Abstract
Yersinia pestis survives and replicates in phagosomes of murine macrophages. Previous studies demonstrated that Y. pestis-containing vacuoles (YCVs) acquire markers of late endosomes or lysosomes in naïve macrophages and that this bacterium can survive in macrophages activated with the cytokine gamma interferon. An autophagic process known as xenophagy, which destroys pathogens in acidic autophagolysosomes, can occur in naïve macrophages and is upregulated in activated macrophages. Studies were undertaken here to investigate the mechanism of Y. pestis survival in phagosomes of naïve and activated macrophages and to determine if the pathogen avoids or co-opts autophagy. Colocalization of the YCV with markers of autophagosomes or acidic lysosomes and the pH of the YCV were determined by microscopic imaging of infected macrophages. Some YCVs contained double membranes characteristic of autophagosomes, as determined by electron microscopy. Fluorescence microscopy showed that ∼40% of YCVs colocalized with green fluorescent protein (GFP)-LC3, a marker of autophagic membranes, and that YCVs failed to acidify below pH 7 in naïve macrophages. Replication of Y. pestis in naïve macrophages caused accumulation of LC3-II, as determined by immunoblotting. While activation of infected macrophages increased LC3-II accumulation, it decreased the percentage of GFP-LC3-positive YCVs (∼30%). A viable count assay showed that Y. pestis survived equally well in macrophages proficient for autophagy and macrophages rendered deficient for this process by Cre-mediated deletion of ATG5, revealing that this pathogen does not require autophagy for intracellular replication. We conclude that although YCVs can acquire an autophagic membrane and accumulate LC3-II, the pathogen avoids xenophagy by preventing vacuole acidification.
Yersinia pestis is a gram-negative bacterium and the cause of plague (32, 34). Zoonotic foci of plague exist in many parts of the world, including North America. Y. pestis infections are most commonly transmitted to humans by infected fleas and typically develop into bubonic plague or, less frequently, into septicemic plague (32). Plague infections in humans can also result from contact with body fluids or from inhalation of respiratory droplets from infected animals or humans. Inhalation of Y. pestis into the lungs can initiate primary pneumonic plague.
Y. pestis is able to survive and replicate in murine macrophages in vitro (5, 6, 18, 35, 36, 44) and in vivo (23) and is therefore classified as a facultative intracellular pathogen. Microscopic examination of tissues of animals experimentally infected with Y. pestis has shown the presence of plague bacilli inside macrophages (11, 23, 25, 50). More often, however, Y, pestis is detected as large numbers of extracellular bacteria in tissues (20, 39, 51). Y. pestis produces several antiphagocytic factors that are upregulated during growth at 37°C. These factors include several Yop proteins and the LcrV protein and their designated type III secretion system encoded on pCD1 (48). In addition, a capsule composed of the F1 protein is maximally expressed after extended growth at 37°C and promotes resistance to phagocytosis (8). When grown at ambient temperatures (e.g., 28°C), Y. pestis is efficiently phagocytosed by macrophages (5). It has been hypothesized that when Y. pestis growing at 28°C is introduced into a mammalian host, it initially survives and replicates within macrophages that internalize the bacteria (5). Subsequently, the bacteria escape or are released from dying macrophages and replicate in an extracellular niche (5).
After a macrophage engulfs a bacterium, the phagosome changes rapidly into a less habitable compartment, termed a phagolysosome, through a series of fusion events with endocytic compartments (49). Within 2 to 5 min after their formation, phagosomes transiently acquire characteristics of early endosomes (49). After 10 to 30 min phagosomes begin to fuse with late endosomes and lysosomes. Late endosomes and lysosomes can be characterized by the presence of components such as antimicrobial peptides, lysosome-associated membrane proteins (LAMPs), and lysosomal proteases (cathepsins B, D, and L). In contrast to late endosomes, only lysosomes and phagolysosomes contain significant amounts of mature proteases. In addition, the pH of lysosomes and phagolysosomes is significantly lower (∼pH 4.5) than the pH in late endosomes (pH 5.5 to 6). The decrease in pH is due to the action of the vacuolar proton ATPase (vATPase).
Early work on the trafficking of Y. pestis-containing vacuoles (YCVs) in primary murine macrophages yielded evidence that YCVs fuse with lysosomes (6, 45). It was therefore suggested that Y. pestis survives in a phagolysosome (45). More recent studies have confirmed that the YCV colocalizes with markers of late endosomes or lysosomes in murine J774A.1 macrophage-like cells (13). Results of experiments that utilized lysosomal tracers or antibodies to the LAMP1 or cathepsin D protein in conjunction with immunofluorescence and thin-section electron microscopy (EM) showed that the YCV colocalizes with these markers between 1.5 and 8 h postinfection (13). In addition, results of ultrastructural analysis by thin-section EM showed that the YCV has a spacious morphology beginning around 8 h postinfection, at which time bacterial replication begins (13). How Y. pestis controls phagosome trafficking and expansion of its vacuole in macrophages is not understood.
Activation by the cytokine gamma interferon (IFN-γ) is known to dramatically alter the trafficking and environment of phagosomes in macrophages (37, 41). Y. pestis is able to survive in primary murine macrophages that are activated with IFN-γ (36). A chromosomally encoded operon termed ripCBA that promotes survival of Y. pestis in activated macrophages has been identified. The ripCBA genes appear to encode novel metabolic enzymes (36). However, it is not known how the Rip proteins promote intracellular survival, and it is unclear if the morphology or trafficking of the YCV is modified in IFN-γ-activated macrophages.
Autophagy is a membrane trafficking process in eukaryotic cells that sequesters cytoplasmic material (e.g., defective mitochondria in the case of macroautophagy) in a vacuole (the autophagosome) and routes the cargo for destruction in an autophagolysosome (19, 21, 28). The autophagy pathway encompasses several different membrane compartments, beginning with the phagophore, which functions to sequester cytoplasmic material. Following trapping of cytoplasmic material by the phagophore, the autophagosome is formed, which matures through interactions with late endosomes and lysosomes into an autophagolysosome, where degradation of luminal components takes place. Autophagy is regulated at multiple steps by several signaling molecules, including the mTOR kinase and class I and class III phosphatidylinositol 3-kinases (19, 21, 28). Recent studies have revealed that autophagy can play an important role in the protective innate immune response to cytoplasmic or vacuolar bacterial pathogens (3, 21, 28). The process that results in breakdown of microorganisms within autophagosomes has been referred to as xenophagy (21). IFN-γ can upregulate autophagy in macrophages (21). In macrophages exposed to IFN-γ, upregulation of autophagy appears to override the phagosome maturation block imposed by Mycobacterium tuberculosis, allowing routing of the pathogen to a microbicidal autophagolysosome-like compartment (21, 28). Alternatively, some intracellular pathogens appear to co-opt autophagy for survival within host cells (26, 28).
In this work we examined the possibility that the replication of Y. pestis in macrophages exposed to IFN-γ resulted from an increase in autophagy and that the pathogen might exploit this process for survival within phagosomes. Our results show that, although the YCV can interact with an autophagy pathway in macrophages, this interaction does not appear to require activation by IFN-γ nor is it required for intracellular survival of Y. pestis. Instead, we find that Y. pestis prevents phagosome acidification, and it is suggested that this process allows Y. pestis to avoid destruction in phagolysosomes or autophagolysosomes of either naïve or activated macrophages.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The Y. pestis strains used (Table 1) were derived from Y. pestis KIM (molecular group 2.MED) (1). Ampicillin resistance (Apr), chloramphenicol resistance (Cmr), and kanamycin resistance (aph-3′ or Knr) cassettes were introduced into Y. pestis strains in compliance with CDC guidelines. Yersinia pseudotuberculosis strain 32777 (previously referred to as strain IP2777) is a serogroup I isolate. Y. pestis strains were routinely grown on heart infusion agar plates or in heart infusion broth at 28°C. Y. pseudotuberculosis was grown in Luria-Bertani broth or on Luria-Bertani agar plates at 28°C. Y. pestis was killed by fixation for 30 min with 2.5% paraformaldehyde, followed by extensive washing with phosphate-buffered saline (PBS).
TABLE 1.
Strains used in this study
| Strain | Relevant characteristics | Reference |
|---|---|---|
| KIM6+ | pCD1− pPCP1+, pgm+ | 12 |
| KIM6+/GFP | pCD1−, pPCP1+, pgm+, pMMB207gfp3.1, Cmr | 36 |
| KIM6+/mCherry | pCD1−, pPCP1+, pgm+, p207mCherry, Cmr | This study |
| KIM6+phoP | pCD1−, pPCP1+, pgm+ ΔphoP | 13 |
| KIM6+ripCBA | pCD1−, pPCP1+, pgm+ ΔripCBA | This study |
| KIM6 | pCD1−, pPCP1+, Δpgm | 22 |
| KIM5+ | pCD1Ap, pPCP1+, pgm+, Apr | This study |
| KIM5+/GFP | pCD1Ap, pPCP1+, pgm+, p207gfp3.1::kan, Knr Apr | This study |
| KIM5 | pCD1Ap, pPCP1+, Δpgm, Apr | 22 |
| KIM5/GFP | pCD1Ap, pPCP1+, Δpgm, pMMB207gfp3.1, Apr Cmr | 13 |
| KIM10 | pCD1−, pPCP1−, Δpgm | 33 |
| 32777 | pYV+ | 42 |
Strain construction.
KIM6+ was converted to the fully virulent background by introducing pCD1 marked with Apr (pCD1Ap) (12) via electroporation as described previously (13). The resulting strain was designated KIM5+ and is handled according to select agent guidelines and using biosafety level 3 conditions. Screening for spontaneous loss of the pgm locus from KIM6+ (13) resulted in KIM6. Introduction of pCD1Ap into KIM6 resulted in KIM5, a strain that is conditionally virulent and exempt from select agent guidelines. Plasmid pMMB207gfp3.1 (Cmr), used to express green fluorescent protein (GFP), was introduced into KIM5 or KIM6+ by conjugation (13). A plasmid expressing mCherry under control of the tac promoter was generated using pRSET-mCherry (40). The mCherry gene was amplified from pRSET-mCherry by PCR using oligonucleotides mCherry-EcoRI-F1 (5′-CCGGAATTCATGGTGAGCAAGGGCGAG-3′) and mCherry-HindIII-R1 (5′-CCCAAGCTTTTACTTGTACAGCTCGTCCATGCC-3′). The mCherry coding region was inserted into pMMB207 (27a) downstream of the tac promoter and between the EcoRI and HindIII sites. The resulting plasmid, p207mCherry, was introduced into KIM6+ by conjugation. GFP or mCherry expression in Y. pestis was obtained by incubation in the presence of 500 μM isopropyl-β-d-thiogalactopyranoside (IPTG) for 1 to 2 h.
To construct a Knr plasmid expressing inducible GFP, pMMB207gfp3.1 was modified to inactivate the Cmr gene and to introduce a Knr cassette (aph-3′). To this end, the Knr cassette was isolated from pBSL86 (2) by restriction digestion with SmaI and inserted into the SnaBI site of pMMB207gfp3.1, resulting in inactivation of the Cmr cassette. The resulting plasmid, p207gfp3.1::kan, was conjugated into KIM5+, resulting in KIM5+/GFP.
Deletion of the ripCBA operon in KIM6+ was performed by allelic recombination. To this end, the ripCBA operon and flanking sequence were obtained by PCR amplification using the KIM6+ chromosome as the template and primers Y2382-F2-PvuII (5′-CAGCTGTTGACAACATCCAGAAATGCAATACCT-3′) and Y2385-R5-XmaI (5′-TCCCCCCGGGTCAGATTAGATGCATTTTTTTGGC-3′). The amplified DNA fragment was cloned into the pGEM-T Easy vector (Promega). The resulting plasmid was digested with AfeI, which recognizes and cleaves sites within ripC and ripA. Upon ligation of the ends of the plasmid, a deletion was obtained that removed the 3′ end of ripC, all of the ripB gene, and the 5′ end of ripA. A DNA fragment with the deletion (ΔripCBA) was liberated from pGEM-T Easy and introduced into the suicide vector pSB890 using NotI restriction sites (30). The resulting plasmid, pSB890ΔripCBA, was conjugated into KIM6+, and allelic exchange was performed as described previously (14), resulting in KIM6+ripCBA.
Cell culture.
Bone marrow-derived macrophages (BMDMs) were isolated and cultured as described previously (35, 36). BMDMs were prepared from femurs of C57BL/6 mice (Jackson Laboratory), ATG5flox/flox-Lyz-Cre mice, or control ATG5flox/flox mice (52). ATG5flox/flox mice were backcrossed five generations to a C57BL/6 line prior to use. 293T cells were cultivated at 37°C in the presence of 5% CO2 in Dulbecco modified Eagle medium (DMEM) with Glutamax-I (Invitrogen) supplemented with 1 mM sodium pyruvate (Invitrogen) and 10% fetal bovine serum (FBS) (HyClone). J774A.1 murine macrophage-like cells were cultured as described previously (13).
Assays for determination of bacterial survival in macrophages.
The procedures used for infecting macrophages with Y. pestis or Y. pseudotuberculosis and for measuring intracellular survival by a CFU or GFP induction assay have been described previously (13, 35, 36).
EM.
BMDMs (1.5 × 105 cells) seeded on plastic coverslips (diameter, 12 mm; Thermanox) were infected with KIM5+ at a multiplicity of infection (MOI) of 10 for 30 min in 200 μl of infection medium. Activation of BMDMs with IFN-γ was performed as described previously (36). At the appropriate time, the infected cells were fixed for 1 h with a mixture of 2% paraformaldehyde and 2.5% glutaraldehyde in 100 mM cacodylate buffer (pH 7.5) supplemented with 2.5 mM CaCl2. Fixative buffer baths were renewed after 30 min of incubation. Fixed samples were washed three times for 5 min with 100 mM cacodylate buffer (pH 7.5) and processed by the Central Microscopy Image Center at Stony Brook University. Digital images of thin sections were acquired with an FEI BioTwinG2 transmission electron microscope.
Detection of LC3 by immunoblotting.
BMDMs (6.5 × 105 cells) seeded in six-well plates were infected with Y. pestis strains as previously described (35), with the following modifications. An MOI of 10 to 15 was used, and BMDMs were infected for 30 min in a small volume (1 ml) of infection medium. BMDMs were activated by adding 150 U/ml IFN-γ to the infected wells as previously described (36). Twenty-four hours postinfection, the infected cells were washed three times with PBS and lysed with 100 μl RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 50 mM Tris [pH 8.0], 0.1% sodium dodecyl sulfate [SDS]) containing protease inhibitors (Complete Mini, EDTA-free; Roche). Cell lysate from each well was collected, passed through a 22-gauge needle, and centrifuged for 10 min at 16,000 × g at 4°C. Supernatants were collected, protein concentrations were determined (bincinchoninic acid or Bradford protein assay), and 25 μg of protein from each sample was separated by SDS-polyacrylamide gel electrophoresis (15% polyacrylamide) and transferred onto a 0.2-μm polyvinylidene difluoride membrane for 2 h at 100 V using a MiniBiorad electrophoresis apparatus. The blots were probed overnight using a polyclonal anti-LC3 antibody (catalog no. NB100-2331; Novus) diluted 1:300 in Tris-buffered saline-0.05% Tween containing 5% dry skim milk. To control for loading, blots were probed with a polyclonal anti-actin antibody (catalog no. 4967; Cell Signaling). Secondary rabbit immunoglobulin G antibody conjugated to horseradish peroxidase (catalog no. 111-035-144; Jackson Immunoresearch) was used at a dilution of 1:5,000. Immunoblots were developed using an enhanced chemiluminescent reagent (Perkin-Elmer Life Science) and Kodak X-MAT film. Band intensities for LC3-II, LC3-I, and β-actin were quantified using QuantityOne software (Bio-Rad), and ratios of LC3-II to LC3-I or LC3-II to actin were calculated.
Transfection of 293T cells and production of retroviral particles.
293T packaging cells (4 × 106 cells) were seeded in 100-mm cell culture-treated dishes 16 h before transfection. Six micrograms of pCL-Eco (Imgenex) and 6 μg of pJC110 expressing GFP-LC3 (a gift from Jean Celli) (7) were added to the cell culture medium using the HEPES-buffered saline calcium phosphate method (31). Seven hours later, the supernatant of transfected 293T cells was replaced with fresh cell culture medium and the preparation was incubated overnight. The supernatant containing retroviral particles was collected, centrifuged for 10 min at 834 × g, and passed through a 0.45-μm filter. A second supernatant was obtained after 8 h of incubation with fresh cell culture medium and processed as described above.
Retroviral transduction of BMDMs.
BMDMs seeded on coverslips in 24-well plates at a concentration of 6 × 104 cells/well and grown for 2 days were transduced with a 2:1 mixture of retrovirus-containing supernatant (first collection) and BMDM culture medium (DMEM with Glutamax-I supplemented with 20% FBS, 1 mM sodium pyruvate, and 45% L-cell supernatant). After 8 h, the supernatant was replaced with fresh BMDM culture medium containing the second retroviral harvest (ratio, 2:1). Twenty-four hours posttransduction, the supernatant of transduced BMDMs was removed and replaced with fresh BMDM infection medium (DMEM with Glutamax-I supplemented with 10% FBS, 15% L-cell-conditioned supernatant, and 1 mM sodium pyruvate), and the preparation was incubated for a minimum of 30 min before infection.
Determination of colocalization of GFP-LC3 with YCVs by fluorescence microscopy.
BMDMs were infected with KIM6+/mCherry and treated or not treated with IFN-γ as described previously (36). IPTG was added to the cell culture media 2 h before the end of a time point to induce mCherry expression. At the time indicated below, the infected cells were fixed with 2.5% paraformaldehyde and processed for immunofluorescence labeling (35). Bacteria were stained with anti-Yersinia primary antibody (35) and a goat anti-rabbit secondary antibody conjugated to Alexa Fluor 633 (Molecular Probes) for confocal microscopy analysis or to Alexa Fluor 594 (Molecular Probes) for epifluorescence analysis. GFP-LC3 was immunolabeled using an anti-GFP monoclonal antibody diluted 1:250 (catalog no. A11120; Invitrogen) and a goat anti-mouse antibody conjugated to Alexa Fluor 488 to enhance detection. Confocal images were acquired using a Zeiss inverted Axiovert 200 M microscope. GFP-LC3-positive YCVs were quantified by epifluorescence microscopy using a Zeiss Axioplan2 microscope equipped with a ×40 objective. Images were captured using a Spot camera (Diagnostic Instruments, Inc.) and assembled in Adobe Photoshop. GFP-LC3-positive YCVs were enumerated using merged images of green fluorescence (combined GFP and Alexa Fluor 488) and red fluorescence (combined mCherry and Alexa Fluor 594), and the results were expressed as a percentage obtained by dividing the number of YCVs positive for LC3-GFP by the total number of YCVs. Approximately 300 YCVs were enumerated for each condition in a single experiment.
Phagosomal pH assays.
Determination of Lysotracker Red DND-99 colocalization with YCVs containing Y. pestis was performed using J774A.1 macrophage-like cells as described previously for Texas Red ovalbumin (13), except that Lysotracker was added at a concentration of 50 nM 1 h prior to fixation and analysis of samples. In some experiments macrophages were incubated in medium containing 30 μg/ml chloramphenicol to inhibit bacterial protein synthesis during infection. Coinfection experiments were performed as follows. KIM5/GFP bacteria were induced with IPTG (500 μM) to express GFP and then fixed. Macrophages were infected at an MOI of 5 with fixed KIM5/GFP and unlabeled live KIM5 (total MOI, 10). At 1.25 h postinfection cells were fixed, permeabilized with saponin, and stained with anti-Yersinia primary antibody (1:1,000) and Alexa Fluor 350 (Molecular Probes) secondary antibody (1:500). Macrophages were labeled with Lysotracker as described above.
The YCV pH was measured by ratiometic imaging as described previously (43), with the following modifications. Y. pestis was labeled with fluorescein isothiocyanate (FITC) at a final concentration of 1 mg/ml in PBS (pH 8) for 30 min. After three washes in PBS, the bacteria were used to infect J774A.1 cells seeded in 35-mm glass coverslip dishes as described previously (13). A Zeiss LSM 510 META microscope was used for live cell analysis. Images were obtained using excitation wavelengths of 488 nm and 458 nm. The pH of YCVs was extrapolated from a calibration curve (pH 4 to 7) generated using 10 μM monensin and 10 μM nigericin (43). Signal intensities of at least 50 phagosomes were determined in three different fields per experiment.
RESULTS
Fully virulent Y. pestis replicates in activated macrophages.
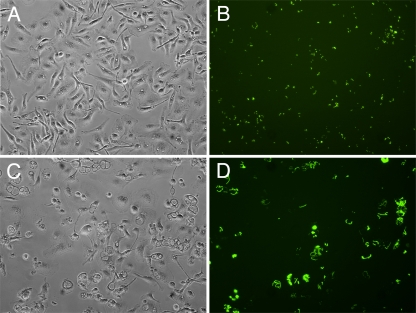
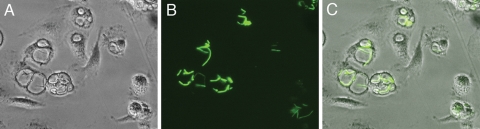
We previously demonstrated that Y. pestis cured of pCD1 (KIM6+) (Table 1) replicates in murine BMDMs activated with IFN-γ (36). To determine if fully virulent, pCD1-containing Y. pestis can survive in activated BMDMs, a GFP induction assay was performed (35). KIM5+/GFP (Table 1) pregrown at 28°C was used to infect BMDMs, and survival of extracellular bacteria was prevented by use of gentamicin (see Materials and Methods). At different time points postinfection de novo synthesis of GFP was induced by addition of IPTG, and live cells were examined using phase-contrast and fluorescence microscopy. As shown in Fig. 1, KIM5+/GFP replicated in activated in BMDMs between 6 and 24 h postinfection. At a higher magnification, GFP-positive bacteria were clearly visible within spacious YCVs of activated BMDMs at 24 h postinfection (Fig. 2), with elongated bacteria typically apposed to the phagosomal membrane.
FIG. 1.
Analysis of Y. pestis replication in activated macrophages by microscopy. BMDMs infected with KIM5+/GFP were activated with IFN-γ and incubated for 6 h (A and B) or 24 h (C and D) before microscopic examination. One hour before examination, IPTG was added to induce de novo expression of GFP in viable intracellular bacteria. (A and C) Representative phase-contrast microscopy images. (B and D) Representative fluorescence microscopy images.
FIG. 2.
Examination of Y. pestis-containing spacious phagosomes in activated macrophages by microscopy. (A and B) Enlargements of regions of panels C and D of Fig. 1, respectively. (C) Overlay of panels A and B.
Ultrastructural analysis of YCV morphology in activated macrophages.
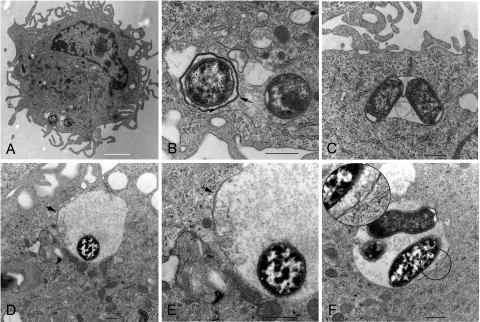
We next looked for evidence that YCVs interact with an autophagy pathway in activated macrophages. Evidence for the interaction of intracellular bacteria with an autophagy pathway can be obtained by analysis of phagosome morphology at the ultrastructural level (19). Nascent autophagosomes typically have a double membrane. Fusion of lysosomes with an autophagosome results in maturation of the vacuole to an autophagolysosome, which has a single delimiting membrane. Therefore, the presence of a double membrane surrounding an intracellular bacterium can be taken as evidence that the pathogen was residing in a nascent autophagosome-like vacuole at the time of cell fixation. Thin-section EM was used to examine the possibility that Y. pestis resides in autophagosome-like vacuoles in activated macrophages. BMDMs were infected with KIM5+ (Table 1) pregrown at 28°C and then exposed to IFN-γ. Analysis of the infected macrophages at 4 h postinfection by thin-section EM (see Materials and Methods) showed that some intracellular Y. pestis bacteria were present within a tight-fitting double membrane (Fig. 3A and B). However, more commonly, a tight-fitting single membrane surrounded intracellular Y. pestis and other vesicular material (Fig. 3C). At a later time point (22 h postinfection) Y. pestis bacteria were found to be present in spacious phagosomes with partial double membranes (Fig. 3D and E and Fig. 3F, inset). These results indicated that, in activated macrophages, Y. pestis resided in autophagosome-like vacuoles, as well as in vacuoles with morphologies similar to those of standard phagosomes or multivesicular bodies.
FIG. 3.
YCV morphology in activated macrophages as determined by thin-section EM. BMDMs were infected with KIM5+ and then treated with IFN-γ. At 4 h (A to C) or 22 h (D to F) the cells were fixed and processed for thin-section EM. (A) BMDM infected with KIM5+ at 4 h postinfection. (B) Magnification of area containing bacteria in panel A. The arrow indicates a double membrane surrounding a single bacterium. (C) BMDM infected with KIM5+ at 4 h postinfection, showing two bacteria and other vesicles in a single-membrane compartment. (D) BMDM infected with KIM5+ for 22 h. The arrow indicates a region with a double membrane. (E) Magnification of a region in panel D. (F) BMDM infected with KIM5+ for 22 h. A region containing a double membrane is magnified in the inset. (A) Bar = 2 μm. (B to F) Bars = 500 nm.
IFN-γ and replication of Y. pestis increase LC3-II levels in macrophages.
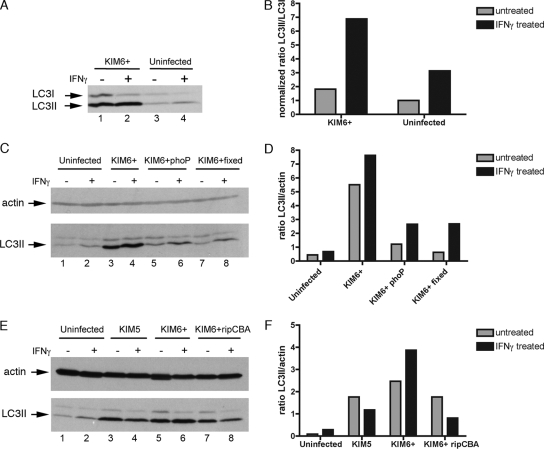
The progress of autophagy is executed by ATG proteins, which comprise two protein conjugation systems. The ATG8 protein (more commonly known as LC3-I in its unconjugated form) becomes conjugated to phosphatidylethanolamine during autophagy, resulting in the formation of LC3-II, which specifically associates with autophagic membranes (19). The conversion of LC3-I to LC3-II and the turnover or degradation of LC3-II within autophagolysosomes can be followed by immunoblotting, and this procedure has become a useful tool for measuring rates of autophagy within eukaryotic cells (19, 27). The steady-state level of LC3-II, as detected by immunoblotting of cell lysates, was used to determine if autophagy was altered in macrophages after 24 h of treatment with IFN-γ and/or infection by Y. pestis KIM6+. The LC3-II signal obtained by immunoblotting was normalized by using two different approaches (19), calculating the ratio of LC3-II to LC3-I and calculating the ratio of LC3-II to a loading control (actin). Treatment of uninfected BMDMs with IFN-γ resulted in an increased ratio of LC3-II to LC3-I compared to that for untreated macrophages (Fig. 4A, compare lanes 3 and 4; Fig. 4B). Interestingly, after infection of BMDMs with Y. pestis KIM6+ in the absence of IFN-γ, there were large increases in the steady-state levels of both LC3-I and LC3-II (Fig. 4A, compare lanes 1 and 3) and there was a slight increase in the LC3-II/LC3-I ratio compared to the data for uninfected macrophages (Fig. 4B). Infection of BMDMs with KIM6+ in the presence of IFN-γ resulted in a further increase in the LC3-II/LC3-I ratio (Fig. 4A, lane 2; Fig. 4B). A time course analysis indicated that the increased LC3-II level was observed beginning at 3 h postinfection (data not shown). To determine if the increase in the LC3-II level required survival of Y. pestis in macrophages, BMDMs were infected with a KIM6+ phoP mutant (Table 1), which is defective for intracellular survival, or with killed formaldehyde-fixed KIM6+. Compared to the data for macrophages infected with live KIM6+, the LC3-II/actin ratios were lower after challenge with the phoP mutant or fixed KIM6+ (Fig. 4C and D). Moreover, the LC3-II/actin ratio was higher in BMDMs infected with a Y. pestis strain containing pCD1 but lacking the pgm locus (KIM5) (Table 1) than in uninfected BMDMs (Fig. 4E and F), although in this case the ratios were higher in nonactivated macrophages than in IFN-γ-treated BMDMs (Fig. 4E and F). We have observed that KIM5 does not replicate in activated BMDMs as well as KIM6+ replicates, in part because it lacks the ripCBA operon in the pgm locus (36). A KIM6+ ripCBA mutant (Table 1) had a phenotype similar to that of KIM5 with respect to LC3-II levels (Fig. 4E and F). These results indicated that the levels of LC3-II in macrophages could be increased by treatment with IFN-γ or by infection with replicating Y. pestis.
FIG. 4.
Immunoblot analysis of LC3 levels in BMDMs exposed to IFN-γ and infected with Y. pestis. BMDMs were not infected or were infected with KIM6+ in the presence or absence of IFN-γ. At 24 h postinfection cell lysates were prepared and subjected to SDS-polyacrylamide gel electrophoresis, followed by immunoblotting with anti-LC3 antibody (A, C, and E) and anti-actin antibody (C and E). For panels C and E, macrophages were infected with the KIM6+ phoP mutant, with KIM6+ fixed with paraformaldehyde, with KIM5, or with the KIM6+ ripCBA mutant. Densitometry was used to quantify band signals for LC3-I and LC3-II (A) or for LC3-II and actin (C and E), and calculated ratios are shown in panels B, D, and F, respectively. For panel B the ratio of LC3-II to LC3-I in uninfected and untreated cells was defined as 1, and other ratios were normalized to this value.
Analysis of GFP-LC3 colocalization with YCVs in macrophages.
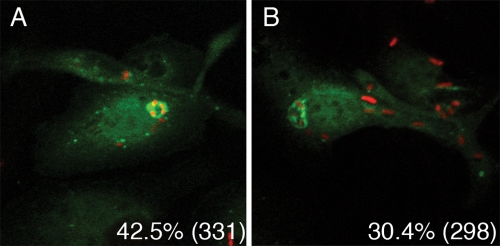
A GFP-LC3 fusion protein can be used as a specific cytological marker for autophagic membranes in eukaryotic cells (19). Fluorescence microscopy was used to quantify the GFP-LC3 in YCVs in macrophages exposed or not exposed to IFN-γ. For this purpose, BMDMs were transduced with a retrovirus producing GFP-LC3 (see Materials and Methods). One day after transduction, BMDMs were infected with KIM6+/mCherry (Table 1) in the presence or absence of IFN-γ. IPTG was added to the infected wells for 2 h to induce de novo mCherry expression in viable intracellular Y. pestis. Eight hours postinfection, the infected wells were fixed and the samples were processed for immunofluorescence microscopy (see Materials and Methods). The colocalization of GFP-LC3 and bacterial signals was determined by confocal fluorescence microscopy. As shown in Fig. 5B, GFP-LC3 could be detected on YCVs in macrophages that were treated with IFN-γ, confirming that an autophagic membrane can be recruited to phagosomes containing viable Y. pestis, as suggested by transmission EM results (Fig. 3B). However, quantification by epifluorescence microscopy showed that only 30.4% of YCVs in activated macrophages were positive for GFP-LC3 (Fig. 5B). In addition, a slightly higher percentage of YCVs colocalized with GFP-LC3 in naïve macrophages (42.5%) (Fig. 5A). Thus, although Y. pestis infection modulated autophagy, based on increased steady-state levels of LC3-II (Fig. 4), the majority of YCVs did not colocalize with the GFP-LC3 marker of autophagic membranes in BMDMs. It was anticipated that the percentage of GFP-LC3-positive YCVs would be highest in activated macrophages, conditions under which maximal LC3-II levels were seen (Fig. 4), but this was not the case (see Discussion).
FIG. 5.
Analysis of GFP-LC3 colocalization with YCVs. BMDMs were transduced with retrovirus producing GFP-LC3. Twenty-four hours posttransduction, the macrophages were infected with KIM6+/mCherry. De novo expression of mCherry was induced in viable intracellular bacteria by addition of IPTG. After 8 h of infection with Y. pestis in the absence (A) or presence (B) of IFN-γ, cells were fixed and processed for examination by fluorescence microscopy. Images were obtained by using confocal fluorescence microscopy. The panels show overlays of GFP-LC3 (green) and mCherry (red) signals from representative images. The percentages of colocalization of signals as determined by epifluorescence microscopy are shown for each condition, and the numbers of phagosomes scored are indicated in parentheses.
Y. pestis inhibits phagosome acidification.
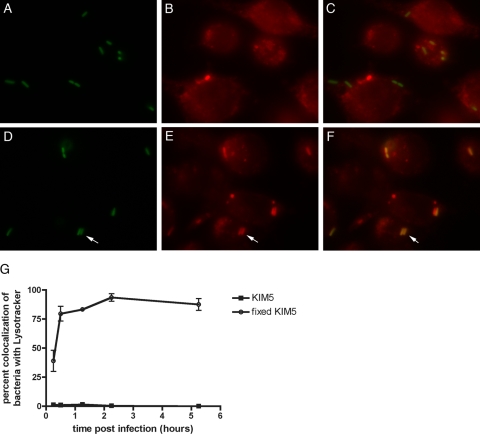
The results described above suggested that the localization of an autophagic membrane to YCVs and the increase in LC3-II levels during infection may not be critical for intracellular survival, but rather a side effect or downstream consequence of the strategy that Y. pestis uses to survive in macrophages. Turnover of LC3-II trapped in autophagosomes is mediated by lysosomal proteases which are activated in autophagolysosomes. Preventing lysosomal degradation by use of protease inhibitors or drugs (such as bafilomycin A1) that inhibit vacuole acidification leads to an increase in the steady-state level of LC3-II (19). Y. pseudotuberculosis, which is closely related to Y. pestis (1), has been shown to inhibit acidification of phagosomes in primary murine macrophages (46). To determine if Y. pestis prevents acidification of phagosomes, colocalization of YCVs with a probe for acidic lysosomes (Lysotracker DND-99) in macrophages was assayed by fluorescence microscopy. When naïve J774A.1 murine macrophage-like cells were infected with KIM5/GFP (Table 1) in the presence of Lysotracker, very few GFP-positive YCVs colocalized with Lysotracker up to 5 h postinfection (Fig. 6A to C and G). In contrast, phagosomes containing killed fixed KIM5/GFP showed increased colocalization with Lysotracker with time, and the value reached 90% by 2.5 h postinfection (Fig. 6D to F and G). This result suggested that live Y. pestis could prevent acidification of the YCVs to values below the value required for detection of Lysotracker (pH ≤5.5).
FIG. 6.
Measurement of YCV acidification using Lysotracker and fluorescence microscopy. J774A.1 macrophages on coverslips were infected with live KIM5/GFP (A to C) or fixed KIM5/GFP (D to F) in the presence of Lysotracker Red DND-99. At 1.25 h postinfection the samples were fixed, and representative images were obtained by using a fluorescence microscope equipped with a digital camera. The images show the GFP signal (A and D), the Lysotracker signal (B and E), or an overlay of the two signals (C and F). (G) Percent colocalization of GFP and Lysotracker signals at the indicated time points, as quantified using three independent experiments. The symbols indicate averages, and the error bars indicate standard deviations.
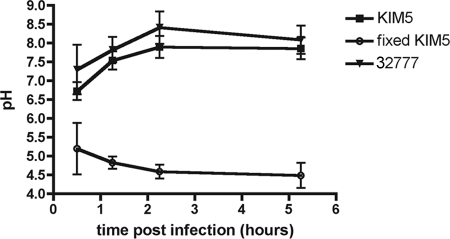
We next used live cell ratiometric imaging and Y. pestis labeled with the pH-sensitive dye FITC to measure the pH of the YCV over a time course (43). As shown in Fig. 7, the pH of phagosomes containing live KIM5 was ∼6.5 at 30 min postinfection. Over the next several hours the pH of the YCV was determined to be ∼8, confirming the finding of the Lysotracker assay that Y. pestis inhibits phagosome acidification. Similar results were obtained for Y. pseudotuberculosis 32777 (Table 1) (Fig. 7), confirming that this property is shared by the two Yersinia species. In contrast, the pH of YCVs containing fixed KIM5 decreased to ∼4.5 within 2.5 h (Fig. 7). These results showed that Y. pestis inhibits acidification of its phagosomes, regardless of whether it acquires an autophagic membrane, and prevents maturation of these vacuoles. Thus, in the YCVs that incorporate an autophagic membrane, the normal turnover of LC3-II may be prevented, resulting in accumulation of this autophagy marker.
FIG. 7.
Measurement of YCV pH using live cell ratiometric imaging. Live KIM5 cells, fixed KIM5 cells, or live 32777 cells labeled with FITC were used to infect J774A.1 macrophages. At the indicated time points live cell imaging was performed using a confocal microscope. Images were obtained by using excitation wavelengths of 488 nm and 458 nm. A calibration curve was generated using the ionophores monensin and nigericin and media buffered at pHs between pH 4 and 7. Comparison of the 488 nm/458 nm ratio to the pH calibration curve was used to estimate the pH of YCVs. The symbols indicate the averages of three experiments (for 32777) or of five experiments (for KIM5 and fixed KIM5), and the error bars indicate standard deviations.
Autophagy is dispensable for survival of Y. pestis in macrophages.
Based on the results described above (Fig. 5 to 7), we predicted that autophagy is dispensable for survival of Y. pestis in macrophages, and to test this hypothesis, we utilized BMDMs deficient for this process. In many studies, 3-methyladenine, an inhibitor of class I and class III phosphatidylinositol 3-kinases, is used to pharmacologically block autophagy in cells. In preliminary control experiments we found that growth of Y. pestis in bacterial culture media was inhibited in the presence of 5 mM 3-methyladenine, a concentration that is typically used to block autophagy in cells. We therefore sought an alternative approach to block autophagy that did not involve the potential nonspecific effects of chemical inhibitors. The ATG5 protein is essential for autophagy, as it is required for the formation of the phagophore (19, 21). Mice lacking ATG5 in myelomonocytic cells have been generated by breeding ATG5flox/flox mice with mice in which Cre recombinase is expressed from the lysozyme M locus (52). BMDMs derived from the ATG5flox/flox-Lyz-Cre mice are defective for autophagy and have been used previously to determine the role of autophagy in coronavirus replication in host cells (52).
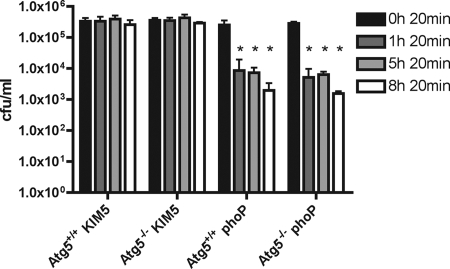
BMDMs prepared from ATG5flox/flox-Lyz-Cre mice or control ATG5flox/flox mice were infected with Y. pestis. Immunoblotting for LC3-I and LC3-II confirmed that the ATG5flox/flox-Lyz-Cre BMDMs were defective for autophagy (data not shown) (52). The BMDMs were infected with KIM5 or the KIM5 phoP mutant, and a CFU assay (see Materials and Methods) was performed at different time points after infection. As shown in Fig. 8, KIM5 survived equally well in BMDMs from ATG5flox/flox-Lyz-Cre mice and BMDMs from control ATG5flox/flox mice. The phoP mutant showed a similar survival defect in both types of macrophages (Fig. 8). Therefore, although YCVs can interact with an autophagy pathway in macrophages, recruitment of an autophagic membrane does not appear to be required for survival of Y. pestis in phagosomes. Instead, a primary mechanism of Y. pestis survival in macrophages seems to be inhibition of YCV acidification.
FIG. 8.
CFU assay of Y. pestis survival in ATG5flox/flox-Lyz-Cre BMDMs or control ATG5flox/flox BMDMs. BMDMs prepared from ATG5flox/flox-Lyz-Cre mice (Atg5−/−) or control ATG5flox/flox mice (Atg5+/+) were infected with KIM5 or the KIM5 phoP mutant at an MOI of 10. After 20 min of infection the macrophages were washed to remove nonadherent bacteria and then incubated in medium containing gentamicin to kill extracellular bacteria. At the indicated time points washed macrophages were lysed in detergent, and serial dilutions were plated to enumerate CFU. The data are values from three independent experiments, and the error bars indicate standard deviations. An asterisk indicates a significant difference (P < 0.01) compared to the data for the 20-min time point for the same group, as determined by one-way analysis of variance with Dunnett's posttest.
Inhibition of phagosome acidification by Y. pestis does not require de novo bacterial protein synthesis.
To determine if de novo bacterial protein synthesis was required for Y. pestis to inhibit phagosome acidification, J774A.1 macrophages were infected in the presence of the bacteriostatic antibiotic chloramphenicol at a final concentration of 30 μg/ml. The results of a Lysotracker assay performed between 1.5 and 5 h postinfection showed that there was no difference in the ability of KIM5/GFP to inhibit phagosome acidification in the presence and in the absence of chloramphenicol (data not shown). To determine if chloramphenicol was effectively inhibiting bacterial protein synthesis under the conditions used for the infection assay, macrophages were infected with KIM5/GFP that had not been preinduced to express GFP. GFP expression was not upregulated in bacteria in chloramphenicol-treated macrophages following addition of IPTG, showing that the antibiotic effectively inhibited de novo protein synthesis. The results of a coinfection experiment with live and fixed KIM5 showed that live bacteria were unable to prevent colocalization of Lysotracker with phagosomes containing fixed bacteria when they were in the same macrophage (data not shown). Taken together, these results suggest that Y. pestis is able to inhibit phagosome acidification using a factor(s) that is produced by the pathogen prior to phagocytosis and that this factor does not act globally within the macrophage to inhibit phagosome acidification.
Inhibition of phagosome acidification by Y. pestis does not require known virulence factors.
KIM10, a Y. pestis strain lacking pCD1, pPCP1, and the pgm locus (Table 1), was tested for the ability to prevent phagosome acidification in J774A.1 cells. The results of a Lysotracker assay with KIM10 showed that genes in pCD1, pPCP1, or the pgm locus were not required to prevent phagosome acidification (data not shown). In addition, although a Y. pestis phoP mutant is defective for intracellular survival, phagosomes containing the KIM6+ phoP mutant did not colocalize with Lysotracker for the limited period during which the bacteria remained intact within macrophages, suggesting that the factor(s) required to inhibit phagosome acidification is not under control of the PhoP regulon.
DISCUSSION
Several species of pathogenic bacteria subvert the function of macrophages in order to survive and replicate within these cells. Examples include Francisella, Brucella, Legionella, Listeria, Mycobacterium, and Salmonella species. These bacteria escape from the phagosome and replicate in the macrophage cytosol or modify the phagosome to reach a replicative niche. Pathogenic bacteria that survive within macrophage phagosomes utilize several different strategies to avoid being killed in a vacuole that could potentially mature into a phagolysosome (9, 24). M. tuberculosis survives within macrophages by stalling maturation of its vacuole at an early stage. The mycobacterial phagosome is characterized by the absence of a number of markers that are usually associated with late endosomes (37, 47). For example, Mycobacterium inhibits acidification of its vacuole by preventing the accumulation of vATPase. Mature lysosomal proteases are also excluded from the mycobacterial phagosome.
We have shown that the YCV has an unusual trafficking pattern as it can acquire markers of late endosomes (LAMP1 and cathepsin D) (13) and autophagosomes (GFP-LC3) (Fig. 5), yet it does not undergo acidification (Fig. 6 and 7). It is thought that acidification is required for efficient phagosome-lysosome or autophagosome-lysosome fusion (17, 28, 49). We hypothesize that the YCV fuses with several vesicular compartments, including late endosomes, multivesicular bodies, and autophagosomes, but fusion with lysosomes and thus full maturation of the YCV are inefficient due to its lack of acidification. The observation that Y. pestis infection results in increased steady-state levels of LC3-II (Fig. 4) is consistent with this model, since LC3-II turnover would be prevented in nonacidified autophagosomes. We previously showed that Y. pestis could replicate in BMDMs exposed to IFN-γ (36). IFN-γ treatment is known to stimulate autophagy, and some pathogens appear to hijack the autophagy pathway for intracellular replication (26, 28). However, in some cases IFN-γ-induced autophagy is protective, leading to efficient killing (i.e., xenophagy) of intracellular bacteria, such as M. tuberculosis. Our results suggest that Y. pestis has a strategy to avoid the protective role of xenophagy in either naïve or activated phagocytes and that the increase in the steady-state levels of LC3-II in Y. pestis-infected macrophages is an effect, rather than a cause, of YCV formation.
Although results described here indicate that autophagy is not required for survival of Y. pestis in macrophage phagosomes (Fig. 8), it is possible that recruitment of an autophagic membrane to the YCV has important consequences for the outcome of the pathogen-host interaction. Autophagosomes may provide a source of membrane, along with late endosomes, for expansion of the YCV into a spacious compartment. Alternatively, recruitment of an autophagic membrane to the YCV may prevent use of the autophagy pathway for its normal functions in macrophages, such as clearing damaged mitochondria or survival during starvation or growth factor withdrawal. There is evidence that death of macrophages infected with Salmonella enterica serovar Typhimurium can occur by autophagy (16). We suggest that recruitment of an autophaghic membrane to the YCV and prevention of LC3-II turnover allow intracellular Y. pestis to slowly compromise the viability of the macrophage. This could provide a mechanism for release of the bacteria from the dying host cell once an intracellular replication cycle has been completed. In this context, we observed that treatment with IFN-γ decreased recruitment of GFP-LC3 to the YCV (Fig. 5), which may represent a protective response on the part of the macrophage to prevent sequestration of autophagic membrane by the pathogen.
The ability to prevent phagosome acidification appears to be an attribute common to several pathogenic bacteria (17). As discussed above for M. tuberculosis, the arrest in maturation of its phagosome precludes acquisition of a sufficient density of vATPase, and the failure to acidify is most likely a consequence of the block in membrane trafficking (17). It is currently unclear how Y. pestis prevents phagosome acidification. It is possible that, like M. tuberculosis, Y. pestis prevents association of the vATPase with the YCV. Alternatively, the vATPase may associate with the YCV but is inactivated, as has been suggested for Y. pseudotuberculosis (46). The use of anti-vATPase antibodies in conjunction with microscopy techniques should allow us to distinguish between these possibilities.
Recently, Listeria monocytogenes has been shown to be capable of replicating slowly in macrophage phagosomes that expand and do not acidify (4). The pore-forming hemolysin listeriolysin O is required for this process, and it was suggested that pore formation by listeriolysin O allows dissipation of the pH gradient (4). Y. pestis is not known to secrete a pore-forming hemolysin, and analysis of the sequenced genome does not reveal predicted proteins with significant homology to pore-forming hemolysins (unpublished observations). The putative factor that Y. pestis secretes to inhibit phagosome acidification appears to be produced by the bacterium prior to macrophage interaction, and it appears to act on or within the phagosome in a local manner. There is precedence for the production of vATPase-inhibiting molecules by microorganisms (e.g., bafilomycin A1), but to our knowledge there is no known bacterial factor that can mediate this activity directly. It is not inconceivable that a bacterial protein secreted in a phagosome could inhibit the vATPase. The V0 domain of the vATPase is exposed on the luminal side of the vacuole, and there is previously obtained evidence that a monoclonal antibody that is specific for the a subunit of the V0 domain can inhibit vATPase activity when this antibody resides in the endosomal compartment (38).
Straley and Harmon showed that genes in pCD1, pPCP1, and the pgm locus were not required for replication of Y. pestis in murine macrophages (44). Using a Lysotracker assay, we found that these genetic elements are also not required for inhibition of phagosome acidification by Y. pestis. The phoP gene is important for survival of Y. pestis in macrophages (29). PhoP-regulated genes important for survival of S. enterica serovar Typhimurium in the phagosomes of macrophages include genes of the pmr operon. The products of the pmr operon function to modify lipid A with aminoarabinose; this increases bacterial resistance to antimicrobial peptides (10, 15). Genes under control of PhoP in Y. pestis that are important for survival in macrophages include ugd, pmrK, and mgtC, which are predicted to promote resistance to antimicrobial peptides (ugd and pmrK) or low-Mg2+ conditions (mgtC) of a phagosome (13). Phagosomes containing Y. pestis phoP mutants do not colocalize with Lysotracker for the period of time that the bacteria remain intact within macrophages. This result indicates that inhibition of phagosome acidification is not sufficient for intracellular survival of Y. pestis; resistance to antimicrobial peptides and resistance to environmental stress encountered within the YCV are also important for replication of the pathogen in macrophages. Further understanding of the mechanism by which Y. pestis inhibits phagosome acidification requires identification of genes essential for this process.
Acknowledgments
We thank John Brumell and Joel Swanson for advice concerning experiments, Wei-Xing Zong, Erica Ullman, and Zhixun Dou for help with the LC3 immunoblotting procedures, Susan VanHorn and Guo-Wei Tian for their assistance with transmission EM and confocal microscopy, Sylvia Samaniego for advice concerning the protocol for retroviral transduction, and Jean Celli for providing the GFP-LC3 retroviral construct. We thank Jens Grabenstein for making the p207gfp3.1::kan plasmid and KIM5+ and James Murtha for technical assistance with construction of the Y. pestis ripCBA mutant. Hana Fukuto is thanked for providing helpful comments to improve the manuscript.
Herbert W. Virgin IV is gratefully acknowledged for advice and financial support (to Z.Z.). This work was supported by NIH grant P01 AI055621 (to J.B.B.) and NIH grant U54 AI057160 Project 6 (to H.W.V.).
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 16 March 2009.
REFERENCES
- 1.Achtman, M., G. Morelli, P. Zhu, T. Wirth, I. Diehl, B. Kusecek, A. J. Vogler, D. M. Wagner, C. J. Allender, W. R. Easterday, V. Chenal-Francisque, P. Worsham, N. R. Thomson, J. Parkhill, L. E. Lindler, E. Carniel, and P. Keim. 2004. Microevolution and history of the plague bacillus, Yersinia pestis. Proc. Natl. Acad. Sci. USA 10117837-17842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexeyev, M. F. 1995. Three kanamycin resistance gene cassettes with different polylinkers. BioTechniques 1852, 54, 56. [PubMed] [Google Scholar]
- 3.Amano, A., I. Nakagawa, and T. Yoshimori. 2006. Autophagy in innate immunity against intracellular bacteria. J. Biochem. 140161-166. [DOI] [PubMed] [Google Scholar]
- 4.Birmingham, C. L., V. Canadien, N. A. Kaniuk, B. E. Steinberg, D. E. Higgins, and J. H. Brumell. 2008. Listeriolysin O allows Listeria monocytogenes replication in macrophage vacuoles. Nature 451350-354. [DOI] [PubMed] [Google Scholar]
- 5.Cavanaugh, D. C., and R. Randall. 1959. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of fleaborne plague. J. Immunol. 85348-363. [PubMed] [Google Scholar]
- 6.Charnetzky, W. T., and W. W. Shuford. 1985. Survival and growth of Yersinia pestis within macrophages and an effect of the loss of the 47-megadalton plasmid on growth in macrophages. Infect. Immun. 47234-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Checroun, C., T. D. Wehrly, E. R. Fischer, S. F. Hayes, and J. Celli. 2006. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc. Natl. Acad. Sci. USA 10314578-14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du, Y., R. Rosqvist, and A. Forsberg. 2002. Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infect. Immun. 701453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duclos, S., and M. Desjardins. 2000. Subversion of a young phagosome: the survival strategies of intracellular pathogens. Cell. Microbiol. 2365-377. [DOI] [PubMed] [Google Scholar]
- 10.Ernst, R. K., T. Guina, and S. I. Miller. 1999. How intracellular bacteria survive: surface modifications that promote resistance to host innate immune responses. J. Infect. Dis. 179S326-S330. [DOI] [PubMed] [Google Scholar]
- 11.Finegold, M. J. 1969. Pneumonic plague in monkeys. An electron microscopic study. Am. J. Pathol. 54167-185. [PMC free article] [PubMed] [Google Scholar]
- 12.Gong, S., S. W. Bearden, V. A. Geoffroy, J. D. Fetherston, and R. D. Perry. 2001. Characterization of the Yersinia pestis Yfu ABC inorganic iron transport system. Infect. Immun. 692829-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grabenstein, J. P., H. S. Fukuto, L. E. Palmer, and J. B. Bliska. 2006. Characterization of phagosome trafficking and identification of PhoP-regulated genes important for survival of Yersinia pestis in macrophages. Infect. Immun. 743727-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grabenstein, J. P., M. Marceau, C. Pujol, M. Simonet, and J. B. Bliska. 2004. The response regulator PhoP of Yersinia pseudotuberculosis is important for replication in macrophages and for virulence. Infect. Immun. 724973-4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groisman, E. A. 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 1831835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez, L. D., M. Pypaert, R. A. Flavell, and J. E. Galan. 2003. A Salmonella protein causes macrophage cell death by inducing autophagy. J. Cell Biol. 1631123-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huynh, K. K., and S. Grinstein. 2007. Regulation of vacuolar pH and its modulation by some microbial species. Microbiol. Mol. Biol. Rev. 71452-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen, W. A., and M. J. Surgalla. 1969. Plague bacillus: survival within host phagocytes. Science 163950-952. [DOI] [PubMed] [Google Scholar]
- 19.Klionsky, D. J., H. Abeliovich, P. Agostinis, D. K. Agrawal, G. Aliev, D. S. Askew, M. Baba, E. H. Baehrecke, B. A. Bahr, A. Ballabio, B. A. Bamber, D. C. Bassham, E. Bergamini, X. Bi, M. Biard-Piechaczyk, J. S. Blum, D. E. Bredesen, J. L. Brodsky, J. H. Brumell, U. T. Brunk, W. Bursch, N. Camougrand, E. Cebollero, F. Cecconi, Y. Chen, L. S. Chin, A. Choi, C. T. Chu, J. Chung, P. G. Clarke, R. S. Clark, S. G. Clarke, C. Clave, J. L. Cleveland, P. Codogno, M. I. Colombo, A. Coto-Montes, J. M. Cregg, A. M. Cuervo, J. Debnath, F. Demarchi, P. B. Dennis, P. A. Dennis, V. Deretic, R. J. Devenish, F. Di Sano, J. F. Dice, M. Difiglia, S. Dinesh-Kumar, C. W. Distelhorst, M. Djavaheri-Mergny, F. C. Dorsey, W. Droge, M. Dron, W. A. Dunn, Jr., M. Duszenko, N. T. Eissa, Z. Elazar, A. Esclatine, E. L. Eskelinen, L. Fesus, K. D. Finley, J. M. Fuentes, J. Fueyo, K. Fujisaki, B. Galliot, F. B. Gao, D. A. Gewirtz, S. B. Gibson, A. Gohla, A. L. Goldberg, R. Gonzalez, C. Gonzalez-Estevez, S. Gorski, R. A. Gottlieb, D. Haussinger, Y. W. He, K. Heidenreich, J. A. Hill, M. Hoyer-Hansen, X. Hu, W. P. Huang, A. Iwasaki, M. Jaattela, W. T. Jackson, X. Jiang, S. Jin, T. Johansen, J. U. Jung, M. Kadowaki, C. Kang, A. Kelekar, D. H. Kessel, J. A. Kiel, H. P. Kim, A. Kimchi, T. J. Kinsella, K. Kiselyov, K. Kitamoto, E. Knecht, M. Komatsu, E. Kominami, S. Kondo, A. L. Kovacs, G. Kroemer, C. Y. Kuan, R. Kumar, M. Kundu, J. Landry, M. Laporte, W. Le, H. Y. Lei, M. J. Lenardo, B. Levine, A. Lieberman, K. L. Lim, F. C. Lin, W. Liou, L. F. Liu, G. Lopez-Berestein, C. Lopez-Otin, B. Lu, K. F. Macleod, W. Malorni, W. Martinet, K. Matsuoka, J. Mautner, A. J. Meijer, A. Melendez, P. Michels, G. Miotto, W. P. Mistiaen, N. Mizushima, B. Mograbi, I. Monastyrska, M. N. Moore, P. I. Moreira, Y. Moriyasu, T. Motyl, C. Munz, L. O. Murphy, N. I. Naqvi, T. P. Neufeld, I. Nishino, R. A. Nixon, T. Noda, B. Nurnberg, M. Ogawa, N. L. Oleinick, L. J. Olsen, B. Ozpolat, S. Paglin, G. E. Palmer, I. Papassideri, M. Parkes, D. H. Perlmutter, G. Perry, M. Piacentini, R. Pinkas-Kramarski, M. Prescott, T. Proikas-Cezanne, N. Raben, A. Rami, F. Reggiori, B. Rohrer, D. C. Rubinsztein, K. M. Ryan, J. Sadoshima, H. Sakagami, Y. Sakai, M. Sandri, C. Sasakawa, M. Sass, C. Schneider, P. O. Seglen, O. Seleverstov, J. Settleman, J. J. Shacka, I. M. Shapiro, A. Sibirny, E. C. Silva-Zacarin, H. U. Simon, C. Simone, A. Simonsen, M. A. Smith, K. Spanel-Borowski, V. Srinivas, M. Steeves, H. Stenmark, P. E. Stromhaug, C. S. Subauste, S. Sugimoto, D. Sulzer, T. Suzuki, M. S. Swanson, I. Tabas, F. Takeshita, N. J. Talbot, Z. Talloczy, K. Tanaka, I. Tanida, G. S. Taylor, J. P. Taylor, A. Terman, G. Tettamanti, C. B. Thompson, M. Thumm, A. M. Tolkovsky, S. A. Tooze, R. Truant, L. V. Tumanovska, Y. Uchiyama, T. Ueno, N. L. Uzcategui, I. van der Klei, E. C. Vaquero, T. Vellai, M. W. Vogel, H. G. Wang, P. Webster, J. W. Wiley, Z. Xi, G. Xiao, J. Yahalom, J. M. Yang, G. Yap, X. M. Yin, T. Yoshimori, L. Yu, Z. Yue, M. Yuzaki, O. Zabirnyk, X. Zheng, X. Zhu, and R. L. Deter. 2008. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4151-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lathem, W. W., S. D. Crosby, V. L. Miller, and W. E. Goldman. 2005. Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proc. Natl. Acad. Sci. USA 10217786-17791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine, B., and V. Deretic. 2007. Unveiling the roles of autophagy in innate and adaptive immunity. Nat. Rev. Immunol. 7767-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lilo, S., Y. Zheng, and J. B. Bliska. 2008. Caspase-1 activation in macrophages infected with Yersinia pestis KIM requires the type III secretion system effector YopJ. Infect. Immun. 763911-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukaszewski, R. A., D. J. Kenny, R. Taylor, D. G. Rees, M. G. Hartley, and P. C. Oyston. 2005. Pathogenesis of Yersinia pestis infection in BALB/c mice: effects on host macrophages and neutrophils. Infect. Immun. 737142-7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meresse, S., O. Steele-Mortimer, E. Moreno, M. Desjardins, B. Finlay, and J. P. Gorvel. 1999. Controlling the maturation of pathogen-containing vacuoles: a matter of life and death. Nat. Cell Biol. 1E183-E188. [DOI] [PubMed] [Google Scholar]
- 25.Meyer, K. F. 1950. Immunity in plague; a critical consideration of some recent studies. J. Immunol. 64139-163. [PubMed] [Google Scholar]
- 26.Mizushima, N., B. Levine, A. M. Cuervo, and D. J. Klionsky. 2008. Autophagy fights disease through cellular self-digestion. Nature 4511069-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizushima, N., and T. Yoshimori. 2007. How to interpret LC3 immunoblotting. Autophagy 3542-545. [DOI] [PubMed] [Google Scholar]
- 27a.Morales, V. M., A. Bäckman, and M. Bugdasarian. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 9739-47. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa, M., and C. Sasakawa. 2006. Bacterial evasion of the autophagic defense system. Curr. Opin. Microbiol. 962-68. [DOI] [PubMed] [Google Scholar]
- 29.Oyston, P. C. F., N. Dorrell, K. Williams, S.-R. Li, M. Green, R. W. Titball, and B. Wren. 2000. The response regulator PhoP is important for survival under conditions of macrophage-induced stress and virulence in Yersinia pestis. Infect. Immun. 683419-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer, L. E., S. Hobbie, J. E. Galan, and J. B. Bliska. 1998. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNFα production and the downregulation of the MAP kinases p38 and JNK. Mol. Microbiol. 27953-965. [DOI] [PubMed] [Google Scholar]
- 31.Pear, W. S., A. M. Scott, and G. P. Nolan (ed.). 1997. Generation of high titer, helper-free retroviruses by transient transfection. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 32.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis—etiologic agent of plague. Clin. Microbiol. Rev. 1035-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry, R. D., M. L. Pendrak, and P. Schuetze. 1990. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J. Bacteriol. 1725929-5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prentice, M. B., and L. Rahalison. 2007. Plague. Lancet 3691196-1207. [DOI] [PubMed] [Google Scholar]
- 35.Pujol, C., and J. B. Bliska. 2003. The ability to replicate in macrophages is conserved between Yersinia pestis and Yersinia pseudotuberculosis. Infect. Immun. 715892-5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pujol, C., J. P. Grabenstein, R. D. Perry, and J. B. Bliska. 2005. Replication of Yersinia pestis in interferon gamma-activated macrophages requires ripA, a gene encoded in the pigmentation locus. Proc. Natl. Acad. Sci. USA 10212909-12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rohde, K., R. M. Yates, G. E. Purdy, and D. G. Russell. 2007. Mycobacterium tuberculosis and the environment within the phagosome. Immunol. Rev. 21937-54. [DOI] [PubMed] [Google Scholar]
- 38.Sato, S. B., and S. Toyama. 1994. Interference with the endosomal acidification by a monoclonal antibody directed toward the 116 (100)-kD subunit of the vacuolar type proton pump. J. Cell Biol. 12739-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sebbane, F., D. Gardner, D. Long, B. B. Gowen, and B. J. Hinnebusch. 2005. Kinetics of disease progression and host response in a rat model of bubonic plague. Am. J. Pathol. 1661427-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaner, N. C., R. E. Campbell, P. A. Steinbach, B. N. Giepmans, A. E. Palmer, and R. Y. Tsien. 2004. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 221567-1572. [DOI] [PubMed] [Google Scholar]
- 41.Shenoy, A. R., B. H. Kim, H. P. Choi, T. Matsuzawa, S. Tiwari, and J. D. MacMicking. 2007. Emerging themes in IFN-gamma-induced macrophage immunity by the p47 and p65 GTPase families. Immunobiology 212771-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simonet, M., and S. Falkow. 1992. Invasin expression in Yersinia pseudotuberculosis. Infect. Immun. 604414-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinberg, T. H., and J. A. Swanson. 1994. Measurement of phagosome-lysosome fusion and phagosomal pH. Methods Enzymol. 236147-160. [DOI] [PubMed] [Google Scholar]
- 44.Straley, S. C., and P. A. Harmon. 1984. Growth in mouse peritoneal macrophages of Yersinia pestis lacking established virulence determinants. Infect. Immun. 45649-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Straley, S. C., and P. A. Harmon. 1984. Yersinia pestis grows within phagolysosomes in mouse peritoneal macrophages. Infect. Immun. 45655-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsukano, H., F. Kura, S. Inoue, S. Sato, H. Izumiya, T. Yasuda, and H. Watanabe. 1999. Yersinia pseudotuberculosis blocks the phagosomal acidification of B10.A mouse macrophages through the inhibition of vacuolar H+-ATPase activity. Microb. Pathog. 27253-263. [DOI] [PubMed] [Google Scholar]
- 47.Vergne, I., J. Chua, S. B. Singh, and V. Deretic. 2004. Cell biology of Mycobacterium tuberculosis phagosome. Annu. Rev. Cell Dev. Biol. 20367-394. [DOI] [PubMed] [Google Scholar]
- 48.Viboud, G. I., and J. B. Bliska. 2005. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 5969-89. [DOI] [PubMed] [Google Scholar]
- 49.Vieira, O. V., R. J. Botelho, and S. Grinstein. 2002. Phagosome maturation: aging gracefully. Biochem. J. 366689-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Welkos, S., M. L. M. Pitt, M. Martinez, A. Friedlander, P. Vogel, and R. Tammariello. 2002. Determination of the virulence of the pigmentation-deficient and pigmentation-/plasminogen activator-deficient strains of Yersinia pestis in non-human primate and mouse models of pneumonic plague. Vaccine 202206-2214. [DOI] [PubMed] [Google Scholar]
- 51.Welkos, S. L., A. M. Friedlander, and K. J. Davis. 1997. Studies on the role of plasminogen activator in systemic infection by virulent Yersinia pestis strain C092. Microb. Pathog. 23211-223. [DOI] [PubMed] [Google Scholar]
- 52.Zhao, Z., L. B. Thackray, B. C. Miller, T. M. Lynn, M. M. Becker, E. Ward, N. N. Mizushima, M. R. Denison, and H. W. T. Virgin. 2007. Coronavirus replication does not require the autophagy gene ATG5. Autophagy 3581-585. [DOI] [PubMed] [Google Scholar]