Abstract
In areas of endemicity pregnancy-associated malaria is an important cause of maternal anemia, stillbirth, and delivery of low-birth-weight children. The syndrome is precipitated by the accumulation of Plasmodium falciparum-infected erythrocytes in the placenta, mediated through an interaction between a parasite protein expressed on erythrocytes named variant surface antigen 2-chondroitin sulfate A (VAR2CSA) and CSA on syncytiotrophoblasts. VAR2CSA is a large polymorphic protein consisting of six Duffy binding-like (DBL), domains and with current constraints on recombinant protein production it is not possible to produce entire VAR2CSA recombinant proteins. Furthermore, the presence of polymorphisms has raised the question of whether it is feasible to define VAR2CSA antigens eliciting broadly protective antibodies. Thus, the challenge for vaccine development is to define smaller parts of the molecule which induce antibodies that inhibit CSA binding of different parasite strains. In this study, we produced a large panel of VAR2CSA proteins and raised antibodies against these antigens. We show that antibodies against the DBL4 domain effectively inhibit parasite binding. As the inhibition was not limited to homologous parasite strains, it seems feasible to base a protective malaria vaccine on a single VAR2CSA DBL domain.
To avoid circulation and splenic clearance, Plasmodium falciparum-infected erythrocytes (IE) bind host receptors on various endothelia through antigens called P. falciparum erythrocyte membrane protein 1 (PfEMP1). Pregnancy-associated malaria (PAM) is caused by IE that sequester in the intervillous space of the placenta by adhering to chondroitin sulfate A (CSA) (8). The clinical outcome is determined by both the host immune response and the presence of parasites (reviewed in reference 18). The interaction between parasite antigens on the surface of the IE and CSA in the placenta is one of the most direct associations between binding phenotype and disease outcome in P. falciparum malaria. Placental parasites and parasite lines selected for CSA binding in vitro express a unique var gene named var2csa (20, 24). Variant surface antigen 2-CSA (VAR2CSA) is a large molecule (350 kDa) consisting of six Duffy binding-like (DBL) domains and several interdomain regions (12). The antigen is expressed on the surface of IE panned on CSA in vitro and on ex vivo IE isolated from infected placentas (13, 19). Parasite clones where the var2csa gene is disrupted lose the ability to bind CSA (25). Furthermore, several domains and regions of VAR2CSA have been shown to bind CSA in vitro (11, 17). Women in areas where malaria is endemic acquire antibodies that protect against PAM as a function of parity (22). The mechanism of protection appears to be antibodies that block binding of IE to CSA (6). Likewise, high anti-VAR2CSA immunoglobulin G (IgG) levels are correlated with protection against the clinical consequences of PAM (19). These findings suggest that it is feasible to develop a VAR2CSA-based vaccine to protect women in areas of malaria endemicity from PAM. However, the presence of multiple CSA binding VAR2CSA domains, combined with the presence of polymorphisms, has raised the question of whether it is feasible to define VAR2CSA antigens eliciting broadly protective antibodies. Thus, a challenge for vaccine development is to define a functional VAR2CSA construct of a size compatible with protein vaccine production. Here we tested a large panel of proteins covering the entire VAR2CSA from strain FCR3 for the ability to induce antibodies that inhibit parasite binding to CSA. The data demonstrate that it is feasible to develop a vaccine based on single domains of VAR2CSA, which elicits pan-reactive antibodies that abrogate binding of parasites in the placenta.
MATERIALS AND METHODS
Plasmodium falciparum cultures.
Parasite cultures were grown as previously described (14). In brief, parasites were maintained in culture using 5% hematocrit of human blood group 0+ blood in parasite medium consisting of RPMI 1640 supplemented with 25 mmol/liter sodium bicarbonate (Sigma-Aldrich), 0.125 μg/ml gentamicin, 0.125 μg/ml Albumax II (Invitrogen), and 2% normal human serum. To select for VAR2CSA expression, IE were repeatedly panned on BeWo cells to maintain a CSA binding phenotype. The PL1 parasite is an isolate recently derived from placental tissue (13). All isolates were mycoplasma negative and were regularly genotyped using nested GLURP and MSP-2 primers in a single PCR step.
Protein production.
All VAR2CSA constructs were based on native FCR3 var2csa and cloned from genomic parasite DNA, except for a single synthetic chimeric construct (DiCo DBL5). The VAR2CSA subdomains were defined by the structural alignment published by Andersen et al. (1), and subdomain definitions were based on the defined subdomains of Pkalfa-DBL (21). To facilitate high levels of expression of DBL4, 30 amino acids of the interdomain (ID4) between DBL4 and DBL5 was included in the DBL4 protein. Control constructs were cloned from DNA from either FCR3 (var1DBL3g) or 3D7 (F08_0141). Gene fragments were cloned into the Baculovirus vector pAcGP67-A (BD Biosciences) modified to contain a V5 epitope upstream of a histidine tag in the C-terminal end of the constructs. Linearized Bakpak6 Baculovirus DNA (BD Biosciences) was cotransfected with pAcGP67-A into Sf9 insect cells for generation of recombinant virus particles. Histidine-tagged recombinant protein was purified on Ni2+-Sepharose columns from the supernatant of Baculovirus infected High-Five insect cells using an ÄKTA-express purification system (GE-Healthcare). Protein sequencing and mass spectrometric peptide mapping was done by Alphalyse (Denmark).
Rat immunizations and IgG preparations.
Rat antisera were produced in Wistar rats by injection of 40 μg of recombinant protein in Freund's complete adjuvant, followed by two booster injections of 40 μg of protein in Freund's incomplete adjuvant at 3-week intervals. Antisera were collected 8 days after the final boosting injection. All immunizations induced antibodies against the recombinant proteins as measured by enzyme-linked immunosorbent assay (ELISA) of the final bleed. The end point titers of selected serum samples and IgG were determined by ELISA (Table 1). IgG was purified on a Hi-Trap protein G HP column according to the manufacturer's recommendations (GE-Healthcare).
TABLE 1.
FCR3 VAR2CSA constructsa
| FCR3 VAR2CSA construct | Amino acid location in FCR3 VAR2CSA (accession no. AY372123) | End point titer of antiserum from rat:
|
||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Single domains | ||||
| DBL1 | 58-438 | 8.1E+04 | 6.9E+05 | 1.3E+06 |
| DBL2 | 545-890 | 2.3E+05 | 2.3E+05 | 1.4E+05 |
| DBL3 | 1210-1587 | 6.4E+05 | 4.2E+04 | 3.3E+05 |
| DBL4 | 1583-1947 | 7.8E+04 | 3.3E+05 | 7.2E+04 |
| DBL5 | 1990-2328 | 4.6E+06 | 1.1E+06 | 2.6E+05 |
| DBL6 | 2307-2641 | >1E+07 | >1E+07 | >1E+07 |
| Interdomain 2 | 929-1223 | 9.5E+04 | 4.6E+05 | 4.3E+04 |
| Subdomains | ||||
| DBL1 Sub1+Sub2 | 58-267 | 8.0E+06 | 9.7E+05 | 7.4E+05 |
| DBL1 Sub3 | 256-438 | 3.8E+04 | 3.7E+05 | 8.2E+04 |
| DBL2 Sub1+Sub2 | 538-754 | 1.6E+05 | 7.7E+05 | 4.1E+05 |
| DBL2 Sub3 | 745-893 | 1.2E+05 | 7.3E+04 | 6.8E+04 |
| DBL3 Sub1+Sub2 | 1210-1449 | 4.5E+05 | 3.2E+06 | 5.3E+05 |
| DBL3 Sub3 | 1444-1587 | 2.8E+04 | 4.8E+05 | 6.7E+05 |
| DBL4 Sub1+Sub2 | 1583-1789 | >1E+04 | >1E+04 | >1E+04 |
| DBL4 Sub2+Sub3 | 1638-1947 | >1E+04 | >1E+04 | >1E+04 |
| DBL4 Sub3 | 1784-1947 | >1E+04 | >1E+04 | >1E+04 |
| Multidomains | ||||
| NTS+DBL1+DBL2 | 1-893 | 2.3E+06 | 6.0E+06 | 1.8E+06 |
| DBL2+DBL3 | 545-1587 | 5.0E+06 | 1.3E+06 | 3.1E+05 |
| DBL3+DBL4 | 1210-1947 | 4.8E+06 | 1.9E+06 | 5.8E+05 |
| DBL5+DBL6 | 1990-2641 | >1E+07 | >1E+07 | 1.6E+06 |
| Chimeric domain DiCo DBL5 | Synthetic gene, GenBank accession no. FJ028815 | 8.6E+05 | 4.2E+05 | Not done |
| Control domains | ||||
| FCR3 var1DBL3g | 1267-1580 | 5.1E+06 | 3.7E+05 | 9.1E+05 |
| 3D7 F08_0141. DBL2b | 861-1193 | 7.1E+05 | 2.4E+06 | 1.8E+07 |
The table describes the antigens cloned from FCR3 VAR2CSA used for immunizations. Antigen boundaries are indicated as start and stop amino acids from the coding sequence of VAR2CSA FCR3 (accession no. AY372123). Three rats were immunized per antigen, and the end point titer of the full bleed is indicated for each rat.
Flow cytometry.
Flow cytometry was used to test the reactivity of rat serum to VAR2CSA on the surface of the IE. In brief, erythrocytes infected with late trophozoite and schizont stage parasites were enriched based on the high contents of hemozoin in these developmental stages on a column placed in a strong magnetic field (16). Aliquots (2 × 105 IE) were labeled with ethidium bromide and sequentially exposed to 10 μl rat serum and 1 μl anti-rat IgG-fluorescein isothiocyanate (Zymax, Invitrogen). Data were acquired using a FC500 flow cytometer (Beckman Coulter). All samples relating to a particular parasite isolate were processed and analyzed in a single assay.
Binding assays.
High-throughput parasite binding assays were done as described previously (14). Briefly, 2 × 105 tritium-labeled late-stage IE and 15 μl rat serum or IgG in a total volume of 120 μl were added in triplicates to wells coated with 2 μg/ml of the commercially available chondroitin sulfate proteoglycan Decorin (D8428; Sigma-Aldrich). After incubation for 90 min at 37°C, unbound IE were washed away by resuspension performed by a pipetting robot (Beckman Coulter). The proportion of adhering IE was determined by liquid scintillation counting on a Topcount NXT (Perkin-Elmer).
To be able to compare results from different laboratories, we performed measurements of inhibition by purified IgG in a standardized petri dish assay adapted to study CSA binding by Fried et al. (10). Briefly, 20 spots in a 100- by 20-mm petri dish (Falcon 351005) were coated overnight with 20 μl Decorin at 2 μg/ml in phosphate-buffered saline. Spots were blocked with 3% bovine serum albumin. Late-stage trophozoites and schizonts were purified from culture by flotation in 0.7% gelatin (Sigma-Aldrich) in RPMI. The final concentration of parasites was 20% parasitemia at 0.5% hematocrit. The parasite suspension was preincubated with purified IgG at different concentrations at 37°C for 30 min. Thereafter duplicate spots were incubated for 15 min at 37°C in a humid chamber. Plates were washed with phosphate-buffered saline on a gyro-turntable (Stuart) until spots were clear of blood and fixed with 1.5% glutaraldehyde. Five fields were counted using a magnification of ×40 on a Nikon microscope.
Parasite binding to Decorin was abrogated by soluble CSA (Sigma-Aldrich) and by chondroitinase (Sigma-Aldrich) treatment of Decorin (data not shown). Parasites that did not express VAR2CSA did not bind to Decorin. Ten and 20 microliters of a pool of serum from women living in an area of malaria endemicity were used to inhibit binding to CSA, and pools of male and Danish serum were used as controls. Ethical clearance and informed consent for the collection group of these human samples from Tanzania was described by Magistrado et al. (13).
Statistical analysis.
Statistical analysis was performed using Sigma Stat software (Systat Software, Inc.).
RESULTS
Proteins expressed in the Baculovirus system.
It has previously been shown that immunizations with single domains of VAR2CSA can induce antibodies reacting with the native protein expressed on IE; however, none of these antibody reagents appeared to effectively inhibit the CSA binding of VAR2CSA-expressing parasites (2, 3, 7, 13, 14). This is likely to be caused by a deficit of antibodies with the right fine specificity, due to either restriction of the animal used as immunization model or a lack of relevant epitopes in the recombinant proteins. To broaden the analysis of the functional capacity of antibodies directed against VAR2CSA, we expressed 21 recombinant VAR2CSA proteins, representing subdomains, single domains, and multidomains from VAR2CSA from the FCR3 parasite strain. In addition, control antigens, as well as a chimeric single domain encompassing different polymorphic types in one construct, were expressed. We used Baculovirus-transfected insect cells as the expressing system and immunized groups of three rats with each recombinant protein (Table 1). All the recombinant proteins were immunogenic, and all ELISA end point IgG titers measured ranged between 1E+04 and >1E+07 (Table 1).
Functional characteristics of VAR2CSA-specific rat sera.
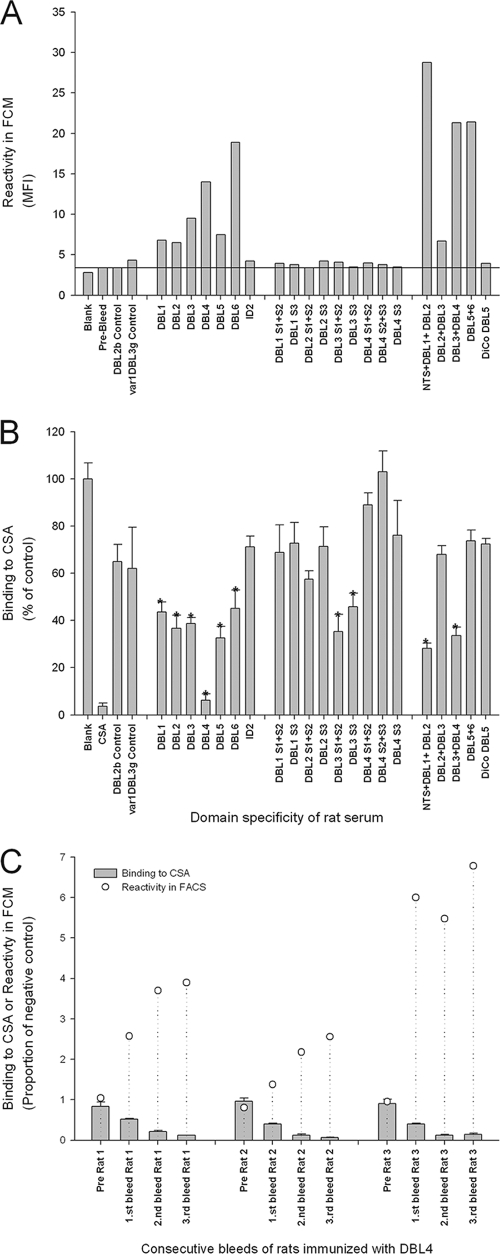
The ability of rat serum IgG to react with native VAR2CSA was tested by flow cytometry using erythrocytes infected with a VAR2CSA-expressing FCR3 parasite line following selection on CSA in vitro. Overall, the multidomains induced higher levels of surface-reactive antibodies than single domains, whereas disruption of the domains into subdomains induced antibodies that reacted poorly with native VAR2CSA (Fig. 1A). Interestingly, we found that DBL4-specific antibodies were surface reactive, which was contrary to published results obtained using rabbits as an immunization model (2, 14).
FIG. 1.
Functional capacity of the anti-VAR2CSA antibodies. (A) Reactivity measured by flow cytometry of antibodies against native VAR2CSA expressed on the surface of erythrocytes infected with an FCR3 parasite line selected for CSA binding. Each column shows the reactivity in rats immunized with the indicated VAR2CSA constructs based on FCR3 DNA. Groups of three rats were immunized with the same constructs, and 10 μl pooled rat serum was used to stain 2 × 105 IE. Samples without primary antibody (blank), prebleed sera, and DBL3g-VAR1 and DBL2b-VAR5 immunizations were used as controls. Bars represent the fluorescein isothiocyanate mean fluorescence intensity from 5000 IE. (B) Ability of immune serum to inhibit binding to CSA by VAR2CSA-expressing FCR3-IE. A high-throughput plate assay was used due to the number of serum samples. Fifteen microliters of pooled rat serum was used in a total volume of 120 μl containing 2 × 105 IE. Statistically significant adhesion inhibitory capacity compared to sera prior to immunization is represented by an asterisk (P < 0.01, t test). Bars represent the mean percent binding compared to binding without serum. Error bars are standard deviations of triplicate measurements. (C) Consecutive bleeds of rats immunized with DBL4 at 3-week intervals. Bars represent the mean proportion of binding compared to binding without serum. Error bars are standard deviations of triplicate measurements. The circles indicate the proportion of mean fluorescence intensity values compared to a negative control serum. All binding assays were performed multiple times with similar results.
In the next series of experiments, we used a static high-throughput assay to measure the ability of the immune sera to inhibit CSA binding of erythrocytes infected with the VAR2CSA-expressing FCR3 parasite line. Preimmunization sera of all rats were tested first to exclude unspecific inhibition, and we observed a mean background inhibition of 15% (confidence interval of mean, 6.4) compared to the binding in medium without serum (data not shown). When comparing all preimmunization serum pools with serum pools from the same rats after immunization with recombinant proteins of FCR3 origin, there was a highly significant increase in the ability to inhibit binding of IE to CSA (P < 0.001, paired t test) (Fig. 1B). Of all the FCR3 VAR2CSA domain combinations tested, 10 sera inhibited binding significantly more than the preimmunization serum (P < 0.01, t test). However, most domains appeared to induce intermediate levels of inhibitory reactivity, while one domain, DBL4, induced highly inhibitory antibodies. Surprised by this result, we tested the reproducibility of the protein production and the immunization in a new group of rats with a new batch of DBL4-FCR3 protein. In this group of animals we tested how single animals developed antibodies reacting with the native protein on the surface of IE and likewise how these animals induced antibodies inhibiting IE binding to CSA (Fig. 1C). All animals developed IgG reacting with the surface of the IE, and, comparable to the first immunizations, we found these sera to be highly adhesion inhibitory. The DBL4 immunogen was further analyzed by N-terminal sequencing and mass spectrometric peptide mapping, and it was verified to be DBL4 from FCR3 VAR2CSA. Proteins expressed in insect cells are glycosylated. We examined the levels of rat-induced antisugar antibodies in ELISA by testing the reactivity against non-VAR2CSA DBL domains produced in the same cells. We found that the induced antibodies did not cross-react with other glycosylated DBL domains, which suggests that the antibodies are protein specific (data not shown).
We next tested whether the highly inhibitory DBL4 antiserum could also inhibit the binding of other parasite lines expressing VAR2CSA. Although the level of inhibition varied between the different parasite lines used (7G8, PL1, or FCR3), the DBL4-FCR3 antibodies clearly reduced the binding to CSA of all three parasite lines (Fig. 2A). In general, the reactivity in VAR2CSA-immunized rats was comparable to the reactivity using a serum pool from past-pregnant women living in a high-transmission area, whereas the reactivity in the control immunized rats was comparable to the reactivity in a serum pool from males living in the same area (Fig. 2B).
FIG. 2.
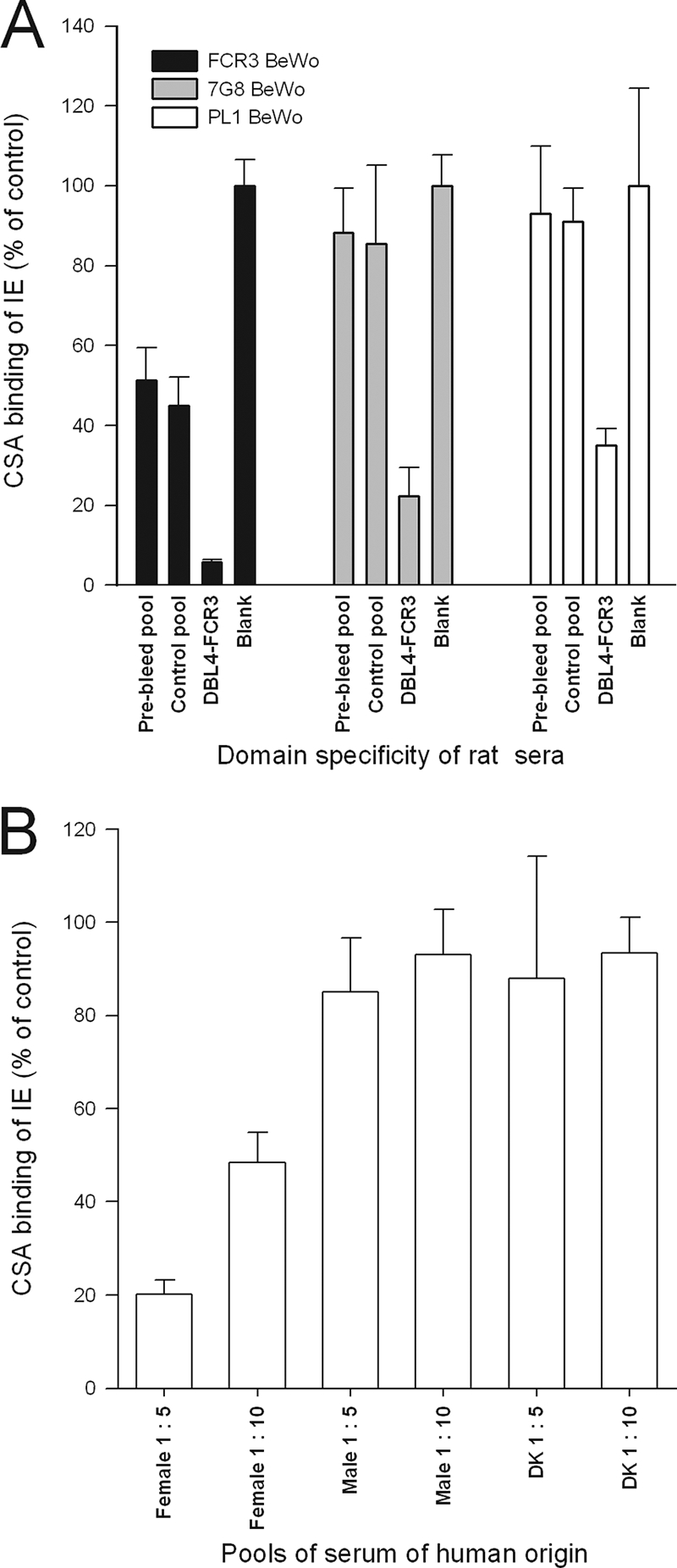
Adhesion-inhibitory antibodies are cross-reactive (A) Ability of antiserum to inhibit the CSA binding of VAR2CSA-expressing erythrocytes infected with FCR3, 7G8, or PL1 parasites. A pool of preimmune and a pool of control-immunized sera were used as controls. (B) Inhibition of adhesion by pools of Tanzanian human serum and Danish nonimmune serum (DK) at different concentrations. All bars represent the mean percentage of binding compared to binding without serum. Error bars are standard deviations of triplicate measurements. The assays were performed multiple times with similar results.
Purified IgG from rats immunized with DBL4-FCR3 inhibits binding.
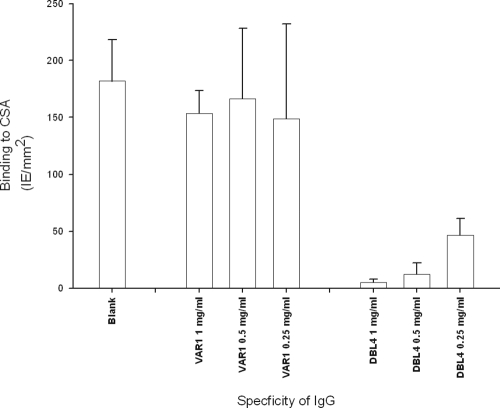
The association between serum reactivity in the flow cytometry assay and activity in the binding inhibition assays indicated that the inhibition of binding was mediated by IgG. To confirm this, IgG from rats immunized with the DBL4 recombinant protein was purified on protein G and tested for binding inhibition activity. Here we used a standardized petri dish assay developed by M. Fried that allows comparison of results obtained by other groups (see Materials and Methods). There was a highly significant difference between the different IgG purifications (P < 0.001, analysis of variance). More specifically, the purified IgG inhibited 97% of the binding at a concentration of 1 mg/ml total IgG compared to binding without IgG (P < 0.001, pairwise multiple comparisons by the Holm-Sidak method), while purified IgG from mock-immunized animals did not significantly inhibit binding at this concentration (Fig. 3) (P = 0.36, pair-wise multiple comparisons by the Holm-Sidak Method).
FIG. 3.
Inhibition of CSA binding using purified IgG from DBL4-VAR2CSA and DBL3g-VAR1 specific sera. All assays were performed using FCR3-IE expressing VAR2CSA in a petri dish assay. Bars are mean numbers of IE per mm2. Error bars are standard deviations from four replicates. The assay was performed multiple times with similar results.
DISCUSSION
Previous reports have shown that antiadhesive IgG is acquired during the first pregnancy and represents a small subset of the IgG reacting with the CSA-binding IE (4). It has also been demonstrated that inhibitory sera from women from Asia can prevent parasites from other regions in the world from adhering to CSA (10), further suggesting that the antigenic targets of protective immunity are conserved.
Here we demonstrate that immunizations with single domains of VAR2CSA can generate adhesion-inhibitory antibodies against genetically diverse parasites. This is encouraging since a vaccine obviously needs to induce neutralizing antibodies against most of the, if not the entire, diverse parasite population. Although other domains also elicited surface-reactive and adhesion-inhibitory antibodies, the most promising candidate of the recombinant proteins used in this study appears to be the DBL4 domain, based on the inhibition data. This is encouraging as this is one of the most conserved DBL domains of VAR2CSA (5). With the aim of defining a smaller region of the DBL4 domain which could induce functional antibodies, we cloned and expressed different combinations of DBL4 subdomains. However, none of these constructs elicited functional antibodies, which indicates that a potential vaccine needs to be based on a full domain.
All rats immunized with recombinant DBL4 FCR3 proteins elicited surface-reactive antibodies, indicating that previously reported failures to induce surface-reactive antibodies with DBL4 domains using rabbits may be due to differences in the species of animals used in the immunization model (3, 14, 15). However, we have previously shown that high levels of antibodies specific for the DBL4 domain are naturally acquired during infection with a placental parasite, indicating that humans can induce specific antibodies against this conserved domain (3).
The region(s) of native VAR2CSA that mediates CSA binding has not been identified with certainty, but when testing recombinant single domains, DBL2, DBL3, and DBL6 have shown the highest affinity for CSA binding (11). This indicates that the native VAR2CSA molecule has several CSA binding epitopes. Antiadhesive antibodies should therefore target all CSA binding sites or at least several of these at the same time to hinder binding. Our findings are at variance with this hypothesis. Inhibition of binding by single-domain-specific antibodies may suggest that such antibodies block the assembly of the quaternary structure of native VAR2CSA, which prevents the protein from engaging with its ligand. It has been shown that the two DBL domains of EBA-175 can form a dimer and that the glycan-ligand interacts at the dimer interface (23); if VAR2CSA glycan interaction is also dependent on domain dimerization, a possible mechanism of the blocking antibodies could be to hinder this dimerization. The hypothesis that blocking antibodies interfere with the quaternary structure is in line with data suggesting that VAR2CSA exists as a globular protein on the surface of IE (1). It also suggests that CSA binding in the native protein may be intrinsically different than that for single recombinant domains.
PAM pathogenesis is aggravated by the inflammatory responses to the placental parasites (9). Although antibodies reacting with native VAR2CSA, which do not have binding inhibition activity, could serve as opsonins for phagocytosis of IE, such antibodies may also fuel harmful inflammatory responses taking place in the placenta. The strategy for PAM vaccine development is therefore to induce IgG, which inhibits the sequestration of parasites in the placenta (6). We have shown here that immunization with single DBL domains can induce antibodies that effectively inhibit CSA adhesion of several genetically distinct parasite lines. Antibodies effectively interfering with CSA binding in vitro were induced in all animals in response to VAR2CSA DBL4 immunizations. The results obviously have direct implications for VAR2CSA-based vaccine development but also for the development of vaccines to protect children against severe malaria syndromes based on other members of the PfEMP1 family.
Acknowledgments
We thank technicians Anne Corfitz, Besim Berisha, Jonas Fjelbye Hansen, Maiken Visti, and Nahla Chehabi for excellent technical assistance.
This study received funding primarily from the PMI2 Gates Malaria Programme grant from the Bill and Melinda Gates Foundation. The work was also supported by the Danish Research Council (SSVF) (grant 22-03-0333), The Novo Nordisk Foundation (grant 10335), the Danish Research Council for Development Research (RUF) (grant 104.Dan.8.L.306 and 8.L.306), and PreMalStruct FP7-HEALTH-2007-A ID 201222.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 23 March 2009.
REFERENCES
- 1.Andersen, P., M. A. Nielsen, M. Resende, T. S. Rask, M. Dahlback, T. Theander, O. Lund, and A. Salanti. 2008. Structural insight into epitopes in the pregnancy-associated malaria protein VAR2CSA. PLoS Pathog. 4e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avril, M., B. R. Kulasekara, S. O. Gose, C. Rowe, M. Dahlback, P. E. Duffy, M. Fried, A. Salanti, L. Misher, D. L. Narum, and J. D. Smith. 2008. Evidence for globally shared, cross-reacting polymorphic epitopes in the pregnancy-associated malaria vaccine candidate VAR2CSA. Infect. Immun. 761791-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barfod, L., M. A. Nielsen, L. Turner, M. Dahlback, A. T. Jensen, L. Hviid, T. G. Theander, and A. Salanti. 2006. Baculovirus-expressed constructs induce immunoglobulin G that recognizes VAR2CSA on Plasmodium falciparum-infected erythrocytes. Infect. Immun. 744357-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beeson, J. G., E. J. Mann, S. R. Elliott, V. M. Lema, E. Tadesse, M. E. Molyneux, G. V. Brown, and S. J. Rogerson. 2004. Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes and adhesion inhibitory antibodies are associated with placental malaria and have overlapping and distinct targets. J. Infect. Dis. 189540-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bockhorst, J., F. Lu, J. H. Janes, J. Keebler, B. Gamain, P. Awadalla, X. Z. Su, R. Samudrala, N. Jojic, and J. D. Smith. 2007. Structural polymorphism and diversifying selection on the pregnancy malaria vaccine candidate VAR2CSA. Mol. Biochem. Parasitol. 155103-112. [DOI] [PubMed] [Google Scholar]
- 6.Duffy, P. E., and M. Fried. 2003. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect. Immun. 716620-6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez, P., N. Kviebig, S. Dechavanne, C. Lepolard, J. Gysin, A. Scherf, and B. Gamain. 2008. Var2CSA DBL6-epsilon domain expressed in HEK293 induces limited cross-reactive and blocking antibodies to CSA binding parasites. Malar. J. 7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried, M., and P. E. Duffy. 1996. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 2721502-1504. [DOI] [PubMed] [Google Scholar]
- 9.Fried, M., R. O. Muga, A. O. Misore, and P. E. Duffy. 1998. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J. Immunol. 1602523-2530. [PubMed] [Google Scholar]
- 10.Fried, M., F. Nosten, A. Brockman, B. J. Brabin, and P. E. Duffy. 1998. Maternal antibodies block malaria. Nature 395851-852. [DOI] [PubMed] [Google Scholar]
- 11.Gamain, B., A. R. Trimnell, C. Scheidig, A. Scherf, L. H. Miller, and J. D. Smith. 2005. Identification of multiple chondroitin sulfate A (CSA)-binding domains in the var2CSA gene transcribed in CSA-binding parasites. J. Infect. Dis. 1911010-1013. [DOI] [PubMed] [Google Scholar]
- 12.Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, A. Pain, K. E. Nelson, S. Bowman, I. T. Paulsen, K. James, J. A. Eisen, K. Rutherford, S. L. Salzberg, A. Craig, S. Kyes, M. S. Chan, V. Nene, S. J. Shallom, B. Suh, J. Peterson, S. Angiuoli, M. Pertea, J. Allen, J. Selengut, D. Haft, M. W. Mather, A. B. Vaidya, D. M. Martin, A. H. Fairlamb, M. J. Fraunholz, D. S. Roos, S. A. Ralph, G. I. McFadden, L. M. Cummings, G. M. Subramanian, C. Mungall, J. C. Venter, D. J. Carucci, S. L. Hoffman, C. Newbold, R. W. Davis, C. M. Fraser, and B. Barrell. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magistrado, P., A. Salanti, N. G. Tuikue Ndam, S. B. Mwakalinga, M. Resende, M. Dahlback, L. Hviid, J. Lusingu, T. G. Theander, and M. A. Nielsen. 2008. VAR2CSA expression on the surface of placenta-derived Plasmodium falciparum-infected erythrocytes. J. Infect. Dis. 1981071-1074. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen, M. A., M. Resende, M. Alifrangis, L. Turner, L. Hviid, T. G. Theander, and A. Salanti. 2007. Plasmodium falciparum: VAR2CSA expressed during pregnancy-associated malaria is partially resistant to proteolytic cleavage by trypsin. Exp. Parasitol. 1171-8. [DOI] [PubMed] [Google Scholar]
- 15.Oleinikov, A. V., S. E. Francis, J. R. Dorfman, E. Rossnagle, S. Balcaitis, T. Getz, M. Avril, S. Gose, J. D. Smith, M. Fried, and P. E. Duffy. 2008. VAR2CSA domains expressed in Escherichia coli induce cross-reactive antibodies to native protein. J. Infect. Dis. 1971119-1123. [DOI] [PubMed] [Google Scholar]
- 16.Paul, F., S. Roath, D. Melville, D. C. Warhurst, and J. O. Osisanya. 1981. Separation of malaria-infected erythrocytes from whole blood: use of a selective high-gradient magnetic separation technique. Lancet ii70-71. [DOI] [PubMed] [Google Scholar]
- 17.Resende, M., M. A. Nielsen, M. Dahlback, S. B. Ditlev, P. Andersen, A. F. Sander, N. T. Ndam, T. G. Theander, and A. Salanti. 2008. Identification of glycosaminoglycan binding regions in the Plasmodium falciparum encoded placental sequestration ligand, VAR2CSA. Malar. J. 7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogerson, S. J., L. Hviid, P. E. Duffy, R. F. Leke, and D. W. Taylor. 2007. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect. Dis. 7105-117. [DOI] [PubMed] [Google Scholar]
- 19.Salanti, A., M. Dahlback, L. Turner, M. A. Nielsen, L. Barfod, P. Magistrado, A. T. Jensen, T. Lavstsen, M. F. Ofori, K. Marsh, L. Hviid, and T. G. Theander. 2004. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 2001197-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salanti, A., T. Staalsoe, T. Lavstsen, A. T. R. Jensen, M. P. K. Sowa, D. E. Arnot, L. Hviid, and T. G. Theander. 2003. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 49179-191. [DOI] [PubMed] [Google Scholar]
- 21.Singh, S. K., R. Hora, H. Belrhali, C. E. Chitnis, and A. Sharma. 2006. Structural basis for Duffy recognition by the malaria parasite Duffy-binding-like domain. Nature 439741-744. [DOI] [PubMed] [Google Scholar]
- 22.Staalsoe, T., C. E. Shulman, J. N. Bulmer, K. Kawuondo, K. Marsh, and L. Hviid. 2004. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet 363283-289. [DOI] [PubMed] [Google Scholar]
- 23.Tolia, N. H., E. J. Enemark, B. K. Sim, and L. Joshua-Tor. 2005. Structural basis for the EBA-175 erythrocyte invasion pathway of the malaria parasite Plasmodium falciparum. Cell 122183-193. [DOI] [PubMed] [Google Scholar]
- 24.Tuikue Ndam, N. G., A. Salanti, G. Bertin, M. Dahlback, N. Fievet, L. Turner, A. Gaye, T. Theander, and P. Deloron. 2005. High level of var2csa transcription by Plasmodium falciparum isolated from the placenta. J. Infect. Dis. 192331-335. [DOI] [PubMed] [Google Scholar]
- 25.Viebig, N. K., B. Gamain, C. Scheidig, C. Lepolard, J. Przyborski, M. Lanzer, J. Gysin, and A. Scherf. 2005. A single member of the Plasmodium falciparum var multigene family determines cytoadhesion to the placental receptor chondroitin sulphate A. EMBO Rep. 6775-781. [DOI] [PMC free article] [PubMed] [Google Scholar]