Abstract
Parasitic worms and molecules derived from them have powerful anti-inflammatory properties and are shown to have therapeutic effects on inflammatory diseases. The helminth Fasciola hepatica has been reported to suppress antigen-specific Th1 responses in concurrent bacterial infections, thus demonstrating its anti-inflammatory ability in vivo. Here, F. hepatica tegumental antigen (Teg) was shown to significantly suppress serum levels of gamma interferon (IFN-γ) and interleukin-12p70 (IL-12p70) in a model of septic shock. Since dendritic cells (DCs) are a good source of IL-12p70 and critical in driving adaptive immunity, we investigated the effects of F. hepatica Teg on the activation and function of murine DCs. While Teg alone did not induce cytokine production or cell surface marker expression on DCs, it significantly suppressed cytokine production (IL-12p70, IL-6, IL-10, tumor necrosis factor alpha, and nitrite) and cell surface marker expression (CD80, CD86, and CD40) in DCs matured with a range of Toll-like receptor (TLR) and non-TLR ligands. Teg works independently of the TLR4 pathway, since it still functioned in DCs generated from TLR4 mutant and knockout mice. It impaired DC function by inhibiting their phagocytic capacity and their ability to prime T cells. It does not appear to target the common components (extracellular signal-regulated kinase, Jun N-terminal protein kinase, or p38) of the TLR pathways; however, it suppressed the active p65 subunit of the transcription factor NF-κB in mature DCs, which could explain the impairment of proinflammatory cytokine production. Overall, our results demonstrate the potent anti-inflammatory properties of F. hepatica Teg and its therapeutic potential as an anti-inflammatory agent.
Dendritic cells (DCs) are highly specialized antigen-presenting cells, important in stimulating the primary immune response and driving inflammatory responses associated with immunopathology. Immature DCs reside in peripheral tissues where they are well situated to recognize foreign pathogens through Toll-like receptors (TLRs)—pattern recognition receptors which directly recognize conserved microbial molecules known as pathogen-associated molecular patterns (30). To date, 13 known mammalian TLRs have been characterized, 10 in humans and 13 in mice, with Toll-like receptor 1 (TLR1) to TLR9 conserved in humans and mice (27). Each receptor can recognize a panel of endogenous and exogenous stimuli that initiates the “classical” maturation of DCs. Lipopolysaccharide (LPS), which binds and signals through TLR4, is the most characterized TLR ligand and has shaped much of our knowledge of DC biology. LPS activation of TLR4 triggers a signaling cascade including mitogen-activated protein kinases (MAPKs) and NF-κB, culminating in the production of proinflammatory cytokines and the differentiation of Th1 cells (27). The activation of these signaling cascades is common to most TLR pathways. This activation process is also accompanied by enhanced expression of costimulatory molecules, such as CD40, CD80, and CD86, as well as major histocompatibility complex (MHC) class II (4). TLR activation is associated with inflammatory disorders, such as inflammatory bowel disease and septic shock (1); thus, mechanisms of suppressing TLR maturation of DCs may lead to the development of potential therapeutics (11, 31).
Parasitic worms (helminths) classically induce highly polarized Th2 and T-regulatory immune responses, correlating with chronic infection but with little associated immune pathology (35). Controlling immune pathology, in particular inflammatory responses, prolongs the worm's survival within the host and thus increases its chances of completing the life cycle. In contrast to bacteria and viruses, little is known about how helminths induce DC maturation; however, studies demonstrate that they differ predominantly by their DC activation status which lacks many of the classical maturation markers, such as costimulatory molecules (CD80, CD86, MHC class II [MHC-II], and CD40) and proinflammatory cytokines (interleukin-12p70 [IL-12p70] and tumor necrosis factor alpha [TNF-α]) (33). Although helminth-matured DCs are classed as being less phenotypically mature, they are still capable of priming naïve T cells to activate Th2/tolerogenic T cells associated with helminth infection (28, 52).
Helminths have also evolved means of evading or suppressing inflammatory responses (17, 34), rendering a host more susceptible to secondary bystander infections, such as malaria, tuberculosis, and human immunodeficiency virus infection, that require Th1 immunity for protection (6, 14, 40). In line with this, helminth antigens have been shown to suppress DC maturation and function when stimulated with TLR ligands. For example, the filarial nematode-secreted product, ES-62, has been shown to significantly suppress LPS-induced production of IL-12p40 and IL-12p70 from DCs and IL-12p40, IL-12p70, TNF-α, and IL-6 production from macrophages (20). Similarly, Schistosoma mansoni egg antigen significantly suppressed TLR ligand-induced production of IL-12p40 and IL-12p70 by DCs in vitro, while simultaneously enhancing LPS-induced IL-10 production (9, 29). With such potent suppression of proinflammatory responses by DCs, helminth antigens have been hypothesized to antagonize the Th1 immunity required to control intracellular pathogens (5), as well as ameliorate certain proinflammatory autoimmune diseases (15, 16, 32, 49).
Fasciola hepatica (liver fluke) is a parasitic helminth and the causative agent of fasciolosis, an emerging important disease in humans (36). F. hepatica induces a dominant Th2/T-regulatory type immune response (10, 18) and suppresses Th1 responses to bystander infections, such as Bordetella pertussis and Mycobacterium tuberculosis during coinfection (7, 19, 44). Fasciola-derived antigens have been shown to mimic this effect by suppressing antigen-specific Th1 immune responses both in vitro and in vivo (45). They also modulate the functions of innate immune cells, inducing alternative activation of macrophages which are thought to inhibit the production of gamma interferon (IFN-γ) by anti-CD3-stimulated naïve CD4+ T cells (12). However, the effect of Fasciola antigen on DC function has yet to be determined. In this study, we have isolated the tegumental coat from F. hepatica and are the first to investigate the effects of F. hepatica tegumental antigen on DC maturation and function.
MATERIALS AND METHODS
Animals and antigens.
C57BL/6j and BALB/c mice, 6 to 8 weeks old, were purchased from Harlan UK Ltd. (Bicester, United Kingdom) and DO11.10 TCR transgenic mice (on a BALB/c background) were either a kind gift from Padraic Fallon (Trinity College Dublin, Ireland) or purchased from Jackson Laboratory (Bar Harbor, ME). All mice were maintained according to the Irish Department of Health guidelines. C3H/HeJ and C3H/HeN bone marrow cells were a gift from Christine Loscher (Dublin City University, Ireland) and TLR4−/− (on a C57BL/6j background) bone marrow cells were a gift from Padraic Fallon. F. hepatica tegumental antigen (Teg) was prepared by adapting a previously published method (26). In brief, F. hepatica adult worms were washed in sterile phosphate-buffered saline (PBS) and incubated in 1% Nonidet P-40 (NP-40 [Sigma Aldrich] in PBS) for 30 min, and supernatant was collected. NP-40 was removed using Extracti-Gel D detergent-removing gel (Pierce), and the remaining supernatant was centrifuged at 14,000 × g for 30 min at 4°C prior to being filtered/concentrated using compressed air, and then stored at −20°C. Protein concentrations were measured using the bicinchoninic acid protein assay kit (Pierce), and endotoxin levels were assessed using the Pyrogene endotoxin detection system (Cambrex) which utilizes recombinant factor C, an endotoxin-sensitive protein. F. hepatica Teg gave endotoxin levels similar to background levels and to complete RPMI 1640 medium (supplemented with 5% heat-inactivated fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 50 μM 2-mercaptoethanol), so the medium was taken to be endotoxin-free.
Septic shock.
Groups of four C57BL/6j mice were injected intraperitoneally (i.p.) with F. hepatica Teg (15 μg for each mouse), either 2.5 h before or after i.p. injection with LPS (Escherichia coli 0111:B4, Alexis; 1 μg for each mouse). Control mice received PBS, F. hepatica Teg, or LPS only (i.p.). Mice were sacrificed by cervical dislocation 6 h later, and blood samples were taken by cardiac puncture. Serum concentrations of IFN-γ, IL-12p40, and IL-12p70 were measured by enzyme-linked immunosorbent assays (ELISAs) (BD OptEIA ELISA sets; BD Biosciences).
Isolation and maturation of bone marrow-derived DCs.
Bone marrow-derived immature DCs were prepared by culturing bone marrow cells, isolated from the femurs and tibia of mice, in complete RPMI 1640 medium with recombinant mouse granulocyte-macrophage colony-stimulating factor (GM-CSF) (20 ng/ml; R&D Systems Ltd., United Kingdom) at 37°C. On days 3 and 6 of culture, fresh medium with GM-CSF (20 ng/ml) was added to the cells. On day 8, cells were harvested, counted, and used for experimental assays.
DCs were seeded into 24-well plates (Nunc) at 106/ml in complete RPMI 1640 medium plus 5 ng/ml GM-CSF. The cells were treated with F. hepatica Teg (15 μg/ml) for 2.5 h prior to stimulation with LPS (Escherichia coli 0111:B4; Alexis and Sigma Aldrich; 100 ng/ml), zymosan A (Saccharomyces cerevisiae; 5 μg/ml), peptidoglycan (PGN) (Staphylococcus aureus peptidoglycan; 5 μg/ml), poly(I:C) (polyinosinic acid·polycytidylic acid, a synthetic analogue of double-stranded RNA; 2.5 μg/ml), flagellin (Salmonella enterica serovar Typhimurium; 0.5 μg/ml), CpG (stimulatory oligonucleotide 1826 [ODN1826]; 5 μg/ml) (all from InvivoGen). or phorbol myristate acetate (PMA; Sigma Aldrich; 50 ng/ml) for 18 h. In time course experiments, DCs were treated with F. hepatica Teg 2.5 h before, at the same time as, or 2.5 h after LPS stimulation. Control cells were treated with medium, Teg, or TLR ligand alone. In the MAPK inhibitor studies, cells were incubated with extracellular signal-regulated kinase (ERK) (U0126; Sigma Aldrich), p38 (SB 202190; Sigma Aldrich), or Jun N-terminal protein kinase (JNK) inhibitors [JNK inhibitor I (L)-form; Calbiochem] (all 5 μM) 1 hour prior to TLR ligand stimulation. Control cells were treated with medium, Teg, or LPS alone and had inhibitors added.
Characterization of matured DCs.
Expression of cell surface markers on DCs was quantified by three-color flow cytometry using phycoerythrin-, fluorescein isothiocyanate (FITC)-, or phycoerythrin-Cy5.5-conjugated antibodies specific for CD80, CD86, CD40, MHC-II (BD Biosciences), and CD11c (Caltag Laboratories). Appropriately labeled isotype-matched antibodies were used as controls. DC purity was >90% positive for expression of CD11c. Acquisition was performed using a FACSCalibur flow cytometer (BD Biosciences), and analysis of results was performed using FlowJo software (Tree Star). Supernatants from cultured DCs were tested for the production of IL-12p70, IL-10, TNF-α, IL-12p40, IL-6 (BD OptEIA ELISA sets; BD Biosciences), and IL-23 (eBioscience ELISA set) by ELISAs, and nitrite was measured using Griess reagent (Promega, Madison, WI).
RNA extraction and RT-PCR.
RNA was recovered from cultured cells by the direct addition of Tri-reagent (Sigma Aldrich) to the wells following aspiration of supernatants. Total cellular RNA was subsequently extracted from the preparation according to the manufacturers' specifications. For reverse transcription-PCR (RT-PCR), first-strand cDNA was produced with oligo(dT) primers from 1 μg total RNA using avian myeloblastosis virus reverse transcriptase (reverse transcription system; Promega) at 42°C for 15 min, followed by 95°C for 5 min and 4°C for 5 min. A 1-μl aliquot of the resultant cDNA was amplified using primers specific for IL-12p35, IL-12p40, and β-actin (42) under the following conditions: 35 cycles, with 1 cycle consisting of 45 s of denaturation at 95°C, 45 s of annealing of primers at 56°C (β-actin and p40) or 51°C (p35), and 60 s of elongation at 72°C; followed by a step of 10 min at 72°C. All PCR products were electrophoresed on 1% agarose gel and visualized by ethidium bromide staining.
Cell viability and apoptosis detection.
Cell viability and growth during experimental conditions was first assessed by seeding DCs into 96-well plates (5 × 104 cells/ml and 1 × 106 cells/ml, respectively) and treating the cells with F. hepatica Teg (15 μg/ml) for 2.5 h prior to stimulation with LPS for 18 h. Control cells were treated with medium, Teg, or LPS alone. Following incubation, cell viability was assessed by adding 40 μl MTS solution [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS), plus phenazine ethosulfate] to each well. Following an incubation of 3 h at 37°C, the absorbance of each well was read at 450 nm. Cell growth was assessed by adding a volume of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] solution equivalent to 10% of culture medium to each well and incubating for 3 h at 37°C. The culture medium was then removed, the resulting MTT formazan crystals were dissolved by adding MTT solvent (equal to the original culture volume), and absorbance was read at 562 nm.
Cell viability was also measured using the annexin V-FITC apoptosis detection kit I (BD Biosciences). Briefly, 1 × 106 cells/ml were seeded into a 24-well plate and treated with F. hepatica Teg (15 μg/ml) and each TLR ligand, either separately or together, or medium alone. Cells were harvested 24 h later, washed twice in cold PBS and incubated in binding buffer (0.1 M HEPES-NaOH, 1.4 M NaCl, 25 mM CaCl2) with annexin V-FITC and propidium iodide for 15 min. Following the addition of more binding buffer, cells were immediately analyzed by flow cytometry.
TLR screening.
F. hepatica Teg-induced stimulation of TLR2 to TLR9 was tested in HEK-293 cells expressing a given TLR protein as well as a reporter gene driven by the NF-κB promoter (InvivoGen). Each 293-TLR cell line was induced with a known specific ligand as a positive control, and a recombinant HEK-293 cell line for the reporter gene only was used as a negative control.
Protein extraction and Western blot analysis.
Total protein was extracted from cell lysates using radioimmunoprecipitation assay (RIPA) buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1.0% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and protease and phosphatase inhibitor cocktails (Sigma Aldrich). Cells were incubated in the extraction buffer on ice for 5 min before being centrifuged at 8,000 × g for 10 min at 4°C. Supernatants were transferred to clean tubes, and protein concentrations were determined using the bicinchoninic acid protein assay kit (Pierce). Protein samples (10 μg) and prestained protein markers (Precision Plus protein standards; Bio-Rad) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto 0.45-μm Immobilon-P polyvinylidene difluoride membrane (Sigma Aldrich). Membranes were blocked for 1 h at room temperature in 5% nonfat dried milk in PBS and incubated overnight at 4°C with anti-NF-κBp65 (Cell Signaling Technology; 1:1,000). Membranes were washed in PBS with 0.05% Tween 20 and incubated for 1 h at room temperature with peroxide-conjugated anti-rabbit immunoglobulin G (IgG) (Sigma Aldrich; 1:2,000). After further washing, proteins were visualized with supersignal (Pierce), exposed to film for 10 to 30 min, and processed using an FPM 100A processor (Fujifilm). Protein bands were quantified using the GeneSnap acquisition and GeneTools analysis software (GeneGenius gel documentation and analysis system; Syngene).
Phagocytosis assay.
The phagocytic ability of DCs was measured using the CytoSelect 96-well phagocytosis assay (Cell Biolabs Inc.). Briefly, DCs from C57BL/6 mice were plated at 0.5 × 106/ml in complete RPMI 1640 medium and incubated overnight at 37°C to allow the cells to adhere to the plate. DCs were then treated with F. hepatica Teg (15 μg/ml), LPS (100 ng/ml), or zymosan A (5 μg/ml) alone or with Teg and LPS or Teg and zymosan at the same time for 2.5 h before the addition of sheep erythrocytes opsonized by IgG at a ratio of 50:1. Supernatants were aspirated after 1 h, and adherent cells were washed with sterile PBS to remove nonphagocytosed erythrocytes. Adherent DCs were then lysed, substrate solution was added, and the amount of engulfed erythrocytes was determined by colorimetric assay using an absorbance of 610 nm. Negative-control cells were treated with 2 μM cytochalasin D to block phagocytosis.
In vivo T-cell assays, cytokine measurements, and proliferation.
For assays of in vivo T-cell priming, DCs isolated from DO11.10 mice were stimulated with medium or F. hepatica Teg (15 μg/ml) in the presence of ovalbumin (OVA) peptide (323-ISQAVHAAHAEINEAGR-329; 100 nM; GenScript Corp.) for 24 h. After the DCs were washed with sterile endotoxin-free PBS, the cells (3 × 105) were delivered into the sternum of naïve DO11.10 mice by subcutaneous injection. After 7 days, skin draining lymph nodes (sdLNs) were removed and a single-cell suspension of cells was plated with medium and OVA peptide (500 nM) or with PMA (25 ng/ml; Sigma Aldrich) and anti-CD3 monoclonal antibody (1 μg/ml; clone 145-2C11; BD Biosciences). After 72 h, supernatants were removed for measurement of IL-4, IFN-γ, and IL-10 by commercial assay (R&D Systems). Lymph node cells as described above were also plated on a 96-well plate, and after 72 h, 1 μCi of [3H]thymidine was added per well. After 4 hours, cells were harvested onto filter plates, and [3H]thymidine uptake was determined via a liquid scintillation counter. Flow cytometric analysis of CD4, CD28, and cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4) (BD Biosciences) expression on sdLN cells was performed as described above.
Statistics.
All data were analyzed for normality prior to statistical testing. When comparisons of the values for multiple groups were made, data were analyzed using one-way analysis of variance. For comparisons of values for two groups, the Student's t test was used. For all tests, a P value of <0.05 was deemed significant.
RESULTS
F. hepatica Teg suppresses proinflammatory cytokines in vivo in a model of septic shock.
Since parasitic helminth infections have been reported to ameliorate symptoms of proinflammatory diseases (15, 16, 32, 49), and F. hepatica infection and antigen have been shown to suppress pathogen-specific Th1 responses (44), we assessed whether F. hepatica Teg could suppress proinflammatory cytokine production in vivo using a model of septic shock. Intraperitoneal injection of LPS alone induced significantly higher levels of serum IFN-γ (P ≤ 0.001), IL-12p70 (P ≤ 0.05), and IL-12p40 (P ≤ 0.001) than in mice injected with PBS (Fig. 1A to C). However, injection of mice with Teg either 2.5 h before or after LPS injection resulted in significantly reduced levels of IFN-γ (Fig. 1A; P ≤ 0.01 and P ≤ 0.05, respectively) and IL-12p70 (Fig. 1B; P ≤ 0.05). In contrast, Teg did not affect the serum level of IL-12p40. Injection of Teg alone induced cytokine responses similar to those induced by PBS (data not shown).
FIG. 1.
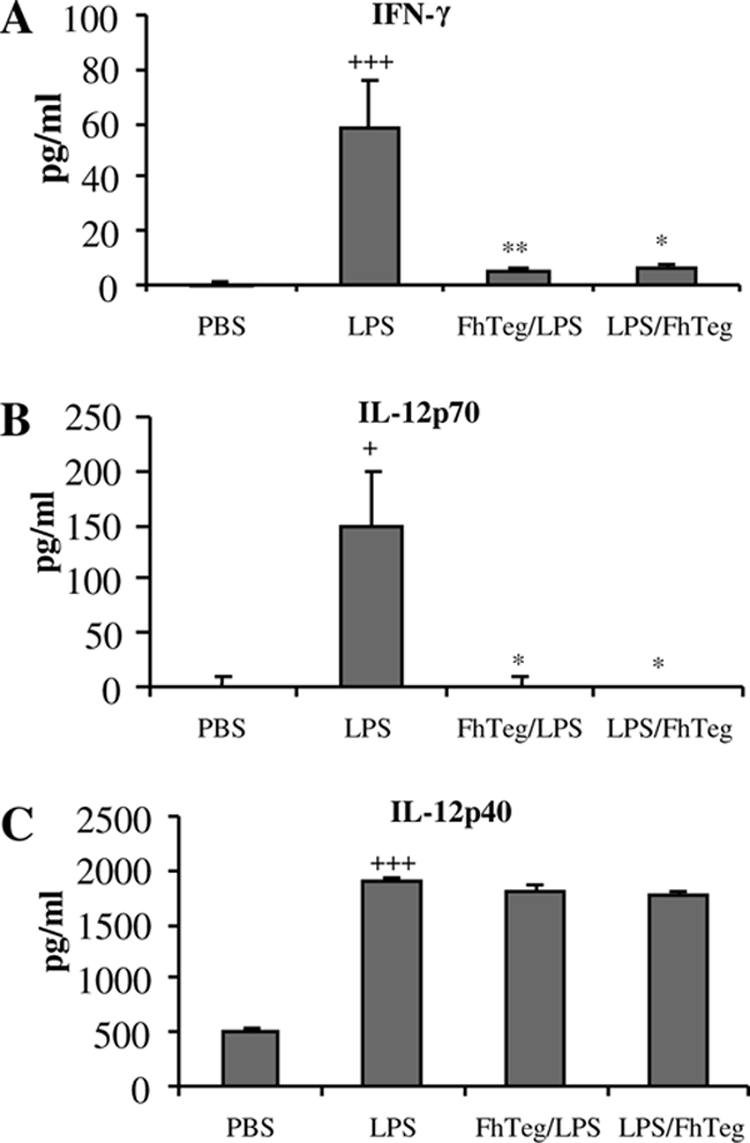
F. hepatica Teg suppresses IFN-γ and IL-12p70 in vivo in a murine model of septic shock. Mice were injected i.p. with F. hepatica Teg (FhTeg) (15 μg), either 2.5 h before or after injection with LPS (1 μg). Control mice were injected with PBS or FhTeg alone (data shown only for PBS). Six hours after LPS injection, mice were sacrificed, and blood was taken by cardiac puncture. Serum IFN-γ (Α), IL-12p70 (B), and IL-12p40 (C) levels were measured by ELISAs. Results are mean values (plus SEM [error bars]) for four mice per group and represent four experiments. Values that were significantly different from the value for the PBS-treated group are indicated as follows: +, P ≤ 0.05, +++, P ≤ 0.001. Values that were significantly different from the value for the LPS-treated group are indicated as follows: *, P ≤ 0.05; **, P ≤ 0.01.
F. hepatica Teg modulates DC cytokine production and cell surface marker expression.
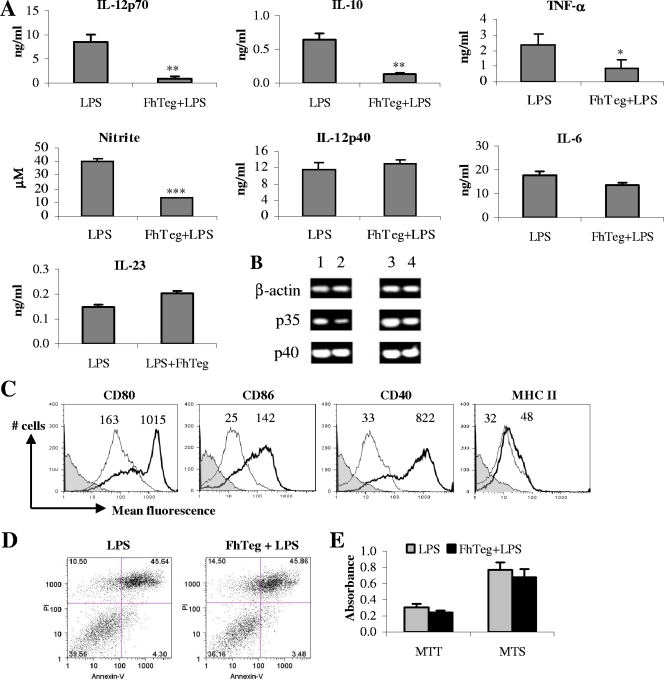
Given the potent anti-inflammatory effect of Teg in vivo, we investigated whether our antigen was influencing the functions of DCs, since these cells, when stimulated in vitro, are excellent producers of proinflammatory cytokines, and previous studies have shown that helminth antigens have immunomodulatory effects on DC maturation, suppressing proinflammatory cytokine production (28, 52). Here, we demonstrate that treatment of DCs with Teg prior to LPS stimulation resulted in significant suppression of IL-12p70 (P ≤ 0.01), IL-10 (P ≤ 0.01), TNF-α (P ≤ 0.05), and nitrite (P ≤ 0.001) production but had no significant effect upon IL-12p40, IL-6, or IL-23 production (Fig. 2A). Since Teg suppressed IL-12p70, but not IL-12p40, from LPS-matured DCs, we wanted to confirm that the p35 subunit, but not the p40 subunit, of IL-12p70 cytokine was downregulated. RNA was extracted from DCs from the experimental groups as described above at 1 and 4 h following LPS stimulation. As expected, the expression of p35, but not p40, was suppressed in DCs in the presence of Teg (Fig. 2B). Both medium and Teg alone were included as controls and gave similar levels of expression (data not shown).
FIG. 2.
F. hepatica Teg modulates LPS-stimulated DC cytokine production and cell surface marker expression without affecting cell viability. DCs were treated with F. hepatica Teg (FhTeg) (15 μg/ml) or medium 2.5 h prior to stimulation with LPS (100 ng/ml) for 18 h. Control DCs were treated with FhTeg or medium alone and consistently gave background levels of cytokines, so the data are not shown. (A) IL-12p70, IL-10, TNF-α, IL-12p40, IL-6, and IL-23 were measured in the supernatants by ELISAs, and nitrite was measured using Griess reagent. Data are the mean values (plus SEM) of three individual experiments. Values that were significantly different from the value for the LPS-treated group are indicated as follows: *, P ≤ 0.05; **, P ≤ 0.01, ***, P ≤ 0.001. (B) Cells were treated as described above for panel A, RNA was extracted from the cells at 1 h and 4 h following LPS stimulation, and the expression of p35, p40, and β-actin was measured by RT-PCR. Data are representative of three separate experiments. The cells were treated with LPS for 1 h (lane 1), FhTeg plus LPS for 1 h (lane 2), LPS for 4 h (lane 3), and FhTeg plus LPS for 4 h (lane 4). (C) Cells treated as described above were harvested and analyzed by three-color flow cytometry for CD80, CD86, CD40, and MHC-II. Cells were gated on CD11c+ cells, and histograms show LPS-stimulated cells (thick line), FhTeg-plus-LPS-stimulated cells (thin line), and isotype controls (solid shaded histogram). The numbers above the histograms represent mean fluorescence intensity for LPS (right peak in each panel) and FhTeg plus LPS (left peak in each panel). (D) Cells were stimulated as described above and stained with annexin V and propidium iodide (PI), and quantification of apoptotic cells was performed by fluorescence-activated cell sorting. The number in each quadrant represents the percentage of cells: living cells (bottom left), dead cells (top left), early apoptotic cells (bottom right), and late apoptotic cells (top right). (E) Cell growth and viability were also assessed in experimental groups using MTT and MTS assays, respectively.
Stimulation of DCs with LPS resulted in a marked increase of CD80, CD86, and CD40 expression (Fig. 2C), which is typical of LPS-induced DC maturation, and a slight increase in MHC-II expression. Treatment of DCs with F. hepatica Teg prior to stimulation with LPS resulted in a dramatic decrease of CD80, CD86, and CD40 expression and a slight decrease in MHC-II expression. DCs treated with medium or Teg alone consistently gave very low to undetectable background levels of cytokines and comparable levels of cell surface marker expression, so these data are not shown.
With such potent inhibitory effects on DC maturation, we measured the influence of F. hepatica Teg on cell viability using an annexin V assay. Cells incubated with LPS and Teg plus LPS exhibited similar profiles of live, early apoptotic, and late apoptotic cells (Fig. 2D), indicating that the difference in cytokine and surface marker expression between the experimental groups may not be attributed to cell death. This was further confirmed with the MTT and MTS assays, which demonstrated that treatment with Teg did not inhibit cell growth or viability (Fig. 2E). Treatment of DCs with medium or Teg alone gave similar results in all experiments (data not shown).
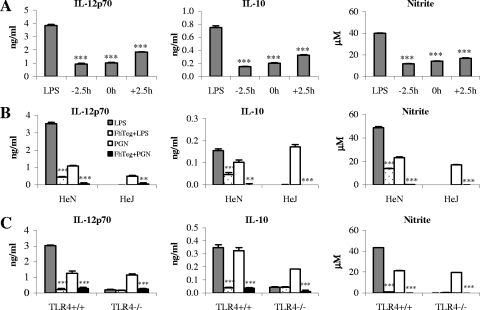
Suppressive effects of F. hepatica Teg are not influenced by the time of exposure to DCs and are not TLR4 dependent.
To investigate whether the suppressive effects of F. hepatica Teg were due to competition with LPS for TLR4 binding, we incubated DCs with Teg 2.5 h prior to, at the same time as, or 2.5 h after LPS stimulation, and the effects on cytokine production and cell surface marker expression were examined. The timing of exposure made no difference to the suppression of IL-12p70, IL-10, and nitrite (Fig. 3A), with levels being significantly reduced at all time points (P ≤ 0.001), thus suggesting that it is unlikely that Teg is interfering with LPS binding. The timing of exposure also made no difference to the suppression of TNF-α and did not alter the lack of suppression of IL-6, IL-12p40, or IL-23 (data not shown). There was also no difference in the suppression of CD80, CD86, and CD40 expression (data not shown).
FIG. 3.
Suppression of LPS-induced DC cytokine production by F. hepatica Teg does not depend upon time of exposure and is not mediated through TLR4. (A) DCs were treated with F. hepatica Teg (15 μg/ml) or medium 2.5 h before (−2.5 h), at the same time as (0 h), or 2.5 h after (+2.5 h) LPS stimulation (100 ng/ml). After 18 h, IL-12p70 and IL-10 were measured in the supernatants by ELISAs, and nitrite was measured using Griess reagent. (B and C) DCs from C3H/HeN (control) and C3H/HeJ (TLR4 mutant) mice (B) and DCs from TLR4 knockout mice (TLR4−/−) and controls (TLR4+/+) (C) were treated with F. hepatica Teg (FhTeg) (15 μg/ml) or medium 2.5 h prior to stimulation with LPS (100 ng/ml) or PGN (5 μg/ml). IL-12p70, IL-10, and nitrite were measured as described above for panel A. Data are the mean values (plus SEM [error bars]) of three individual experiments. Values that were significantly different from the value for the LPS- or PGN-treated group are indicated as follows: **, P ≤ 0.01; ***, P ≤ 0.001.
To demonstrate that F. hepatica Teg does not exert its suppressive effects through TLR4, the experiment was repeated in DCs from C3H/HeJ and C3H/HeN mice and TLR4 knockout (TLR4−/−) mice. C3H/HeJ mice exhibit a point mutation in the TIR domain of TLR4 (47), and although they express the receptor, they fail to produce a response to LPS. As suspected and in support of the timing study, the suppressive effects of Teg were not lost in DCs from HeJ or TLR4−/− mice (Fig. 3B and C). DCs from control HeN and TLR4+/+ mice produced significant levels of IL-12p70, IL-10, and nitrite in response to LPS and PGN (TLR2) compared to the levels produced in response to medium alone (P ≤ 0.001; medium data not shown) and demonstrated significant reductions when treated with Teg prior to stimulation (P ≤ 0.001 and P ≤ 0.01, respectively). DCs from HeJ and TLR4−/− mice failed to respond to LPS, but the Teg-mediated suppression of PGN-induced IL-12p70, IL-10, and nitrite was still intact. There was also no difference in the suppression of PGN-induced CD80, CD86, and CD40 expression (data not shown). These results indicate that not only does Teg not require TLR4 to exert its suppressive effects but it may have a broad suppressive effect on DC function, rather than targeting a single TLR pathway.
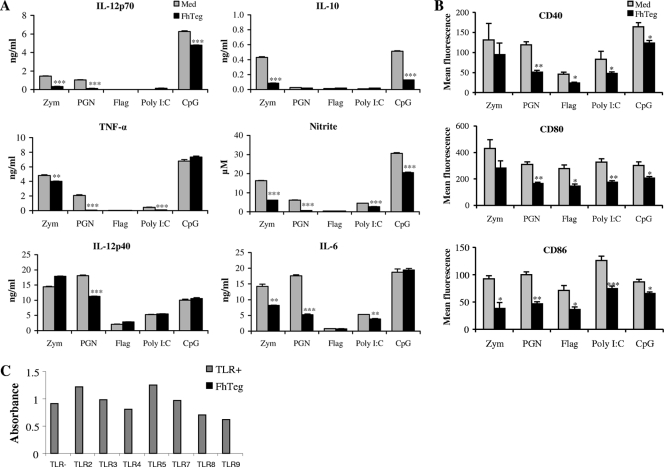
F. hepatica Teg targets multiple TLR pathways in DCs.
To determine whether F. hepatica Teg has a broad suppressive effect on DC function rather than targeting a single TLR pathway, DCs were cultured with Teg for 2.5 h prior to TLR ligand stimulation with zymosan (5 μg/ml), PGN (5 μg/ml), flagellin (0.5 μg/ml), poly(I:C) (100 μg/ml), or CpG-ODN1826 (2.5 μg/ml). As published previously (13), each ligand demonstrated differential secretion of cytokines, with zymosan and CpG inducing all cytokines tested (Fig. 4A). In all cases where the TLR ligand induced a cytokine response [except TNF-α in response to CpG and IL-12p40 in response to zymosan, flagellin, poly(I:C), and CpG], prior incubation with Teg resulted in significant suppression (Fig. 4A). When the DCs were stimulated with Teg or medium alone, no cytokines were produced (data not shown).
FIG. 4.
F. hepatica Teg targets multiple TLR pathways in DCs. (A) DCs were cultured with zymosan A (Zym) (5 μg/ml), PGN (5 μg/ml), flagellin (Flag) (0.5 μg/ml), poly(I:C) (100 μg/ml), or CpG-ODN1826 (2.5 μg/ml) in the presence or absence of F. hepatica Teg (FhTeg) (15 μg/ml), added 2.5 h before TLR stimulation, or with medium (Med) alone as a control. Supernatants were harvested 18 h after TLR ligand stimulation, IL-12p70, IL-10, TNF-α, IL-12p40, and IL-6 levels were measured using sandwich ELISAs, and nitrite was measured using Griess reagent in the medium. (B) DCs were matured with TLR ligands as described above for panel A, and cell surface expression of CD40, CD80, and CD86 was measured by flow cytometry after 18 h. DCs cultured in medium alone and gated on a live CD11c+ population were used as controls. Data are the mean (plus SEM [error bars]) of three independent experiments. Values that were significantly different from the value for the group stimulated with TLR only are indicated as follows: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. (C) Recombinant HEK-293 cells, functionally expressing TLR protein as well as a reporter gene, were stimulated with a specific agonist (TLR+) or FhTeg (15 μg/ml).
Cell surface marker expression of CD40, CD80, and CD86 was measured by flow cytometry and in all cases was significantly suppressed in response to F. hepatica Teg (Fig. 4B). The lack of involvement of TLR activation in Teg-mediated suppression was further demonstrated by the absence of stimulation of HEK-293 cells which functionally express TLR proteins (Fig. 4C).
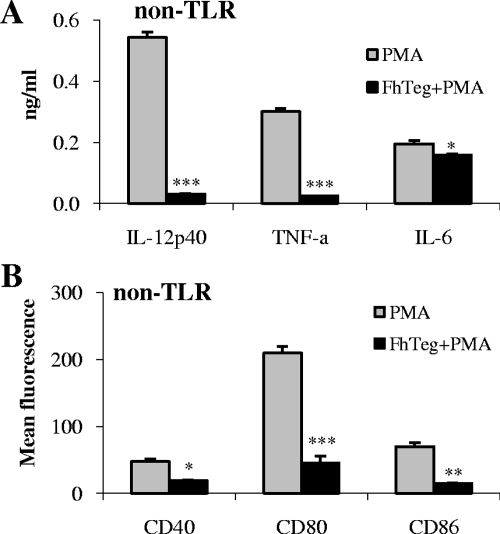
Suppressive effects of F. hepatica Teg are not unique to TLR-stimulated DCs.
To determine whether F. hepatica Teg had a suppressive effect on a non-TLR pathway, DCs were cultured with Teg for 2.5 h prior to stimulation with PMA. DCs were stimulated with PMA or medium alone as controls. PMA stimulated DCs to produce IL-12p40, TNF-α, and IL-6, and these cytokines were significantly suppressed in the presence of Teg (Fig. 5; P ≤ 0.001, P ≤ 0.001, and P ≤ 0.05, respectively). PMA also induced expression of the cell surface markers CD40, CD80, and CD86, which were all significantly suppressed in the presence of Teg (P ≤ 0.05, P ≤ 0.001, and P ≤ 0.01, respectively).
FIG. 5.
F. hepatica Teg targets a non-TLR pathway. To determine whether F. hepatica Teg (FhTeg) could also suppress DC maturation following non-TLR stimulation, DCs were cultured for 2.5 h prior to stimulation with PMA (50 ng/ml). DCs were stimulated with PMA or medium alone as controls. After 18 h, supernatants were harvested, and the levels of IL-12p40, TNF-α (TNF-a), and IL-6 were measured using sandwich ELISAs (A), and cell surface marker expression of CD40, CD80, and CD86 was measured by flow cytometry (B). Data are the means (plus SEM [error bars]) of three independent experiments. Values that were significantly different from the value for the group stimulated with PMA only are indicated as follows: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
F. hepatica Teg suppresses phagocytosis by DCs.
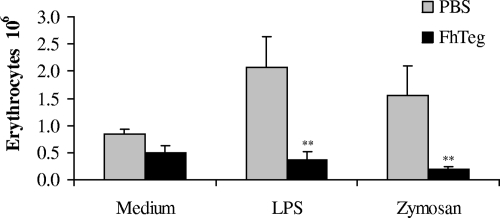
Phagocytosis is an integral part of DC biology, allowing these key cells of the innate immune system to ingest foreign pathogens and present them to naïve T cells, thus driving the host's response to infection (4). To determine whether Teg was interfering with the phagocytic ability of DCs, DCs were cultured with medium, LPS, or zymosan in the presence or absence of Teg prior to exposure to opsonized erythrocytes. The results demonstrated that incubation with Teg alone did not induce phagocytosis of red blood cells by DCs (Fig. 6). Furthermore, incubation with Teg significantly suppressed the phagocytic ability of DCs in response to both LPS and zymosan (Fig. 6; P ≤ 0.01).
FIG. 6.
F. hepatica Teg suppresses phagocytosis by DCs. DCs were cultured with medium, LPS (100 ng/ml), or zymosan A (5 μg/ml) in the presence or absence of F. hepatica Teg (FhTeg) (15 μg/ml) prior to the addition of sheep erythrocytes opsonized by IgG. Negative-control cells were treated with 2 μM cytochalasin D to block phagocytosis (data not shown). Data are the mean values (plus SEM [error bars]) for five wells and are representative of three experiments. Values that were significantly different (P ≤ 0.01) from the value for the non-FhTeg-treated group (PBS) are indicated (**).
F. hepatica Teg inhibits the ability of DCs to prime T cells.
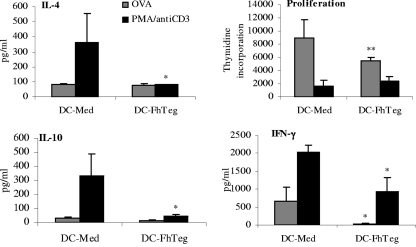
Since F. hepatica Teg significantly suppressed cytokine production and costimulatory marker expression from stimulated DCs, its effects on the ability of differentially activated DCs to prime T-cell responses in sdLNs was investigated in DO11.10 mice. DCs were cultured with OVA peptide in the presence or absence of Teg prior to their inoculation into naïve DO11.10 mice. T-cell priming was measured 7 days later by restimulation of sdLN cells with OVA peptide or with PMA or anti-CD3 (PMA/anti-CD3). DCs primed with medium induced IFN-γ, IL-10, and IL-4 production from sdLN cells in response to OVA stimulation (Fig. 7). Although the levels of IL-4 and IL-10 were low in comparison to PMA/anti-CD3-stimulated cells, they were comparable to the values published in the literature (28). DCs primed with Teg induced significantly less OVA-specific IFN-γ from sdLN cells than control DCs primed with medium did (Fig. 7; P ≤ 0.05), indicating that DCs exposed to Teg suppress local Th1 responses in vivo. There was a slight suppression in IL-10 levels, but this was not significant. Unlike other helminth antigens, such as S. mansoni 0-3hRP (28), in this model system, F. hepatica Teg did not appear to drive local Th2 responses, with IL-10 and IL-4 levels from DC-Teg recipients similar to those of DC-medium recipients (Fig. 7). Interestingly, sdLN cells from DC-Teg recipients, stimulated with PMA/anti-CD3, produced significantly decreased levels of all cytokines (Fig. 7; P ≤ 0.05) than cells from DC-medium recipients, indicating that Teg had a general suppressive effect on the nonspecific T-cell responses. This suppressive effect was also observed when we examined proliferation, since sdLN cells from DC-Teg recipients proliferated significantly less in response to OVA stimulation than cells from DC-medium recipients (Fig. 7). However, there was no significant difference in proliferation of cells stimulated with PMA/anti-CD3 (Fig. 7).
FIG. 7.
F. hepatica Teg alters the ability of DCs to prime T-cell responses. DCs from DO11.10 mice were cultured with OVA (100 nM) in the presence or absence of F. hepatica Teg (FhTeg) (15 μg/ml) overnight at 37°C. Stimulated DCs were thoroughly washed and subcutaneously injected over the sternum of naïve DO11.10 mice. After 7 days, sdLN cells were removed for restimulation in vitro with OVA (500 nM) or with PMA (25 ng/ml) and anti-CD3 (1 μg/ml). After 72 h, supernatants were analyzed by ELISAs for IFN-γ, IL-10, and IL-4. Data are the mean values (plus SEM [error bars]) of three individual wells for four individual mice and are representative of three experiments. Values that were significantly different (P ≤ 0.01) from the value for the appropriate group of DCs treated with medium (DC-Med) are indicated as follows: *, P ≤ 0.05; **, P ≤ 0.01.
CD28 and CTLA-4 are related receptors that differentially regulate T-cell activation. While CD28 enhances T-cell proliferation and survival, in contrast, CTLA-4 inhibits T-cell responses (23). We measured the expression of CD28 and CTLA-4 on CD4-positive cells and found that F. hepatica Teg did not impact on the expression of these molecules (data not shown). The suppression of cell proliferation or cytokine production can therefore not be attributed to changes in the expression of these cell surface markers.
F. hepatica Teg suppresses NF-κBp65 activation, but the activation of JNK, p38, or ERK cannot account for the broad suppressive effect of Teg on DCs.
Since the suppressive effects of F. hepatica Teg are not mediated through TLR4 binding and are not limited to one specific receptor, we investigated whether it is targeting a common component of TLR pathways, such as the MAPKs. To address this question, DCs were treated with MAPK inhibitors specific for ERK, p38, and JNK following exposure to Teg but prior to stimulation with different TLR ligands. Although the inhibition of specific MAPK inhibitors abrogated the Teg-mediated suppression of a select number of cytokines, the effect was not uniform (data not shown). For example, treatment with ERK or p38 inhibitors did not alter the suppression of any of the measured cytokines or cell surface markers, whereas the suppression of LPS-induced TNF-α production by Teg was significantly reversed in the presence of JNK inhibitor. We did not observe any abrogation of the suppressive effect of Teg on cell surface marker expression following the inhibition of the ERK, JNK, or p38 pathway (data not shown).
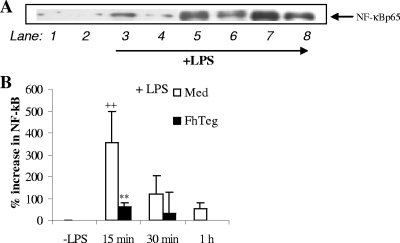
The transcription factor NF-κB is primarily associated with TLR activation and is associated with regulating the expression of a large number of genes associated with proinflammatory processes and Th1 immune responses (25). The active nuclear form of the NF-κB transcription factor complex is composed of two DNA binding subunits, p65 and p50, both of which are critical for DNA binding (25). Here, we investigated whether F. hepatica Teg could modulate NF-κBp65 expression in resting DCs and in DCs matured with LPS. As anticipated, treatment of DCs with LPS led to a significant increase in NF-κBp65 at 15 min which gradually decreased over time (a representative blot shown in Fig. 8A). However, densitometric analysis on blots from several experiments revealed that this was significant only at 15 min (Fig. 8B). Similarly, pretreatment of DCs with Teg suppressed LPS-induced NF-κBp65 at all time points, but again this was significant only at 15 min (Fig. 8A and B). Activation of NF-κBp65 was consistently increased in resting DCs and following exposure to Teg alone, but there were no significant differences for the values of these two groups at any time point.
FIG. 8.
F. hepatica Teg suppresses NF-κBp65 activation, but the activation of JNK, p38, or ERK cannot account for the broad suppressive effect of F. hepatica Teg (FhTeg) on DCs. DCs were treated as described in the legend to Fig. 2. (A) Cells were harvested 0 to 2 h after LPS stimulation, and NF-κBp65 was determined in whole-cell lysates by Western blot analysis. A representative blot is shown. The cells were treated with medium (lane 1), FhTeg (lane 2), LPS for 15 min (lane 3), FhTeg plus LPS for 15 min (lane 4), LPS for 30 min (lane 5), FhTeg plus LPS for 30 min (lane 6), LPS for 1 h (lane 7), and FhTeg plus LPS for 1 h (lane 8). (B) Densitometric analysis was performed on all immunoblots, and NF-κBp65 was expressed in arbitrary units as a percentage increase over the group treated with medium only (control) group. Values that were significantly different (P ≤ 0.01) from the value for the LPS-treated group are indicated (**). Values that were significantly different (P ≤ 0.01) from the value for the group treated with medium (control) are indicated (++).
DISCUSSION
The tegumental coat of F. hepatica is a metabolically active layer that is in intimate contact with the host tissues and body fluids. It is here that much of the immune interplay between the fluke and host takes place. The tegument is shed every 2 to 3 h during the course of infection, thus representing a constant source of antigen in direct contact with the host's immune cells. Studies have shown that by shedding its tegument, the fluke can also shed immune complexes that have formed on the surface, thereby evading a damaging immune response, and the shed antigen acts as a decoy “mopping up” host immune cells (24, 39). This present study demonstrates the powerful suppressive effect of F. hepatica Teg on DC maturation and function.
In initial experiments, the modulatory role of F. hepatica Teg in vivo in a model of septic shock was examined. Mice injected with F. hepatica Teg either prior to or following LPS exposure demonstrated significantly suppressed levels of serum IL-12p70 and IFN-γ, but not IL-12p40. The suppression of proinflammatory cytokines in vivo carries implications for the control of concurrent infections with F. hepatica, as studies have shown that it suppresses IFN-γ, altering the immune response to tuberculosis (45) and delaying bacterial clearance in concurrent infections with Salmonella enterica serovar Dublin or Bordetella pertussis (2, 7). Since DCs are key players in driving adaptive immunity, we went on to examine the effects of Teg on these cells.
First, we investigated the effect of F. hepatica Teg on DC maturation alone, and our results supported other studies which demonstrate that exposure of immature DCs to helminth-derived antigens failed to induce DC maturation, as these cells did not secrete cytokines or increase costimulatory marker expression in response to Teg (3, 46). However, other studies have shown that helminth-derived antigens, such as the filaria-derived antigen, ES-62, which induces IL-12p40, IL-6, and TNF-α production from DCs, can partially induce DC maturation (21) and Schistosoma mansoni larva antigen, which can induce low, yet significant, increases in IL-12p40 and IL-6 from DCs (28). Despite the inactivation or partial activation of DCs, many helminth-matured DCs are still capable of driving potent Th2 immune responses (28, 52). We have yet to determine whether Teg drives Th2 or T-regulatory immune responses.
Although F. hepatica Teg failed to induce cytokine production and cell surface marker expression, treatment of DCs with Teg rendered them hyporesponsive to a range of TLR ligands with significant decreases in cytokine production (IL-12p70, IL-10, IL-6, TNF-α, and nitrite) and costimulatory marker expression (CD80, CD86, and CD40). A decrease in proinflammatory cytokines is in keeping with a helminth's need to suppress inflammatory processes (21), and since IL-12p70 is an important polarizing cytokine known to drive Th1 differentiation (50), this suppression may result in the Th1 suppression observed during F. hepatica coinfections. The decrease in IL-10 observed was not anticipated, since this cytokine is traditionally associated with an anti-inflammatory or regulatory response (37). However, recent studies show IL-10 production by Th1 cells, emphasizing the versatile role that IL-10 plays during infection (43). Cytokine suppression by Teg was selective, given that in general IL-12p40 and IL-23 production were not affected. This is of interest given that the p40 subunit is shared by IL-12 and IL-23 and both cytokines belong to the same family of proinflammatory cytokines (51).
In addition to cytokine production, costimulation is essential for the successful activation and differentiation of naïve CD4+ T cells, and similarly, the suppression of costimulatory markers could influence Th1 cell differentiation. This was confirmed in the T-cell priming studies, which demonstrated that F. hepatica Teg-primed DCs significantly suppressed local Th1 immune responses. These results indicate that exposure to Teg, and the subsequent lack of cytokine production and costimulatory molecule expression, can interfere with the function of DCs and their ability to prime naïve T cells. The effect of our antigen on DC function was further confirmed by the demonstration of reduced phagocytic ability by Teg-primed DCs which would also impact on the development of the adaptive immune response. We hypothesize, therefore, that Teg maintains the DCs in an immature state, impairing their function and ultimately modulating the development of adaptive T-cell responses. Our data support previous findings which demonstrate that F. hepatica infection and antigens suppress Th1 immune responses in vivo (7, 19). Furthermore, the suppression of Th1 responses is likely to be a survival mechanism for the worm, since vaccine trials in domestic livestock have shown the importance of inducing strong Th1 immune responses in the host to protect against challenge infection (38).
While the exact mechanism of F. hepatica Teg-mediated suppression remains to be elucidated, the timing of exposure of DCs to our antigen did not affect the suppressive effects, suggesting that it does not compete with LPS for binding and that it is not exerting its effects through TLR4 itself. This was confirmed by the fact that Teg is still effective in DCs from TLR4 mutant and knockout mice. Furthermore, Teg was able to suppress the effects of all TLR ligands tested (which included both MyD88-dependent and -independent pathways), in addition to non-TLR ligands. While we would not directly rule out the involvement of other TLRs, it seems likely that Teg utilizes a pathway common to these receptors, particularly since it did not activate HEK-296 cells functionally expressing a range of TLRs.
The MAPK pathway is a highly conserved pathway involved in the initiation of DC maturation through all known TLR ligands (13). Activation of the three main mammalian groups, JNK, ERK, and p38, culminates in the release of cytokines from DCs following the downstream activation of a signaling cascade involving adaptor proteins, such as MyD88 and Mal (41). Helminth-mediated suppression of cytokine production by antigen-presenting cells has been shown to be modulated by MAPK pathways. For example, ES-62-mediated suppression of LPS-induced IL-12p40 by macrophages can be reversed with the addition of the ERK inhibitor (22), and S. mansoni egg antigen dramatically reduces LPS-stimulated phosphorylation of p38 (29). However, in the present study, we have shown that F. hepatica Teg does not target these pathways, since MAPK pathways are involved in the suppressive effect of only a small number of select cytokines.
Instead, we found that F. hepatica Teg targeted the transcription factor NF-κBp65, one of the active subunits of the NF-κB complex involved in NF-κB binding to DNA. This effect on downstream signaling events could explain the observed decrease in proinflammatory cytokines demonstrated in this study. Activation of NF-κB is also inhibited by other parasites, such as Brugia malayi (48) and Toxoplasma gondii (8), where NF-κB translocation is blocked from entering the nucleus. We have yet to determine whether Teg can suppress other members of the NF-κB family (such as the p50 subunit) or other transcription factors, such as interferon regulatory factor 3, which are also activated following LPS stimulation.
In summary, this is the first study to report that F. hepatica tegumental antigen modulates DC maturation and function, which may go some way to explaining its reported immunomodulatory properties in vivo. F. hepatica tegumental antigen is a heterogeneous group of molecules, and preliminary proteomic studies have already pointed to a number of potential molecules that may be involved in its anti-inflammatory capacity (R. M. Morphew and P. M. Brophy, unpublished data). These proteomic studies also show that the profile observed for the tegument differs from that of its F. hepatica excretory-secretory products, and preliminary studies using excretory-secretory products demonstrate that it has a different modulatory effect on DC maturation (C. M. Hamilton, D. J. Dowling, and S. M. O'Neill, unpublished data). Although the precise immunological scenario is not completely understood, it is clear that Teg maintains the DCs in an immature state, impairing their function and the subsequent development of adaptive immunity. Given the powerful modulatory effect that Teg exhibits, understanding its exact mechanisms may lead to the development of novel immune therapeutics for the treatment of Th1-mediated inflammatory diseases (15, 49).
Acknowledgments
This work was supported by the Dublin City University Faculty of Science and Health Targeted Research Development Fund, European Union (DELIVER) (grant FOOD-CT-2005-023025), and BBSRC (grant BB/C503638/2).
We thank Carolyn Wilson (Dublin City University [DCU]) for technical support. We also thank Anthony Ryan and Eve Draper (DCU) and Bernie Mahon and Ciaran Skerry (National University of Ireland, Maynooth) for assistance with experiments and Cariosa Noone (DCU) for critical reading of the manuscript.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 30 March 2009.
REFERENCES
- 1.Abreu, M. T., and M. Arditi. 2004. Innate immunity and Toll-like receptors: clinical implications of basic science research. J. Pediatr. 144421-429. [DOI] [PubMed] [Google Scholar]
- 2.Aitken, M. M., P. W. Jones, G. A. Hall, D. L. Hughes, and G. T. Brown. 1981. Responses of fluke-infected and fluke-free cattle to experimental reinfection with Salmonella dublin. Res. Vet. Sci. 31120-126. [PubMed] [Google Scholar]
- 3.Balic, A., Y. Harcus, M. J. Holland, and R. M. Maizels. 2004. Selective maturation of dendritic cells by Nippostrongylus brasiliensis-secreted proteins drives Th2 immune responses. Eur. J. Immunol. 343047-3059. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392245-252. [DOI] [PubMed] [Google Scholar]
- 5.Beutler, B. 2004. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430257-263. [DOI] [PubMed] [Google Scholar]
- 6.Borkow, G., and Z. Bentwich. 2006. HIV and helminth co-infection: is deworming necessary? Parasite Immunol. 28605-612. [DOI] [PubMed] [Google Scholar]
- 7.Brady, M. T., S. M. O'Neill, J. P. Dalton, and K. H. G. Mills. 1999. Fasciola hepatica suppresses a protective Th1 response against Bordetella pertussis. Infect. Immun. 675372-5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butcher, B. A., L. Kim, P. E. Johnson, and E. Y. Denkers. 2001. Toxoplasma gondii tachyzoites inhibit proinflammatory cytokine induction in infected macrophages by preventing nuclear translocation of the transcription factor NF-kappa B. J. Immunol. 1672193-2201. [DOI] [PubMed] [Google Scholar]
- 9.Cervi, L., A. S. MacDonald, C. Kane, F. Dzierszinski, and E. J. Pearce. 2004. Dendritic cells copulsed with microbial and helminth antigens undergo modified maturation, segregate the antigens to distinct intracellular compartments, and concurrently induce microbe-specific Th1 and helminth-specific Th2 responses. J. Immunol. 1722016-2020. [DOI] [PubMed] [Google Scholar]
- 10.Clery, D., P. Torgerson, and G. Mulcahy. 1996. Immune responses of chronically infected adult cattle to Fasciola hepatica. Vet. Parasitol. 6271-82. [DOI] [PubMed] [Google Scholar]
- 11.Daubeuf, B., J. Mathison, S. Spiller, S. Hugues, F. Herren, S. Ferlin, and M. Kosco-Vilbois. 2007. TLR4/MD-2 monoclonal antibody therapy affords protection in experimental models of septic shock. J. Immunol. 1796107-6114. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly, S., S. M. O'Neill, M. Sekiya, G. Mulcahy, and J. P. Dalton. 2005. Thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages. Infect. Immun. 73166-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowling, D. J., C. M. Hamilton, and S. M. O'Neill. 2008. A comparative analysis of cytokine responses, cell surface marker expression and MAPKs in DCs matured with LPS compared with a panel of TLR ligands. Cytokine 41254-262. [DOI] [PubMed] [Google Scholar]
- 14.Elias, D., S. Britton, A. Kassu, and H. Akuffo. 2007. Chronic helminth infections may negatively influence immunity against tuberculosis and other diseases of public health importance. Expert Rev. Anti. Infect. Ther. 5475-484. [DOI] [PubMed] [Google Scholar]
- 15.Elliott, D. E., J. Li, A. Blum, A. Metwali, K. Qadir, J. F. Urban, Jr., and J. V. Weinstock. 2003. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 284G385-G391. [DOI] [PubMed] [Google Scholar]
- 16.Elliott, D. E., T. Setiawan, A. Metwali, A. Blum, J. F. Urban, Jr., and J. V. Weinstock. 2004. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur. J. Immunol. 342690-2698. [DOI] [PubMed] [Google Scholar]
- 17.Fallon, P. G., E. J. Richardson, P. Smith, and D. W. Dunne. 2000. Elevated type 1, diminished type 2 cytokines and impaired antibody response are associated with hepatotoxicity and mortalities during Schistosoma mansoni infection of CD4-depleted mice. Eur. J. Immunol. 30470-480. [DOI] [PubMed] [Google Scholar]
- 18.Flynn, R. J., and G. Mulcahy. 2008. The roles of IL-10 and TGF-β in controlling IL-4 and IFN-γ production during experimental Fasciola hepatica infection. Int. J. Parasitol. 381673-1680. [DOI] [PubMed] [Google Scholar]
- 19.Flynn, R. J., C. Mannion, O. Golden, O. Hacariz, and G. Mulcahy. 2007. Experimental Fasciola hepatica infection alters responses to tests used for diagnosis of bovine tuberculosis. Infect. Immun. 751373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodridge, H. S., E. H. Wilson, W. Harnett, C. C. Campbell, M. M. Harnett, and F. Y. Liew. 2001. Modulation of macrophage cytokine production by ES-62, a secreted product of the filarial nematode Acanthocheilonema viteae. J. Immunol. 167940-945. [DOI] [PubMed] [Google Scholar]
- 21.Goodridge, H. S., F. A. Marshall, K. J. Else, K. M. Houston, C. Egan, L. Al-Riyami, and F. Y. Liew. 2005. Immunomodulation via novel use of TLR4 by the filarial nematode phosphorylcholine-containing secreted product, ES-62. J. Immunol. 174284-293. [DOI] [PubMed] [Google Scholar]
- 22.Goodridge, H. S., W. Harnett, F. Y. Liew, and M. M. Harnett. 2003. Differential regulation of interleukin-12 p40 and p35 induction via Erk mitogen-activated protein kinase-dependent and -independent mechanisms and the implications for bioactive IL-12 and IL-23 responses. Immunology 109415-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green, J. M. 2000. The B7/CD28/CTLA-4 T cell activation pathway: implications for inflammatory lung disease. Am. J. Respir. Cell Mol. Biol. 22261-264. [DOI] [PubMed] [Google Scholar]
- 24.Halton, D. 2004. Microscopy and the helminth parasite. Micron 35361-390. [DOI] [PubMed] [Google Scholar]
- 25.Hayden, M. S., A. P. West, and S. Ghosh. 2006. NF-κB and the immune response. Oncogene 256758-6780. [DOI] [PubMed] [Google Scholar]
- 26.Hillyer, G. V. 1980. Isolation of Fasciola hepatica tegument antigens. J. Clin. Microbiol. 12695-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janeway, C. A., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20197-216. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins, S. J., and A. P. Mountford. 2005. Dendritic cells activated with products released by schistosome larvae drive Th2-type immune responses, which can be inhibited by manipulation of CD40 costimulation. Infect. Immun. 73395-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kane, C. M., L. Cervi, J. Sun, A. S. McKee, K. S. Masek, S. Shapira, C. A. Hunter, and E. J. Pearce. 2004. Helminth antigens modulate TLR-initiated dendritic cell activation. J. Immunol. 1737454-7461. [DOI] [PubMed] [Google Scholar]
- 30.Kapsenberg, M. 2003. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 3984-993. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi, M., M. N. Kweon, H. Kuwata, R. D. Schreiber, H. Kiyono, K. Takeda, and S. Akira. 2003. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J. Clin. Investig. 1111297-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.La Flamme, A. C., K. Ruddenklau, and B. T. Bäckström. 2003. Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infect. Immun. 714996-5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacDonald, A. S., and R. M. Maizels. 2008. Alarming dendritic cells for Th2 induction. J. Exp. Med. 20513-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maizels, R. M., A. Balic, N. Gomez-Escobar, M. Nair, M. D. Taylor, and J. D. Allen. 2004. Helminth parasites—masters of immune regulation. Immunol. Rev. 20189-116. [DOI] [PubMed] [Google Scholar]
- 35.Maizels, R. M., and M. Yazdanbakhsh. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3733-744. [DOI] [PubMed] [Google Scholar]
- 36.Mas-Coma, S., M. D. Barques, and J. G. Esteban. 1999. Human fasciolosis, p. 411-447. In J. P. Dalton (ed.), Fasciolosis. CAB International, Oxon, United Kingdom.
- 37.Moore, K. W., R. L. de Waal Malefyt, R. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19683-765. [DOI] [PubMed] [Google Scholar]
- 38.Mulcahy, G., F. O'Connor, S. McGonigle, A. Dowd, D. G. Clery, S. J. Andrews, and J. P. Dalton. 1998. Correlation of specific antibody titre and avidity with protection in cattle immunized against Fasciola hepatica. Vaccine 16932-939. [DOI] [PubMed] [Google Scholar]
- 39.Mulcahy, G., P. Joyce, and J. P. Dalton. 1999. Immunology of Fasciola hepatica infection, p. 341-375. In J. P. Dalton (ed.), Fasciolosis. CAB International, Oxon, United Kingdom.
- 40.Mwangi, T. W., J. M. Bethony, and S. Brooker. 2006. Malaria and helminth interactions in humans: an epidemiological viewpoint. Ann. Trop. Med. Parasitol. 100551-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakahara, T., Y. Moroi, H. Uchi, and M. Furue. 2006. Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement. J. Dermatol. Sci. 421-11. [DOI] [PubMed] [Google Scholar]
- 42.Noone, C. M., E. A. Lewis, A. B. Frawely, R. W. Newman, B. P. Mahon, K. H. Mills, and P. A. Johnson. 2005. Novel mechanism of immunosuppression by influenza virus haemagglutinin: selective suppression of interleukin 12 p35 transcription in murine bone marrow-derived dendritic cells. J. Gen. Virol. 861885-1890. [DOI] [PubMed] [Google Scholar]
- 43.O'Garra, A., and P. Vieira. 2007. TH1 cells control themselves by producing interleukin-10. Nat. Rev. Immunol. 7425-428. [DOI] [PubMed] [Google Scholar]
- 44.O'Neill, S. M., M. T. Brady, J. J. Callanan, G. Mulcahy, P. Joyce, K. H. G. Mills, and J. P. Dalton. 2000. Fasciola hepatica infection downregulates Th1 responses in mice. Parasite Immunol. 22147-155. [DOI] [PubMed] [Google Scholar]
- 45.O'Neill, S. M., K. H. Mills, and J. P. Dalton. 2001. Fasciola hepatica cathepsin L cysteine proteinase suppresses Bordetella pertussis-specific interferon-gamma production in vivo. Parasite Immunol. 23541-547. [DOI] [PubMed] [Google Scholar]
- 46.Perona-Wright, G., S. J. Jenkins, and A. S. MacDonald. 2006. Dendritic cell activation and function in response to Schistosoma mansoni. Int. J. Parasitol. 36711-721. [DOI] [PubMed] [Google Scholar]
- 47.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, and D. Birdwell. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 2822085-2088. [DOI] [PubMed] [Google Scholar]
- 48.Semnani, R. T., P. G. Venugopal, C. A. Leifer, S. Mostböck, H. Sabzevari, and T. B. Nutman. 2008. Inhibition of TLR3 and TLR4 function and expression in human dendritic cells by helminth parasites. Blood 1121290-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Summers, R. W., D. E. Elliot, J. F. Urban, R. Thompson, and J. V. Weinstock. 2005. Trichuris suis therapy in Crohn's disease. Gut 5487-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3133-146. [DOI] [PubMed] [Google Scholar]
- 51.Waibler, Z., U. Kalinke, J. Will, M. H. Juan, J. M. Pfeilschifter, and H. H. Radeke. 2007. TLR-ligand stimulated interleukin-23 subunit expression and assembly is regulated differentially in murine plasmacytoid and myeloid dendritic cells. Mol. Immunol. 441483-1489. [DOI] [PubMed] [Google Scholar]
- 52.Whelan, M., M. M. Harnett, K. M. Houston, V. Patel, W. Harnett, and K. P. Rigley. 2000. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J. Immunol. 1646453-6460. [DOI] [PubMed] [Google Scholar]