using Graphpad Prism (GraphPad Software, Inc.).
RESULTS
Expression profiles of ISL 929 and ISL 1373 in tick salivary glands.
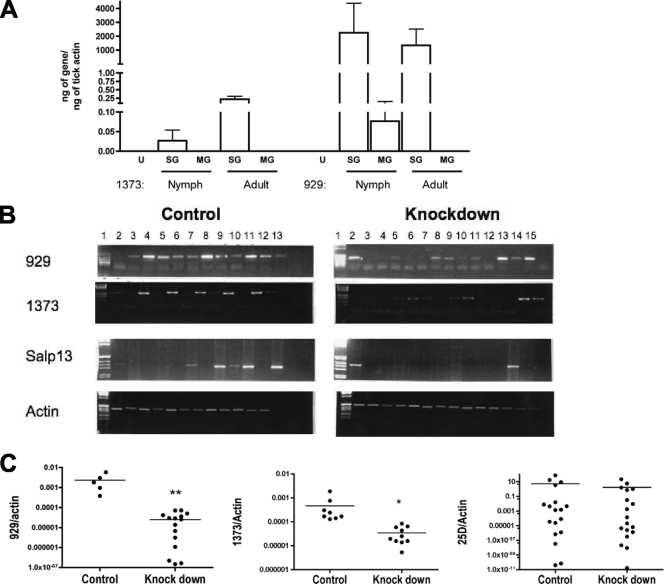
The recent description of 735 clones from an Ixodes salivary gland cDNA library revealed numerous proteins, including some that might act to disrupt host integrin function. The predicted sequences of ISL 929 and ISL 1373 were identified based on homology to the cysteine-rich domains of ADAMTS (a disintegrin and metalloprotease with thrombospondin motifs) metalloproteases, which are postulated to interact with integrins through cysteine-rich domains (44, 61). These two candidate inhibitors from Ixodes contain eight conserved cysteine residues, lack RGD sequences, and have similarities to the cysteine-rich domains of ADAMTS proteins from snake venoms (19). We assessed the expression of ISL 929 and ISL 1373 by real-time PCR in Ixodes tick salivary glands and midguts. Spatial and temporal analysis of the expression profiles showed that both ISL 929 and ISL 1373 were preferentially expressed in salivary glands, with little or no expression in the midguts of either nymphal or adult ticks (Fig. 1A). By examining expression before and during the ticks' feeding, we assessed the kinetics of expression that may indicate the likely timing of their function. In unfed nymphs, the transcripts for both genes were undetectable. Expression of ISL 929 and ISL 1373 was induced during tick feeding (Fig. 1A), suggesting a predominant role for these gene products at the vector-host interface.
FIG. 1.
Expression profiles of ISL 929 and ISL 1373 in ticks and knockdown by RNAi. (A) Nymphal and adult I. scapularis ticks were allowed to feed on naïve mice, and tick salivary glands (SG) and midguts (MG) were harvested after 72 h of feeding. Expression of ISL 929 and ISL 1373 was assessed by RT-PCR as described in Materials and Methods and normalized to tick actin. U, unfed nymphs. (B) Adult female I. scapularis ticks were injected with 0.5 μl buffer alone or dsRNA corresponding to a shared coding region of ISL 929, ISL 1373, and Salp 13. RNAs extracted from salivary glands of control-injected (left) and dsRNA-injected (right) ticks were reverse transcribed and PCR amplified to assess the efficiency of gene knockdown by agarose gel electrophoresis of the PCR products using actin as a control. (C) Selected ticks from panel B were assessed by qPCR for the efficiency of knockdown. Significance compared to control-injected mice: *, P < 0.0001; **, P = 0.0012; Mann-Whitney U test.
To assess the effects of Ixodes salivary proteins directly, we employed RNAi technology to knock down the expression of ISL 929 and ISL 1373 in the salivary glands prior to the harvesting of saliva from ticks. A dsRNA sequence (Table 1) was designed to target a shared interior domain from the ISL 929 and ISL 1373 genes and the related salp 13 salivary gene (7). The goal was to knock down all three genes simultaneously, as targeting individual members of closely related paralogous families often provides a weak phenotype because the related members compensate for the loss of function. This is consistent with our earlier work demonstrating that paralogous family members may be simultaneously silenced when conserved regions are targeted (36). While we cannot entirely rule out the possibility of off-target silencing, a protein BLAST search with each of these three genes against the I. scapularis genome database showed limited (26 to 30%) identity with ISCW014580-PA, ISCW02368, and ISCW004535, three structurally related members of this gene family. We did not expect these genes to be altered in the described RNAi experiments, since distinct 21-nucleotide stretches of identity between the designed dsRNA and these genes were absent (41).
TABLE 1.
Alignment of ISL 929, ISL 1373, and Salp 13 predicted proteinsa
| Protein | Sequence | Position |
|---|---|---|
| ISL 929 | MQLALFMIMVTFTLLSCEEQSEASPDIFGVMKYLPEDCKVNIKKQIEDKCSGNPYQPQLL | 60 |
| Salp 13 | MQLTLFIVIVTFTHLSCEVQSDSNPLISGKMEKLPQDCKDTLIQQMRNKCGESPFQTQLV | 60 |
| ISL 1373 | MQLALFLVVATFIYVSCGEKSESGLVIYKEFESLQEGCKQKLRDEMEQRCSEHPFQPELV | 60 |
| ***:**:::.**:**:*::. *:: *:.** .: .::.::*. *:*.:*: | ||
| ISL 929 | EVKDCTIICGDWHDNGVTKAITRHIINLKDGTPCGHSRVCIKGKCFDTCQMTFV------- | 114 |
| Salp 13 | EVKDCSFACGEWHNNGQTMGTSRQTTNLKDGTSCGYRKICVGGHCVQQCLVDFA------- | 114 |
| ISL 1373 | EVLQCKFKCGNEHSNGKTLLISGQYINLNDGTPCGPNKICIDGQCVPRCSMPFVKGLKGRI | 121 |
| **:*.: **: *.** *:: **:***.** ::*: *:*. * : *. |
Asterisks designate identities, colons indicate conserved substitutions, and periods indicate semiconserved substitutions. The region complementary to the dsRNA is underlined.
Ticks were microinjected with dsRNA (4.5 nl/tick; 1 × 1012 molecules/μl) in the body using glass capillary needles (36, 37). Although expression was not uniform, both ISL 929 and ISL 1373 were detected in most ticks injected with buffer alone (control), with ISL 929 being detected in a higher proportion of individual ticks (Fig. 1B). After injection with dsRNA, RT-PCR showed that mRNAs for ISL 929 and ISL 1373 were knocked down in about 50% of the treated ticks (Fig. 1B), in keeping with previous results (37). Expression of the targeted ISL 929 and ISL 1373 genes, but not the unrelated salp 25D salivary gene, was significantly reduced by dsRNA treatment, as shown by qPCR of selected ticks (Fig. 1C). Western blotting of tick salivary gland extracts was insufficiently sensitive to demonstrate reduction in protein expression of ISL 929 and ISL 1373 after dsRNA treatment of ticks (data not shown). While mRNA was reduced by RNAi treatment, mRNA and protein levels are not always correlated, and thus, we relied on differences in the biological effects of the saliva from dsRNA-treated ticks to indicate whether protein levels were affected. To control for variation in individual ticks both in initial expression of the proteins and in the efficiency of dsRNA knockdown, we collected and stored saliva from each tick individually and correlated each tick with the expression level. Experiments were conducted with control saliva corresponding to positive ISL 929 and ISL 1373 expression and knockdown saliva corresponding to negative ISL 929 and ISL 1373 expression.
Saliva inhibitors downregulate human PMN surface expression of β2 integrin.
Previously, we showed a dose-dependent downregulation of CD18 expression on human PMN in response to treatment with Ixodes tick saliva (32). To determine the roles of candidate salivary inhibitors in the downregulation of PMN integrins, we assessed the expression of CD18 on PMN treated with saliva from control- or dsRNA-injected ticks. As expected, saliva from control-injected ticks resulted in reduction of CD18 on PMN by 50% (P < 0.0005; paired t test) (Table 2). CD18 levels on PMN treated with saliva from ticks that had been injected with dsRNA were still lower than on untreated cells, but they were significantly higher than on PMN treated with control saliva (P < 0.01) (Table 2), suggesting roles for ISL 929 and ISL 1373 in downregulation of PMN integrins. Attempts to reproduce the effect on CD18 expression using salivary gland extracts did not yield similar results (data not shown).
TABLE 2.
Inhibition of PMN integrin expression by saliva and recombinant salivary proteinsa
| Treatment | CD18 | CD11b |
|---|---|---|
| Saliva | ||
| Untreated | 142.9 ± 11.2 | |
| Control (1:10) | 71.4 ± 9.2b | |
| dsRNA (1:10) | 100.8 ± 7.5b | |
| Control (1:20) | 90.9 ± 10.1b | |
| dsRNA (1:20) | 122.5 ± 11.7b | |
| Protein | ||
| Untreated | 134.1 ± 6.1 | 1,285 ± 88.7 |
| ISL 929 (2 μg) | 127.4 ± 7.1b | 1,206 ± 87.5b |
| ISL 929 (20μg) | 101.4 ± 7.7c | 871.6 ± 70.7c |
| ISL 1373 (2 μg) | 127.2 ± 6.6b | 1,229 ± 89.2b |
| ISL 1373 (20 μg) | 120.2 ± 7.0c | 1,027 ± 77.3c |
Freshly isolated human PMN were pretreated with saliva from control- or dsRNA-injected ticks or with recombinant ISL 929 or ISL 1373 prior to stimulation by TNF-α (20 ng/ml). Expression of CD11b and CD18 was quantified by FACS. The data show the average MFI ± SEM for saliva (n = 9) or ISL 929 and ISL 1373 (CD18, n = 8; CD11b, n = 14). CD15 levels were not significantly changed by incubation with 20 μg ISL 929 or ISL 1373 (average MFI, 19.15 ± 7.29, 20.04 ± 5.60, and 19.72 ± 5.42 respectively; differences NS; n = 3).
Significant reduction in staining (P ≤ 0.04) in surface integrin compared to untreated PMN (paired t test).
Significant reduction in staining (P ≤ 0.003) in surface integrin compared to untreated PMN (paired t test).
The effects of tick salivary inhibitors in reducing integrin expression on PMN were assessed directly by treating PMN with purified recombinant ISL 929 and ISL 1373 produced in Drosophila S2 cells (42). Upon stimulation with TNF-α, levels of both CD18 (common β-chain) and CD11b (α-chain) were increased as expected (average mean fluorescence intensity [MFI] resting and after TNF treatment for CD18, 87.89 ± 4.58 and 134.07 ± 6.10, n = 8; for CD11b, 669.34 ± 51.53 and 1,285.20 ± 88.72, n = 14). Both CD18 and CD11b were downregulated by incubation with ISL 929 and ISL 1373 in cells stimulated by TNF-α, as seen previously with native saliva (32). Levels of CD18 were significantly reduced in a dose-dependent manner by both recombinant proteins (Table 1) (P < 0.04 at 2 μg; P < 0.003 at 20 μg; paired t test). Control treatments of PMN with culture medium from S2 cells processed in parallel with ISL 929 and ISL 1373 did not change the levels of integrins, while incubation with an unrelated protein also produced in S2 cells (West Nile virus envelope protein [26]) increased the expression of CD11b and CD18 (data not shown). The reduction in integrins noted with ISL 929 and ISL 1373 is especially relevant, as PMN are highly reactive cells and are easily triggered; thus, impurities in the preparation might be expected to increase integrin expression on PMN rather than decrease it. We noted reduced integrin expression after pretreatment with ISL 929 or ISL 1373 in both the presence and absence of TNF-α and a similar reduction of CD11b and CD18 when monocytes were incubated with ISL 929 and ISL 1373 (data not shown), suggesting that the tick proteins have similar effects in monocytes and that inhibition may be broad based in vivo. Although our study did not identify the mechanism of action, the relatively short incubation time required suggests that ISL 929 and ISL 1373 may increase the internalization of integrins or their disappearance from the cell surface.
Inhibition of PMN O2− release by salivary proteins.
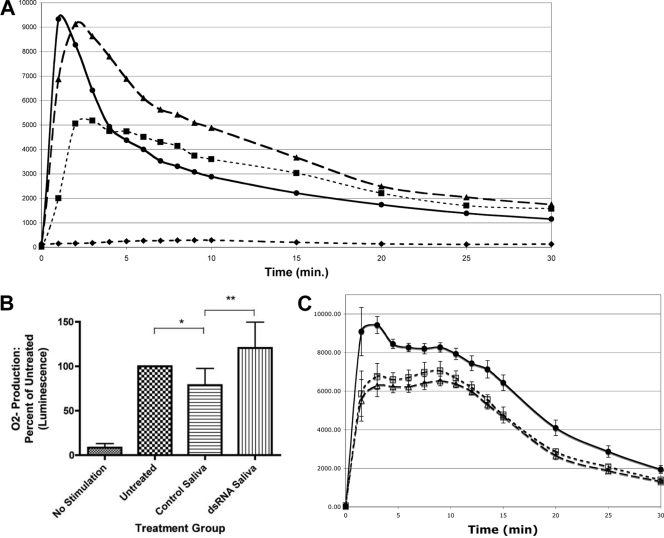
Saliva from Ixodes ticks has been shown previously to inhibit O2− production by rat neutrophils (47). We assessed the effects of tick saliva on PMA-stimulated production of O2− by human PMN. Production of O2− from PMN treated with tick saliva was reduced approximately 45% (Fig. 2A), as expected from studies of tick saliva and PMN of other species. However, no inhibition of production of O2− was detected when PMN were treated with saliva from dsRNA-injected ticks containing reduced or absent levels of ISL 929 and ISL 1373 (Fig. 2A).
FIG. 2.
Effects of ISL 929 and ISL 1373 on salivary inhibition of O2− production by PMN. Freshly isolated PMN were pretreated with saliva (1:10 dilution for 1 h at 37°C) from control-injected or dsRNA-injected ticks and assessed for O2− release by chemiluminescence assay following stimulation with PMA. (A) Representative plot showing O2− production by PMN in the presence (•) and absence (⧫) of stimulation by PMA and inhibition of O2− production by treatment with control (▪) but not by treatment with dsRNA (▴) saliva. (B) The peak value of O2− production (total up to 10 min) by the treated groups relative to that of the untreated group shows inhibition of O2− production by control but not by dsRNA saliva. *, P < 0.03 for PMN treated with control saliva versus untreated PMN; **, P < 0.004 for PMN treated with control versus dsRNA-treated saliva; there were no significant differences in O2− production by untreated and dsRNA-treated groups (paired t test; n = 5). The error bars indicate standard deviations. (C) PMN were treated with recombinant ISL 929 and ISL 1373 (2 μg/106 cells) prior to stimulation by PMA and O2− assay. •, PMA stimulation; ▵, pretreatment with ISL 929; □, pretreatment with ISL 1373. The data shown are the means ± SEM; P < 0.001 for each protein treatment compared to no treatment at peak stimulation (paired t test; n = 6).
The peak values integrated from the first 10 min of O2− production show that the PMN treated with saliva from control-injected ticks produced significantly less O2− (78%) than either untreated PMN or PMN treated with saliva from the dsRNA-injected ticks (Fig. 2B) (n = 5; P < 0.05). The difference between saliva from control- and dsRNA-injected ticks was statistically significant when either was compared to untreated cells or to the other saliva (Fig. 2B). The comparable production of O2− by untreated PMN and PMN treated with knockdown saliva suggests that ISL 929 and ISL 1373 play roles in the inhibition of O2− production.
To confirm the roles of these proteins in saliva's inhibition of PMN function, we quantified O2− production from PMN pretreated with the recombinant salivary proteins ISL 929 and ISL 1373 before stimulation by PMA. Both proteins significantly reduced the production of O2− by PMN at all time points during the 1.5- to 35-min assay (Fig. 2C). As with the decrease after treatment with saliva, the peak values integrated from the first 10 min of O2− production show that the PMN treated with ISL 929 and ISL 1373 produced significantly less O2− (73% and 78%, respectively) than untreated PMN (Fig. 2C) (paired t test; n = 6; P < 0.001). The inhibition effect is dose dependent and was also noted when O2− production was triggered by a distinct mechanism using a monoclonal antibody directed against the PMN component myeloperoxidase from TNF-α-primed neutrophils (43) (n = 3; P < 0.05; data not shown). This finding is consistent with the downregulation of CD18/CD11b (Table 1), as CD18 is required for anti-myeloperoxidase-induced O2− production (43).
Effects of ISL 929 and ISL 1373 on PMN accumulation at the tick attachment site in vivo.
The reduction in PMN adherence noted with saliva treatment in vitro would be expected to reduce migration in tissue. To assess the effects of the inhibitory proteins in vivo, we immunized mice with a cocktail of recombinant ISL 929 and ISL 1373 proteins. Immunoblots performed 2 weeks after the final immunization showed that antisera from all the ISL 929/1373-immunized mice, but not from control BSA-immunized mice, reacted with the recombinant proteins; an enzyme-linked immunosorbent assay showed that sera from immunized mice recognized recombinant ISL 929 and ISL 1373 at a dilution of >1:300,000. Native ISL 929/1373 proteins in salivary gland extracts were below detection by Western blotting. Thus, while we cannot rule out a contribution from cross-reactive antibodies, antisera from ISL 929/1373-immunized mice did not recognize any of a panel of other tick proteins tested, such as salp 9, salp 15, salp 25D, or rMIF; there was slight cross-reactivity to salp 13, as might be expected (18, 35).
Naïve ticks were allowed to feed on BSA- or ISL 929/1373-immunized mice for 3 days, and then ears were harvested to assess PMN accumulation at the site of tick attachment. The ticks had comparable engorgement weights when feeding on either group of mice (average weights ± standard errors of the mean [SEM]: BSA immunized, 3.2 ± 0.2 mg, n = 45 ticks; ISL 929/1373 immunized, 2.9 ± 0.2 mg, n = 38 ticks; differences not significant [NS]; paired t test) indicating that interference with these proteins did not reduce feeding efficiency, in contrast to our earlier studies with the knockdown of tick anticoagulants (36).
To assess the effects of immunization with ISL 929/1373 on PMN accumulation in vivo, serial sections of the external ear at the site of tick attachment were stained with hematoxylin and eosin, and approximately 300 to 450 fields from the 1st, 5th, 10, 15th, and 20th sections of 35 total sections per ear were examined at low power. All foci of inflammation above background were examined at high power (×40) to assess the specific nature of the inflammation. Histologic examination revealed an extensive inflammatory infiltrate in both groups of mice that was focused within the subjacent dermis and subcutis and extended through the underlying muscle to the pinna cartilage (Fig. 3). Additional changes included edema, myonecrosis and loss, vascular congestion, and hemorrhage, similar to dermatologic changes noted in human patients after tick bites (5). In the ear pinnae of mice immunized with BSA, the character of the inflammatory infiltrate consisted predominantly of mononuclear cells (lymphocytes, plasma cells, and macrophages). In contrast, in mice immunized with ISL 929/1373, abundant PMN were detected at the site of tick attachment; PMN were infrequent in BSA-immunized mice (Fig. 3). The severity and extent of the inflammatory infiltrate were quantified using a scale from 0 to 5 and were significantly greater in mice immunized with ISL 929/1373 than in mice immunized with BSA alone, i.e., the ISL 929/1373-immunized mice were more able to respond to injury at the bite site (severity, BSA immunized, 1.46 ± 0.3; ISL 929/1373 immunized, 2.3 ± 0.3; n = 14/group; P = 0.006; paired t test). The increased accumulation of PMN in the skin of ISL 929/1373-immunized mice suggests that when these proteins were blocked, PMN were more able to respond to injury at the bite site. This supports an important role for these inhibitory proteins in the modulation of PMN function in vivo.
Effects of ISL 929 and ISL 1373 on transmission of B. burgdorferi.
As PMN are the first responding innate immune cells to arrive at the site of the tick bite, inhibition of PMN function by tick saliva, including reduction in killing capacity (32), may be expected to influence the transmission of spirochetes to the vertebrate host or the course of spirochete infection. We assessed the effect of blocking ISL 929 and ISL 1373 on the course of murine infection with B. burgdorferi. B. burgdorferi-infected nymphal ticks were allowed to feed on mice immunized with BSA or recombinant ISL 929/1373. The engorgement weights of Borrelia-infected nymphs were comparable in the two groups of mice (average weights ± SEM for ticks that fed on BSA-immunized mice, 2.3 ± 0.3 mg; for those that fed on ISL 929/1373-immunized mice, 2.0 ± 0.2 mg; n = 12/group; differences NS; paired t test). Similarly, no significant differences were detected by qPCR of the B. burgdorferi burden in ticks that fed on animals immunized with BSA or with recombinant ISL 929/1373. Spirochetes from whole ticks (ng of flaB/ng of tick actin, n = 5 to 7/group: BSA fed, 12.0 ± 10.4 versus ISL 929/1373 fed, 7.6 ± 3.9; all differences NS; paired t test) and from salivary glands or midguts quantified separately (n = 6/group, salivary glands, BSA fed, 14.51 ± 7.5 versus ISL 929/1373 fed, 14.3 ± 5.2; midguts, BSA fed, 19.2 ± 6.6 versus ISL 929/1373 fed, 39.7 ± 7.6; all differences NS; paired t test) were equivalent. The comparable level of B. burgdorferi infection suggests that spirochete maintenance was not affected by feeding on immunized mice, although we cannot rule out possible effects on the migration of spirochetes through the ticks after feeding.
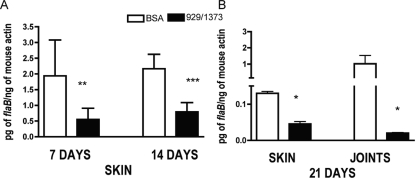
We assessed the course of infection by detection of the spirochete load after 4, 7, 14, and 21 days of infection (earlier time points were below detection). PCR analysis of skin at early time points prior to the dissemination of spirochetes indicated that the spirochete burden was lower in animals immunized with ISL 929/1373 than in BSA-immunized mice (Fig. 4A). Analysis of selected murine tissues at the peak of infection (21 days) revealed reduced spirochete burdens in both the skin and joints of ISL 929/1373-immunized mice compared to BSA-immunized mice; this reached significance in skin samples (Fig. 4B). The lower spirochete burden in animals in which ISL 929 and ISL 1373 were blocked suggests a more efficient clearance of spirochetes and that salivary protein inhibition of PMN function may play a role in the initial clearance of spirochetes. The spirochete burdens in the heart at 21 days were not significantly different in the control and immunized groups (data not shown), perhaps reflecting different kinetics of dissemination to the heart or that spirochete replication in the heart tissue overcomes any initial impairment.
FIG. 4.
Salivary disintegrins modulate infection with B. burgdorferi. B. burgdorferi-infected nymphal ticks were allowed to feed on mice immunized with BSA or ISL 929/1373 (5 or 6 infected or 15 to 20 uninfected ticks per mouse; n = 2). The spirochete burden was assessed by PCR in skin at days 7 and 14 (A) and in skin and joints at day 21 (B) of infection. Significance compared to mice immunized with BSA: *, 0.01; **, 0.02; ***, 0.006; Mann-Whitney U test. The error bars indicate standard errors of the means.
DISCUSSION
The tick sialome includes two cysteine-rich peptides with homology to the cysteine-rich domain of disintegrin metalloproteinases, a family of structurally and evolutionarily related negative regulators of integrins, including ADAMs and ADAMTS (29, 44, 57, 61). Disintegrins bind to integrins and interfere with ligand binding, probably through competitive inhibition; the cysteine-rich domain of ADAM/ADAMTS serves to target the molecule to the substrates and facilitates enzymatic functions (53). Disintegrins in tick saliva inhibit platelet aggregation and are important for successful tick feeding (8, 11, 16). ISL 929 and ISL 1373 belong to the ixostatin family and have been proposed to be involved in angiogenesis and repair inhibition (44). Although they downregulate PMN integrin expression, several lines of evidence suggest that the ISL 929 and ISL 1373 proteins differ from disintegrins. Predicted ISL 929 and ISL 1373 structures lack homology to disintegrins and also lack the Arg-Gly-Asp (RGD) motif, a common sequence in disintegrins that resides within a β-loop structure and interferes with integrin binding to their ligands (17, 20). The RGD tripeptide sequence is the prototype recognition motif among cell adhesion sequences recognized by integrins, such as clotting factors, collagen, the von Willebrand factor, fibronectin, fibrinogen, and thrombospondin, and mediates their adhesive functions (17). Some disintegrins are potent inhibitors of cell adhesion and produce an anticoagulant effect through competitive inhibition of platelet glycoprotein IIb-IIIa by attaching to the metal ion in the binding pocket (17). There are a significant number of ligands for integrins that do not contain an RGD sequence, including naturally occurring host proteins, such as ICAM-1, a common ligand for LFA-1 and MAC-1. Neutrophil (PMN) inhibitory factor (NIF), found in hookworms (33); atrolysin A, found in snake venom (20); obtustatin, found in snake venom (30); and disagregin, found in the tick O. moubata (22), have all been shown to inhibit integrins independently of RGD motifs. The manner in which these non-RGD disintegrins bind to integrins and inhibit their function is not fully understood.
Here, we have shown that two salivary proteins are induced upon tick feeding and predominantly expressed in the salivary glands of adult and nymphal Ixodes ticks. ISL 929 and ISL 1373 significantly reduced the expression of CD18 and inhibited production of O2− by PMN in vitro. The effect of this inhibition was noted both with purified recombinant proteins and with saliva harvested from ticks with reduced levels of ISL 929 and ISL 1373 through targeted RNAi knockdown. While the studies with recombinant ISL 929 and ISL 1373 proteins confirmed that the most likely result of the knockdown was the targeted genes, we cannot rule out the contribution of unintended knockdowns or off-target silencing. Taken together, these results suggest that ISL 929 and ISL 1373 from tick saliva contribute to the inhibition of PMN functions shown previously with tick saliva (32, 47).
We examined the roles of salivary inhibitory proteins in PMN function in vivo in murine skin. Although we have previously shown that saliva does not interfere with the orientation and locomotion per se of PMN in vitro in response to a chemotactic stimulus, saliva reduces PMN adherence in vitro (32). In murine skin, immunization against ISL 929 and ISL 1373 promoted PMN accumulation at the site of tick attachment, suggesting that these proteins inhibit PMN recruitment or accumulation in vivo. Saliva and salivary gland extracts from several tick species have been shown to affect PMN recruitment by interference with chemokine signaling, including downregulation of the chemokine receptor CCR5 and production of chemokine binding proteins (9, 39, 40), and interference with chemotaxis by RGD-containing disintegrins from snake venom has been noted (6, 28). In our studies, mice with immunity to ISL 929 and ISL 1373 also had a significant reduction in the spirochete burden in the skin after tick-transmitted infection with B. burgdorferi, both at early time points and at the peak of infection, suggesting that antibody-mediated inactivation of these proteins promoted PMN clearance of spirochetes in the skin. Previously, when PMN recruitment was increased through expression of PMN chemokines by transfected B. burgdorferi, spirochetes were also cleared more rapidly (62).
Our results show that two tick salivary proteins can inhibit PMN function and may contribute to both efficient tick feeding and transmission of pathogens. Although the spirochete burden alone need not be directly correlated with disease (63), saliva has been shown to influence the kinetics of infection (54), and Salp 15 has been shown to enhance spirochete survival and effect joint inflammation in a murine model in vivo (42). Our increased understanding of the impressive complexity of arthropod saliva and the interaction of pathogens with vectors and hosts has begun to reveal mechanisms for the control of infection. Our results illustrate one mechanism of tick control of the host immune function at the inoculation site and identify two proteins that may be suitable targets for therapeutic control of inflammation in the skin.
Acknowledgments
This work was supported in part by the NIH (AI-069156 and AI-057940).
We are grateful to Jose Ribeiro and Ivo Francischetti of the NIH Laboratory of Parasitic Diseases for cDNAs of ISL 929 and ISL 1373; to John Anderson of the Connecticut Agricultural Experiment Station for ticks; to MaryLou Breitenstein, Donna Caranno, Michael Schadt, and Lin Zhang for valuable assistance; and to Kok-Fai Kong, Stephen Malawista, and Feng Qian for insightful discussions.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 30 March 2009.
REFERENCES
- 1.Alekseev, A. N., E. A. Arumova, and I. S. Vasilieva. 1995. Borrelia burgdorferi sensu lato in female cement plug of Ixodes persulcatus ticks (Acari, Ixodiae). Exp. Appl. Acarol. 19519-522. [DOI] [PubMed] [Google Scholar]
- 2.Amsden, J. R., S. Warmack, and P. O. Gubbins. 2005. Tick-borne bacterial, rickettsial, spirochetal, and protozoal infectious diseases in the United States: a comprehensive review. Pharmacotherapy 25191-210. [DOI] [PubMed] [Google Scholar]
- 3.Anguita, J., N. Ramamoorthi, J. W. Hovius, S. Das, V. Thomas, R. Persinski, D. Conze, P. W. Askenase, M. Rincon, F. S. Kantor, and E. Fikrig. 2002. Salp15, an Ixodes scapularis salivary protein, inhibits CD4+ T cell activation. Immunity 16849-859. [DOI] [PubMed] [Google Scholar]
- 4.Barthold, S. W., M. de Souza, E. Fikrig, and D. H. Persing. 1992. Lyme borreliosis in the laboratory mouse, p. 223-242. In S. E. Schutzer (ed.), Lyme disease: molecular and immunologic approaches. Cold Spring Harbor Press, Cold Spring Harbor, NY.
- 5.Castelli, E., V. Caputo, V. Morello, and R. M. Tomasino. 2008. Local reactions to tick bites. Am. J. Dermatopathol. 30241-248. [DOI] [PubMed] [Google Scholar]
- 6.Coelho, A. L., M. S. De Freitas, A. Mariano-Oliveira, A. L. Oliveira-Carvalho, R. B. Zingali, and C. Barja-Fidalgo. 2001. Interaction of disintegrins with human neutrophils induces cytoskeleton reorganization, focal adhesion kinase activation, and extracellular-regulated kinase-2 nuclear translocation, interfering with the chemotactic function. FASEB J. 151643-1645. [DOI] [PubMed] [Google Scholar]
- 7.Das, S., G. Banerjee, K. DePonte, N. Marcantonio, F. S. Kantor, and E. Fikrig. 2001. Salp25D, an Ixodes scapularis antioxidant, is 1 of 14 immunodominant antigens in engorged tick salivary glands. J. Infect. Dis. 1841056-1064. [DOI] [PubMed] [Google Scholar]
- 8.Decrem, Y., J. Beaufays, V. Blasioli, K. Lahaye, M. Brossard, L. Vanhamme, and E. Godfroid. 2008. A family of putative metalloproteases in the salivary glands of the tick Ixodes ricinus. FEBS J. 2751485-1499. [DOI] [PubMed] [Google Scholar]
- 9.Deruaz, M., A. Frauenschuh, A. L. Alessandri, J. M. Dias, F. M. Coelho, R. C. Russo, B. R. Ferreira, G. J. Graham, J. P. Shaw, T. N. Wells, M. M. Teixeira, C. A. Power, and A. E. Proudfoot. 2008. Ticks produce highly selective chemokine binding proteins with antiinflammatory activity. J. Exp. Med. 2052019-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francischetti, I. M., T. N. Mather, and J. M. Ribeiro. 2004. Penthalaris, a novel recombinant five-Kunitz tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick vector of Lyme disease, Ixodes scapularis. Thrombosis Haemostasis 91886-898. [DOI] [PubMed] [Google Scholar]
- 11.Francischetti, I. M., V. My Pham, B. J. Mans, J. F. Andersen, T. N. Mather, R. S. Lane, and J. M. Ribeiro. 2005. The transcriptome of the salivary glands of the female western black-legged tick Ixodes pacificus (Acari: Ixodidae). Insect Biochem. Mol. Biol. 351142-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francischetti, I. M., J. G. Valenzuela, J. F. Andersen, T. N. Mather, and J. M. Ribeiro. 2002. Ixolaris, a novel recombinant tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick, Ixodes scapularis: identification of factor X and factor Xa as scaffolds for the inhibition of factor VIIa/tissue factor complex. Blood 993602-3612. [DOI] [PubMed] [Google Scholar]
- 13.Garg, R., I. J. Juncadella, N. Ramamoorthi, S. K. Ananthanarayanan, V. Thomas, M. Rincon, J. K. Krueger, E. Fikrig, C. M. Yengo, and J. Anguita. 2006. Cutting edge: CD4 is the receptor for the tick saliva immunosuppressor, salp15. J. Immunol. 1776579-6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillespie, R. D., M. C. Dolan, J. Piesman, and R. G. Titus. 2001. Identification of an IL-2 binding protein in the saliva of the Lyme disease vector tick, Ixodes scapularis. J. Immunol. 1664319-4326. [DOI] [PubMed] [Google Scholar]
- 15.Gillespie, R. D., M. L. Mbow, and R. G. Titus. 2000. The immunomodulatory factors of bloodfeeding arthropod saliva. Parasite Immunol. 22319-331. [DOI] [PubMed] [Google Scholar]
- 16.Harnnoi, T., T. Sakaguchi, Y. Nishikawa, X. Xuan, and K. Fujisaki. 2007. Molecular characterization and comparative study of 6 salivary gland metalloproteases from the hard tick, Haemaphysalis longicornis. Comp. Biochem. Physiol. B 14793-101. [DOI] [PubMed] [Google Scholar]
- 17.Hynes, R. O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110673-687. [DOI] [PubMed] [Google Scholar]
- 18.Jaworski, D. C., A. Jasinskas, C. N. Metz, R. Bucala, and A. G. Barbour. 2001. Identification and characterization of a homologue of the pro- inflammatory cytokine macrophage migration inhibitory factor in the tick, Amblyomma americanum. Insect Mol. Biol. 10323-331. [DOI] [PubMed] [Google Scholar]
- 19.Jia, L. G., K. Shimokawa, J. B. Bjarnason, and J. W. Fox. 1996. Snake venom metalloproteinases: structure, function and relationship to the ADAMs family of proteins. Toxicon 341269-1276. [DOI] [PubMed] [Google Scholar]
- 20.Jia, L. G., X. M. Wang, J. D. Shannon, J. B. Bjarnason, and J. W. Fox. 1997. Function of disintegrin-like/cysteine-rich domains of atrolysin A. Inhibition of platelet aggregation by recombinant protein and peptide antagonists. J. Biol. Chem. 27213094-13102. [DOI] [PubMed] [Google Scholar]
- 21.Juncadella, I. J., R. Garg, S. K. Ananthnarayanan, C. M. Yengo, and J. Anguita. 2007. T-cell signaling pathways inhibited by the tick saliva immunosuppressor, Salp15. FEMS Immunol. Med. Microbiol. 49433-438. [DOI] [PubMed] [Google Scholar]
- 22.Karczewski, J., R. Endris, and T. M. Connolly. 1994. Disagregin is a fibrinogen receptor antagonist lacking the Arg-Gly-Asp sequence from the tick, Ornithodoros moubata. J. Biol. Chem. 2696702-6708. [PubMed] [Google Scholar]
- 23.Kishimoto, T. K., E. T. Baldwin, and D. C. Anderson. 1999. The Role of b2 integrins in inflammation, p. 537-569. In J. I. Gallin and R. Snyderman (ed.), Inflammation: basic principals and clinical correlates, 3rd ed. Lippincott, Williams & Wilkins, Philadelphia, PA.
- 24.Kopecky, J., and M. Kuthejlova. 1998. Suppressive effect of Ixodes ricinus salivary gland extract on mechanisms of natural immunity in vitro. Parasite Immunol. 20169-174. [PubMed] [Google Scholar]
- 25.Kotsyfakis, M., A. Sa-Nunes, I. M. Francischetti, T. N. Mather, J. F. Andersen, and J. M. Ribeiro. 2006. Antiinflammatory and immunosuppressive activity of sialostatin L, a salivary cystatin from the tick Ixodes scapularis. J. Biol. Chem. 28126298-26307. [DOI] [PubMed] [Google Scholar]
- 26.Ledizet, M., K. Kar, H. G. Foellmer, T. Wang, S. L. Bushmich, J. F. Anderson, E. Fikrig, and R. A. Koski. 2005. A recombinant envelope protein vaccine against West Nile virus. Vaccine 233915-3924. [DOI] [PubMed] [Google Scholar]
- 27.Lusitani, D. L., S. E. Malawista, and R. R. Montgomery. 2003. Calprotectin, an abundant cytosolic protein from human polymorphonuclear leukocytes, inhibits the growth of Borrelia burgdorferi. Infect. Immun. 714711-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mariano-Oliveira, A., A. L. Coelho, C. H. Terruggi, H. S. Selistre-de-Araujo, C. Barja-Fidalgo, and M. S. De Freitas. 2003. Alternagin-C, a nonRGD-disintegrin, induces neutrophil migration via integrin signaling. Europ. J. Biochem. 2704799-4808. [DOI] [PubMed] [Google Scholar]
- 29.McLane, M. A., E. E. Sanchez, A. Wong, C. Paquette-Straub, and J. C. Perez. 2004. Disintegrins. Curr. Drug Targets Cardiovasc. Haematol. Disord. 4327-355. [DOI] [PubMed] [Google Scholar]
- 30.Monleon, D., M. P. Moreno-Murciano, H. Kovacs, C. Marcinkiewicz, J. J. Calvete, and B. Celda. 2003. Concerted motions of the integrin-binding loop and the C-terminal tail of the non-RGD disintegrin obtustatin. J. Biol. Chem. 27845570-45576. [DOI] [PubMed] [Google Scholar]
- 31.Montgomery, R. R., C. J. Booth, X. Wang, V. A. Blaho, S. E. Malawista, and C. R. Brown. 2007. Recruitment of macrophages and PMN in Lyme carditis. Infect. Immun. 75613-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montgomery, R. R., D. Lusitani, A. de Boisfleury Chevance, and S. E. Malawista. 2004. Tick saliva reduces adherence and area of human neutrophils. Infect. Immun. 722989-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moyle, M., D. L. Foster, D. E. McGrath, S. M. Brown, Y. Laroche, J. De Meutter, P. Stanssens, C. A. Bogowitz, V. A. Fried, and J. A. Ely. 1994. A hookworm glycoprotein that inhibits neutrophil function is a ligand of the integrin CD11b/CD18. J. Biol. Chem. 26910008-10015. [PubMed] [Google Scholar]
- 34.Narasimhan, S., K. Deponte, N. Marcantonio, X. Liang, T. E. Royce, K. F. Nelson, C. J. Booth, B. Koski, J. F. Anderson, F. Kantor, and E. Fikrig. 2007. Immunity against Ixodes scapularis salivary proteins expressed within 24 hours of attachment thwarts tick feeding and impairs Borrelia transmission. PLoS ONE 2e451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narasimhan, S., R. A. Koski, B. Beaulieu, J. F. Anderson, N. Ramamoorthi, F. Kantor, M. Cappello, and E. Fikrig. 2002. A novel family of anticoagulants from the saliva of Ixodes scapularis. Insect Mol. Biol. 11641-650. [DOI] [PubMed] [Google Scholar]
- 36.Narasimhan, S., R. R. Montgomery, K. Deponte, C. Tschudi, N. Marcantonio, J. F. Anderson, J. R. Sauer, M. Cappello, F. S. Kantor, and E. Fikrig. 2004. Disruption of Ixodes scapularis anticoagulation using RNA interference. Proc. Natl. Acad. Sci. USA 1011141-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narasimhan, S., B. Sukumaran, U. Bozdogan, V. Thomas, X. Liang, K. DePonte, N. Marcantonio, R. A. Koski, J. F. Anderson, F. S. Kantor, and E. Fikrig. 2007. A tick antioxidant facilitates the Lyme disease agent's successful migration from the mammalian host to the arthropod vector. Cell Host Microbe 27-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nuttall, P. A., G. C. Paesen, C. H. Lawrie, and H. Wang. 2000. Vector-host interactions in disease transmission. J. Mol. Microbiol. Biotechnol. 2381-386. [PubMed] [Google Scholar]
- 39.Oliveira, C. J., K. A. Cavassani, D. D. More, G. P. Garlet, J. C. Aliberti, J. S. Silva, and B. R. Ferreira. 2008. Tick saliva inhibits the chemotactic function of MIP-1α and selectively impairs chemotaxis of immature dendritic cells by down-regulating cell-surface CCR5. Int. J. Parasitol. 38705-716. [DOI] [PubMed] [Google Scholar]
- 40.Peterkova, K., I. Vancova, V. Hajnicka, M. Slovak, L. Simo, and P. A. Nuttall. 2008. Immunomodulatory arsenal of nymphal ticks. Med. Vet. Entomol. 22167-171. [DOI] [PubMed] [Google Scholar]
- 41.Qiu, S., C. M. Adema, and T. Lane. 2005. A computational study of off-target effects of RNA interference. Nucleic Acids Res. 331834-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramamoorthi, N. R., S. Narasimhan, U. Pal, F. Bao, X. F. Yang, J. Anguita, M. V. Norgard, F. S. Kantor, J. Anderson, R. A. Koski, and E. Fikrig. 2005. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436573-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reumaux, D., P. L. Hordijk, P. Duthilleul, and D. Roos. 2006. Priming by tumor necrosis factor-alpha of human neutrophil NADPH-oxidase activity induced by anti-proteinase-3 or anti-myeloperoxidase antibodies. J. Leukoc. Biol. 801424-1433. [DOI] [PubMed] [Google Scholar]
- 44.Ribeiro, J. M., F. Alarcon-Chaidez, I. M. Francischetti, B. J. Mans, T. N. Mather, J. G. Valenzuela, and S. K. Wikel. 2006. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem. Mol. Biol. 36111-129. [DOI] [PubMed] [Google Scholar]
- 45.Ribeiro, J. M., and I. M. Francischetti. 2003. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu. Rev. Entomol. 4873-88. [DOI] [PubMed] [Google Scholar]
- 46.Ribeiro, J. M. C. 1995. Blood-feeding arthropods: Live syringes or invertebrate pharmacologists? Infect. Agents Dis. 4143-152. [PubMed] [Google Scholar]
- 47.Ribeiro, J. M. C., J. J. Weis, and S. R. Telford III. 1990. Saliva of the tick Ixodes dammini inhibits neutrophil function. Exp. Parasitol. 70382-388. [DOI] [PubMed] [Google Scholar]
- 48.Sa-Nunes, A., A. Bafica, D. A. Lucas, T. P. Conrads, T. D. Veenstra, J. F. Andersen, T. N. Mather, J. M. Ribeiro, and I. M. Francischetti. 2007. Prostaglandin E2 is a major inhibitor of dendritic cell maturation and function in Ixodes scapularis saliva. J. Immunol. 1791497-1505. [DOI] [PubMed] [Google Scholar]
- 49.Schoeler, G. B., and S. K. Wikel. 2001. Modulation of host immunity by haematophagous arthropods. Ann. Trop. Med. Parasitol. 95755-771. [DOI] [PubMed] [Google Scholar]
- 50.Schuijt, T. J., J. W. Hovius, N. D. van Burgel, N. Ramamoorthi, E. Fikrig, and A. P. van Dam. 2008. The tick salivary protein Salp15 inhibits the killing of serum-sensitive Borrelia burgdorferi sensu lato isolates. Infect. Immun. 762888-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwan, T. G., and J. Piesman. 2002. Vector interactions and molecular adaptations of Lyme disease and relapsing fever spirochetes associated with transmission by ticks. Emerg. Infect. Dis. 8115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schymeinsky, J., A. Mocsai, and B. Walzog. 2007. Neutrophil activation via β2 integrins (CD11/CD18): molecular mechanisms and clinical implications. Thrombosis Haemostasis 98262-273. [PubMed] [Google Scholar]
- 53.Serrano, S. M., J. Kim, D. Wang, B. Dragulev, J. D. Shannon, H. H. Mann, G. Veit, R. Wagener, M. Koch, and J. W. Fox. 2006. The cysteine-rich domain of snake venom metalloproteinases is a ligand for von Willebrand factor A domains: role in substrate targeting. J. Biol. Chem. 28139746-39756. [DOI] [PubMed] [Google Scholar]
- 54.Shih, C.-M., and L.-P. Liu. 1996. Accelerated infectivity of tick-transmitted Lyme diesase spirochetes to vector ticks. J. Clin. Microbiol. 342297-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skallova, A., G. Iezzi, F. Ampenberger, M. Kopf, and J. Kopecky. 2008. Tick saliva inhibits dendritic cell migration, maturation, and function while promoting development of Th2 responses. J. Immunol. 1806186-6192. [DOI] [PubMed] [Google Scholar]
- 56.Sonenshine, D. E. 1991. Biology of ticks, vol. I. Oxford University Press, New York, NY.
- 57.Tang, B. L. 2001. ADAMTS: a novel family of extracellular matrix proteases. Int. J. Biochem. Cell Biol. 3333-44. [DOI] [PubMed] [Google Scholar]
- 58.Tseng, Y. L., H. C. Peng, and T. F. Huang. 2004. Rhodostomin, a disintegrin, inhibits adhesion of neutrophils to fibrinogen and attenuates superoxide production. J. Biomed. Sci. 11683-691. [DOI] [PubMed] [Google Scholar]
- 59.Tyson, K., C. Elkins, H. Patterson, E. Fikrig, and A. de Silva. 2007. Biochemical and functional characterization of Salp20, an Ixodes scapularis tick salivary protein that inhibits the complement pathway. Insect Mol. Biol. 16469-479. [DOI] [PubMed] [Google Scholar]
- 60.Valenzuela, J. G. 2004. Exploring tick saliva: from biochemistry to ‘sialomes’ and functional genomics. Parasitology 129(Suppl.)S83-S94. [DOI] [PubMed] [Google Scholar]
- 61.Valenzuela, J. G., I. M. Francischetti, V. M. Pham, M. K. Garfield, T. N. Mather, and J. M. Ribeiro. 2002. Exploring the sialome of the tick Ixodes scapularis. J. Exp. Biol. 2052843-2864. [DOI] [PubMed] [Google Scholar]
- 62.Xu, Q., S. V. Seemanapalli, K. E. Reif, C. R. Brown, and F. T. Liang. 2007. Increasing the recruitment of neutrophils to the site of infection dramatically attenuates Borrelia burgdorferi infectivity. J. Immunol. 1785109-5115. [DOI] [PubMed] [Google Scholar]
- 63.Yang, L., J. H. Weis, E. Eichwald, C. P. Kolbert, D. H. Persing, and J. J. Weis. 1994. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect. Immun. 62492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeidner, N. S., B. S. Schneider, M. S. Nuncio, L. Gern, and J. Piesman. 2002. Coinoculation of Borrelia spp. with tick salivary gland lysate enhances spirochete load in mice and is tick species-specific. J. Parasitol. 881276-1278. [DOI] [PubMed] [Google Scholar]