Abstract
NK cells, in addition to possessing antitumor and antiviral activity, exhibit perforin-dependent microbicidal activity against the opportunistic pathogen Cryptococcus neoformans. However, the factors controlling this response, particularly whether the pathogen itself provides an activation or rearming signal, are largely unknown. The current studies were performed to determine whether exposure to this fungus alters subsequent NK cell anticryptococcal activity. NK cells lost perforin and mobilized lysosome-associated membrane protein 1 to the cell surface following incubation with the fungus, indicating that degranulation had occurred. Despite a reduced perforin content during killing, NK cells acquired an enhanced ability to kill C. neoformans, as demonstrated using auxotrophs that allowed independent assessment of the killing of two strains. De novo protein synthesis was required for optimal killing; however, there was no evidence that a soluble factor contributed to the enhanced anticryptococcal activity. Exposure of NK cells to C. neoformans caused the cells to rearm, as demonstrated by increased perforin mRNA levels and enhanced loss of perforin when transcription was blocked. Degranulation alone was insufficient to provide the activation signal as NK cells lost anticryptococcal activity following treatment with strontium chloride. However, NK cells regained the activity upon prolonged exposure to C. neoformans, which is consistent with activation by the microbe. The enhanced cytotoxicity did not extend to tumor killing since NK cells exposed to C. neoformans failed to kill NK-sensitive tumor targets (K562 cells). These studies demonstrate that there is contact-mediated microbe-specific rearming and activation of microbicidal activity that are necessary for optimal killing of C. neoformans.
Recently, it has become apparent that NK cells and their cytolytic products possess the ability to kill a vast array of microbes directly, including bacteria, parasites, and fungi (2, 22, 49, 53, 60). In particular, NK cells exhibit direct microbicidal activity against Cryptococcus neoformans, a devastating opportunistic pathogen that infects immunocompromised individuals, including patients afflicted with human immunodeficiency virus and AIDS (31, 32, 37). Remarkably, NK cells are able to kill this organism without prior exposure or the aid of accessory cells. Indeed, NK cells are important during the early immune responses to C. neoformans. Mice lacking functional NK cells have a reduced ability to control an infection compared to mice possessing functional NK cells (15). Despite the importance of NK cells, little is known about the factors regulating NK cell anticryptococcal activity and, in particular, the effect of NK cell interactions with C. neoformans on subsequent NK cell activity.
Much of the work examining the mechanisms of NK cell anticryptococcal activity is based on observations made during NK cell tumor cytolysis. While there are similarities between NK cell antitumor and anticryptococcal activities, there are also substantial differences. In particular, at least some of the signaling molecules that NK cells require to elicit responses to these two targets are conserved. NK cells require phosphoinositol 3-kinase and extracellular signal-regulated kinase 1/2 phosphorylation for both tumor lysis and killing of C. neoformans (4, 6, 21, 58, 61, 62). However, NK cells bind maximally to tumor targets after 20 min of exposure through formation of an intimate synapse involving LFA-1, and subsequent killing is detected 10 to 15 min following binding (43). In contrast, NK cell contact with C. neoformans is not intimate but is characterized by NK cell microvilli penetrating the fungal capsule and binding to the underlying cell wall of the fungus. Maximal binding of NK cells to C. neoformans does not occur until after 2 h and is independent of LFA-1, and inhibition of fungal growth is not detectable until 4 h following maximal binding (14, 23, 43, 44, 46). With these similarities and differences in mind, we wondered whether NK cell microbicidal activity with C. neoformans is influenced in response to C. neoformans as it is in response to other target types.
Exposure of NK cells to specific target cells has been shown to result in either enhancement or suppression of subsequent killing (19, 20). Rat NK cells exposed to fixed tumor targets were “primed” to kill a second tumor target more efficiently than NK cells that were not previously exposed. This was a result of gamma interferon (IFN-γ) secretion and enhanced expression of perforin (8). Parenthetically, NK cells produce cytokines, including IFN-γ, following exposure to C. neoformans that may have the ability to induce perforin transcription (33). Notably, the perforin promoter region contains binding sites for cytokine signaling molecules, including signal transducer and activator of transcription 1α (STAT1α), STAT3, and STAT5a/b (63). Thus, multiple cytokines, including IFN-γ, interleukin-10 (IL-10), and IL-2, may have the ability to induce perforin transcription (29, 35, 55). It follows that perforin expression is important during NK cell antitumor activity. NK cells kill tumor targets until their perforin stores are depleted. Only after subsequent IL-2 treatment, which is correlated with restored perforin expression, is NK cell antitumor activity restored (3). Likewise, NK cell anticryptococcal activity is perforin dependent (37). Therefore, it follows that enhanced perforin production may be induced by the release of immune modulatory cytokines following fungal exposure, enhancing subsequent anticryptococcal activity.
In contrast, NK cells can become inactivated and loose their cytotoxic function following exposure to tumor targets (19, 20). Likewise, exposure to C. neoformans may be able to abrogate subsequent NK cell anticryptococcal activity. In this regard, C. neoformans can modulate immune responses by blocking phagocytosis, inducing lymphocyte apoptosis, and inducing immunosuppressive cytokine secretion (42, 54, 59). Importantly, C. neoformans is able to skew the cytokine milieu to a Th2-type response (1). This prolongs the survival of the fungus as a Th1 immune response is necessary for optimal host defense against the fungus (17).
Alternatively, modulation of NK cell anticryptococcal activity may also be cytokine independent and thus a consequence of direct fungal contact. NK cells possess a wide variety of pattern recognition receptors. While the NK cell receptor responsible for recognition of C. neoformans remains elusive, it is possible that binding of C. neoformans to NK cells may directly influence subsequent anticryptococcal activity. It has been demonstrated that lipopolysaccharide activation of Toll-like receptor 4 can lead to increased perforin expression (50). While this effect was demonstrated in CD4+ T cells, it provides evidence of perforin regulation through pattern recognition receptor stimulation. However, whether the increased perforin expression was a result of cytokine release following activation of Toll-like receptor 4 by lipopolysaccharide is unknown. On the other hand, evidence of reduced NK cell activity following exposure to specific targets has been obtained, and this reduced activity is linked to loss of perforin, down-modulation of activating receptors, and inactivation of signaling pathways due to constant stimulation (3, 51). Thus, NK cell binding to C. neoformans may induce a cytokine-independent signaling cascade resulting in perforin production, enhancing subsequent anticryptococcal activity, or it may result in NK cell exhaustion.
The objective of this study was to determine if exposure to C. neoformans regulates subsequent killing of this fungus. Specifically, the objective was to determine if NK cells are stimulated following fungal exposure, resulting in enhanced killing of C. neoformans and rearming of NK cells. In order to determine the effect of fungal exposure on NK cell anticryptococcal activity, we studied cryptococcal viability by examining the number of CFU and perforin expression at both the protein and transcript levels, and we determined if the effect was the result of a soluble mediator or direct fungal contact. If the mechanisms involved and the consequences of NK cell interactions with C. neoformans are understood, it may be possible to identify alternative therapeutic approaches for the treatment of the devastating infections caused by this fungus.
MATERIALS AND METHODS
Preparation of C. neoformans.
C. neoformans strains B3501 (= ATCC 34873; lightly encapsulated; serotype D), CAP67 (= ATCC 52817; acapsular mutant of B3501), H99 (= ATCC 208821; encapsulated; serotype A), and 145 (= ATCC 62070; moderately encapsulated; serotype A) were all obtained from the American Type Culture Collection (Manassas, VA). Strain FOA-01-11-2 (= Ura5; uracil-dependent mutant of B3501) was generously provided by J. Kwon-Chung (NIH, Bethesda, MD). All strains were maintained on Sabouraud's dextrose agar slants (Difco, Detroit, MI) and passaged on fresh slants every month, as previously described (40).
Cells and cell lines.
YT cells (human NK cell-like thymic leukemia cells) were a gift from C. Clayberger (Stanford University, Stanford, CA) and cultured in complete media containing RPMI 1640, 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids (all obtained from Invitrogen Life Technologies, Burlington, Canada).
Primary NK cells were isolated from peripheral blood mononuclear cells (PBMC) as previously described (41). Briefly, peripheral blood was obtained by venipuncture from healthy volunteers. PBMC were isolated by centrifugation at 800 × g for 30 min (Sorvall Legend RT; Sorvall Heraeus) over a Ficoll-Hypaque density gradient (Sigma-Aldrich, St. Louis, MO). PBMC were harvested and washed three times in Hanks balanced salt solution (Invitrogen Life Technologies). The NK cells were isolated from PBMC by using the MACS negative selection system of an AutoMACS cell sorter according to the manufacturer's protocol (Miltenyi Biotec, Bergisch Gladbach, Germany). The NK cells were cultured in complete media as described above.
K562 cells were a gift from J. Deans (University of Calgary, Calgary, Canada) and were cultured in complete media as described above.
Anticryptococcal activity assays.
The anticryptococcal activity was assessed by determination of the number of CFU as previously described (30, 31, 39). In brief, C. neoformans cells (5 × 103 cells/ml) were incubated with YT cells at an effector cell/target cell (E/T) ratio of 10:1 to 1,000:1 or with NK cells at an E/T ratio of 250:1 to 1,000:1 at the start of the assay to account for the 10- to 500-fold growth of the organisms during the course of the assay. The number of CFU of C. neoformans per well was determined at 0, 24, and 48 h by lysing the effector cells in water, followed by dilution and spotting onto Sabouraud's dextrose agar plates.
In some cases, NK cells were incubated with 25 mM strontium chloride (SrCl2) (Sigma-Aldrich) to assess the effect of degranulation and reduced perforin expression on NK cell killing of C. neoformans. NK cells were treated with SrCl2 for 48 h, washed three times with complete media, added to C. neoformans cells, and incubated for an additional 24 and 48 h in order to assess killing. The viability of NK cells was not altered by treatment with SrCl2, as determined by trypan blue exclusion. In addition, NK cell perforin levels were determined from 24 to 96 h following incubation in the presence of SrCl2, as described below.
In other experiments, NK or YT cells were cultured in media containing 10 μg/ml brefeldin A (BFA) (Sigma-Aldrich) or the equivalent volume of ethanol as a solvent control for 24 h. The NK cells were washed three times with complete media and incubated with C. neoformans for an additional 24 and 48 h in the presence of 0.5 μg/ml BFA or ethanol.
To determine if a soluble factor that can enhance subsequent NK anticryptococcal activity is released from NK cells during fungal contact, a polyethylene terephthalate cell culture insert (BD Biosciences, Mississauga, Canada) was placed into the wells of 24-well culture plates. The wells of the plate then had two chambers that were separated by a membrane with a pore size of 1.0 μm, which allowed soluble factors to move between the chambers while the cells in each chamber were separated. NK cells were placed in the lower chambers, and the upper chambers were inoculated with NK cells, C. neoformans cells, or both types of cells at an E/T ratio of 1:10. The plates were then cultured for 48 h, and then the NK cells from the bottom chamber were harvested and their anticryptococcal activity was assessed.
Finally, NK cells were preincubated with the Ura5 strain of C. neoformans for 48 h at an E/T ratio of 1:10. The majority of the Ura5 cells were removed (there was a 100-fold reduction) by Ficoll-Hypaque density gradient centrifugation for 30 min at 300 × g, and the NK cells were harvested. The NK cells were then incubated with CAP67, and killing of CAP67 was assessed by lysing the NK cells and spotting them on minimal yeast agar plates without uracil containing yeast nitrogen base without amino acids (Sigma-Aldrich), d-glucose (BDH Inc., Toronto, Canada), and Bacto agar (BD Biosciences), which does not support growth of strain Ura5.
Perforin labeling.
NK cells were incubated with C. neoformans for 0, 24, and 48 h at an E/T ratio of 1:100. The NK cells were fixed and made permeable using a Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's protocol. The NK cells were then labeled with a fluorescein isothiocyanate (FITC)-conjugated antiperforin antibody or isotype control (BD Biosciences), and perforin labeling was measured by flow cytometry with a Guava EasyCyte flow cytometer (Guava Technologies, San Francisco, CA). NK cells were labeled with a phycoerythrin-Cy5-conjugated anti-CD11a antibody in order to distinguish NK cells from C. neoformans cells (BD Biosciences).
For some experiments NK cells were preincubated with 1 μg/ml actinomycin D (Sigma-Aldrich) for 1 h and then incubated with C. neoformans for an additional 24 h before perforin labeling was assessed. The actinomycin D remained in the media for the duration of the experiment. The viability of NK cells was not altered, as determined by trypan blue exclusion.
LAMP-1 immunofluorescence microscopy.
NK cells were incubated with C. neoformans strain CAP67 at an E/T ratio of 1:10 in the presence of an anti-lysosome-associated membrane protein 1 (LAMP-1)-FITC antibody (BD Biosciences). After 1 h of incubation, l μl Golgi-Stop containing monensin used according to the manufacturer's directions (BD Biosciences) was added and incubated for an additional 48 h in the presence of 5% CO2 at 37°C in a flat-bottom 96-well plate (Corning Life Sciences, New York, NY). After incubation, 10 μl of the sample was removed and placed on a chamber slide (Lab-Tek; Thermo Fisher Scientific, Rochester, NY). The slide was then dried in the dark at room temperature overnight, and the chambers were removed. Cells were treated with 10 μl Prolong Gold antifade reagent (Invitrogen Life Sciences) and allowed to cure for 2 h at 4°C.
Immunofluorescence microscopy was performed with an Olympus IX70 microscope equipped with digital convolution (DeltaVision; Applied Precision). Fluorescence images were recorded with objective magnification of ×60 using phycoerythrin-Cy5 and FITC filter sets. Images of cells were also obtained by using transmitted light. Image analysis (Softworks software) was used to perform full iterative or nearest-neighbor deconvolution, and the images are single z-section images. NK cells were considered positive for LAMP-1 if there was at least a threefold difference between the minimum and the maximum.
Quantitative real-time PCR.
NK cells were incubated with C. neoformans, IL-10, or IL-2 plus IL-12 for 24 h (all interleukins were obtained from eBioscience, San Diego, CA). The NK cells were lysed, and the RNA was isolated using a Qiagen RNeasy minikit according to the manufacturer's protocol (Qiagen, Mississauga, Canada). The Improm-II reverse transcription system was then utilized to convert the RNA to cDNA according to the manufacturer's directions (Promega, Madison, WI).
Real-time PCR analyses were performed with an ABI Prism 7000 using the ABI Prism 7000 SDS software (Applied Biosystems, Foster City, CA). Perforin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe-primer sets were obtained and used according to the manufacturer's instructions (Applied Biosystems). In brief, a master mixture containing 1.25 μl of the probe primer mixture, 12.5 μl TaqMan universal PCR master mixture (Applied Biosystems), and 6.25 μl DNase- and RNase-free water (Invitrogen Life Sciences) was prepared. The master mixture was then added to 5 μl of cDNA, and the cDNA was amplified by heating the mixture to 50°C for 2 min, followed by 10 min of incubation at 95°C and then 40 cycles of 95°C for 15 s and 60°C for 1 min. The perforin probe contained the 6-carboxyfluorescein and 6-carboxytetramethylrhodamine reporter and quencher dyes, while the GAPDH probe was labeled with VIC fluorochrome (Applied Biosystems, Foster City, CA) and a minor groove binder. PCR efficiency was confirmed for all genes.
Tumor cytotoxicity.
NK cells were either preincubated with C. neoformans for 48 h at an E/T ratio of 1:10 or cultured in media alone. The number of yeast cells was then reduced 100-fold by Ficoll-Hypaque density gradient centrifugation, and the NK cells were incubated with K562 cells for 4 h. The antitumor activity of NK cells was measured by using a Guava CellToxicity kit according to the manufacturer's protocol (Guava Technologies). In brief, K562 cells were counted and labeled in 1 ml phosphate-buffered saline at 37°C in the presence of 5% CO2 for 15 min using 2.5 μg/ml (final concentration) carboxyfluorescein succinimidyl ester (CFSE) (CellToxicity kit; Guava Technologies). The cells were washed three times with phosphate-buffered saline, transferred to complete media, and incubated for 30 min at 37°C in the presence of 5% CO2 before the CFSE-labeled K562 cells were added to NK cells in a 96-well, round-bottom plate. The plate was centrifuged at 50 × g for 2 min and incubated for 4 h at 37°C in the presence of 5% CO2. After 4 h of incubation, 40 μl 7 amino-actinomycin D (7-AAD) (CellToxicity kit; Guava Technologies) was added to each well and incubated for 10 min in the dark. The membrane permeability of the cells was then analyzed by assessing the uptake of 7-AAD using Guava EasyCyte.
Statistics.
Values are expressed below as means ± standard errors of the means for quadruplicate samples. Experiments were performed using different donors on different days. The Student t test with a Bonferroni correction was used to determine statistical significance in pairwise comparisons, and a P value of <0.05 was considered statistically significant.
RESULTS
Exposure to C. neoformans induces an initial decrease in the perforin content and results in mobilization of LAMP-1 to the cell surface of primary NK cells.
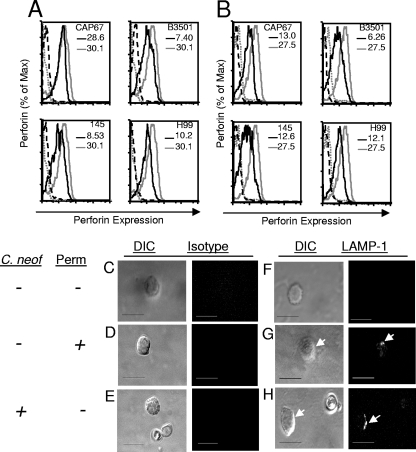
To determine whether there is a loss of perforin expression in response to C. neoformans, primary NK cells were incubated with various strains of C. neoformans, including strains CAP67 (an acapsular mutant), B3501 (serotype D and the parent of CAP67), 145 (serotype A), and H99 (serotype A). The NK cells were incubated with the strains of C. neoformans for 24 h (Fig. 1A) and 48 h (Fig. 1B). Regardless of the strain utilized, there was a decrease in intracellular perforin labeling compared to that in NK cells incubated in media alone. The decrease in the perforin content was observed at 24 h and was sustained after 48 h in the presence of the fungus.
FIG. 1.
Multiple strains of C. neoformans induce loss of the perforin protein in primary NK cells and expose LAMP-1 on the cell surface. NK cells were incubated with C. neoformans strains CAP67, B3501, 145, and H99 for 24 h (A) or 48 h (B) at an E/T ratio of 1:100. NK cells alone were incubated with isotype-matched antibody (negative control) (dashed gray line) or with anti-perforin-FITC antibody (solid gray line), or NK cells were incubated with a strain of C. neoformans and labeled with an isotype-matched antibody (dashed black line) or with anti-perforin-FITC antibody (black line). The MFI for cells with and without C. neoformans are indicated in each panel and reflect the intracellular NK cell perforin levels. (C to H) NK cell degranulation was analyzed by labeling LAMP-1 exposed at the cell surface. Primary NK cells were labeled with an isotype-matched antibody (negative control) when they were not treated (C), permeablized (D), or in the presence of C. neoformans (E), or they were labeled with an anti-LAMP-1-FITC antibody after no treatment (F) or after permeablization (positive control) (G), or they were incubated with C. neoformans strain CAP67 at an E/T ratio of 1:10 for 48 h in the presence of monensin and an anti-LAMP-1-FITC antibody (H). The panels on the left show differential interference contrast (DIC) images, while the panels on the right show either isotype labeling or LAMP-1 expression. NK cell LAMP-1 labeling is indicated by arrows. Bars = 10 μm. The results are representative results of three experiments in which similar results were obtained. C. neof, C. neoformans; Perm, permeablized.
The most likely explanation for the loss of perforin in the presence of C. neoformans is release of perforin stores via degranulation. To determine if NK cells degranulated in response to fungal stimulation, NK cells were exposed to C. neoformans strain CAP67 for 48 h in the presence of an anti-LAMP-1-FITC antibody. When granules fuse with the extracellular membrane in the degranulation process, LAMP-1 is exposed on the surface of a NK cell, allowing an anti-LAMP-1 antibody to bind. The antibody then becomes internalized as the organelle is recycled, and monensin prevents quenching of the fluorophore in the acidic compartments. Thus, the presence of fluorescence suggests that degranulation of NK cells has occurred. NK cells that had been exposed to C. neoformans were compared to cells that had not been exposed to this organism. The anti-LAMP-1 antibody was bound by NK cells after incubation with C. neoformans (Fig. 1H), while it was not bound by NK cells that had not been incubated with C. neoformans (Fig. 1F), indicating that LAMP-1 had been expressed on the cell surface and that the cells had undergone degranulation. As a positive control, NK cells were made permeable and labeled with anti-LAMP-1, which labeled intracellular LAMP-1 (Fig. 1G). Additionally, NK cells that were incubated with an isotype control antibody either alone (Fig. 1C), after they were made permeable (Fig. 1D), or while they were being incubated with C. neoformans (Fig. 1E) did not exhibit fluorescence and served as negative controls.
Preincubating primary NK cells with C. neoformans enhances anticryptococcal activity.
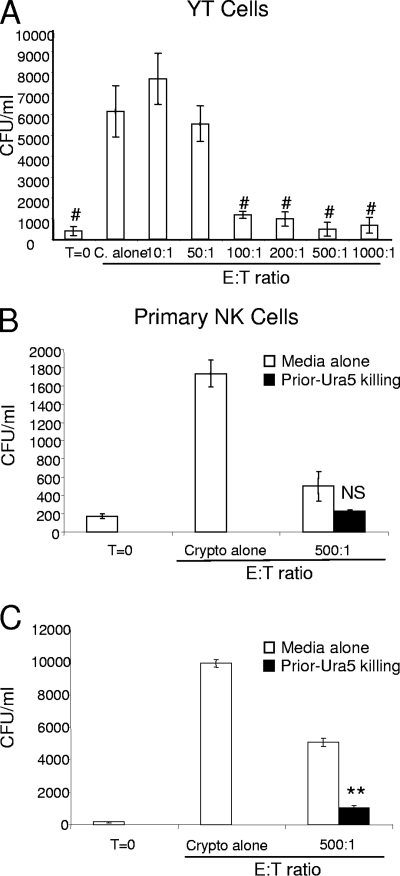
NK cells can serially kill multiple tumor targets; however, this process eventually results in exhaustion and diminished antitumor activity. The reduced antitumor activity is accompanied by a reduction in perforin labeling (3). To determine if the original reduction in NK cell perforin labeling was accompanied by a decrease in anticryptococcal activity, a cryptococcal auxotroph was employed. This enabled us to stimulate the NK cells and assess the killing of a second strain without having to completely remove the stimulating strain. We first needed to demonstrate that the uracil-dependent auxotroph of C. neoformans (Ura5) was a susceptible target. We accomplished this by analyzing the numbers of Ura5 CFU in the presence of increasing numbers of cells of an NK cell line (YT cells). As expected, YT cells displayed significant anticryptococcal activity against Ura5 with increasing E/T ratios (Fig. 2A). Under the same conditions, primary NK cells respond to Ura5, causing a reduction in NK cell perforin labeling (data not shown).
FIG. 2.
Preincubation of NK cells with a C. neoformans auxotroph resulted in enhanced killing of a second strain of C. neoformans. (A) YT cells were incubated with C. neoformans strain Ura5 for 48 h at different E/T ratios, and Ura5 viability analyzed by determining the number of CFU. T=0, zero time; C. alone, incubation in the absence of NK cells. (B and C) Primary NK cells were preincubated either with Ura5 at an E/T ratio of 1:10 (Prior-Ura5 killing) or in media alone. The Ura5 strain was then removed at 48 h, and CAP67 was added. The numbers of CFU were determined at zero time and at 24 h (B) and 48 h (C) in the absence (Crypto alone) or presence (500:1) of NK cells. The results are representative of the results of two experiments performed in quadruplicate in which similar results were obtained. #, P < 0.01 compared to C. neoformans alone; NS, not significant; **, P < 0.01 compared to media alone.
To determine if NK cells display enhanced or reduced fungal killing following exposure to C. neoformans, NK cells were incubated with Ura5 before they were incubated with the uracil-independent strain CAP67. This allowed us to assess the NK cell killing of CAP67 cells at various times following killing of cells of the primary Ura5 strain. Primary NK cells were incubated with Ura5 for 48 h at an E/T ratio of 1:10 in order to ensure maximal NK cell stimulation. The majority of Ura5 cells were then removed by Ficoll-Hypaque density gradient centrifugation (the numbers of Ura5 cells were reduced 100-fold). Subsequently, CAP67 was added at an E/T ratio of 500:1 and incubated for another 24 and 48 h. Surprisingly, despite the high numbers of Ura5 organisms, which ought to have depleted the anticryptococcal activity, the NK cells remained microbicidal for cryptococci at 24 h (Fig. 2B). Furthermore, the activity with the uracil-independent strain increased after 48 h (Fig. 2C), indicating that there was significant activation and potentiation of the microbicidal activity in response to the pathogen. It is important to note that no cytokines were added to the media to stimulate the NK cells.
NK cell anticryptococcal activity requires a functional Golgi apparatus.
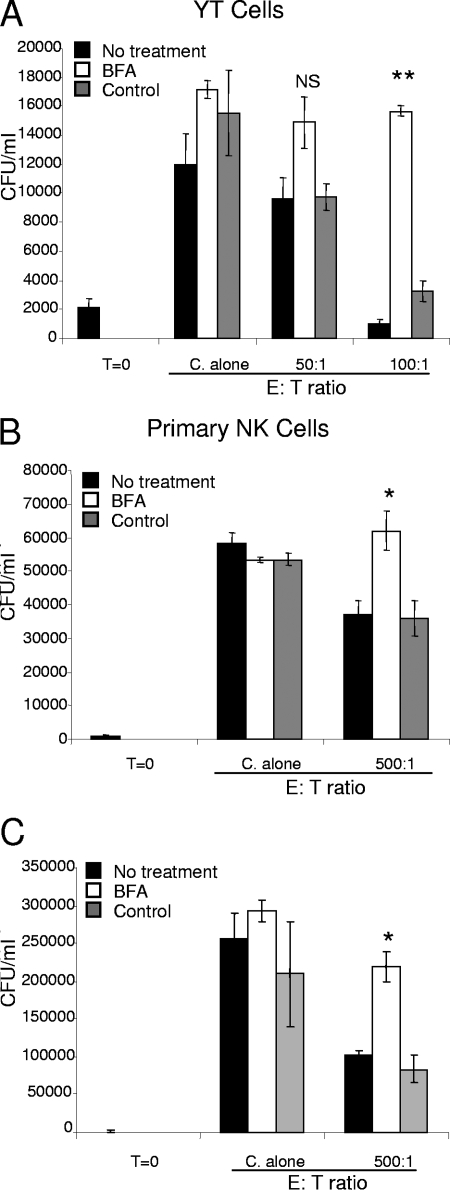
We were interested in determining the process responsible for the enhanced killing. To determine whether de novo proteins were required for NK cell anticryptococcal activity, NK cells were treated with BFA before incubation with C. neoformans. BFA is a fungal metabolite that disrupts the Golgi complex, effectively trapping new proteins in the endoplasmic reticulum (ER) and preventing the proteins from being secreted. Importantly, BFA does not affect the release of preformed NK cell granules, allowing the release of perforin stores (18). Thus, BFA allows the initial round of killing but prevents cytokine release and/or rearming of effector molecules. Here, we demonstrated that YT cell anticryptococcal activity was reduced at 48 h following treatment with BFA (Fig. 3A). Likewise, NK cells displayed reduced anticryptococcal activity after preincubation with BFA at both 24 and 48 h (Fig. 3B and C). Neither the viability of the YT cells nor the viability of the NK cells was affected by BFA, as determined by trypan blue exclusion. This suggests that de novo-synthesized proteins are required for NK cell anticryptococcal activity.
FIG. 3.
BFA blocked the ability of YT and NK cells to kill C. neoformans. YT cells (A) or primary NK cells (B and C) were preincubated with either BFA (10 μg/ml), media alone, or ethanol as a solvent control for 24 h. The cells were washed three times, and C. neoformans was added along with either media alone (No treatment), 0.5 μg/ml of BFA (BFA), or ethanol (Control). The numbers of C. neoformans CFU were determined 24 h (B) or 48 h (A and C) later. The results are representative of the results of three experiments performed in quadruplicate in which similar results were obtained. NS, not significant compared to either no treatment or the control for each E/T ratio; *, P < 0.05 compared to either no treatment or the control; **, P < 0.01 compared to either no treatment or the control. C. alone, incubation of C. neoformans in the absence of NK cells. T=0, zero time.
Fungal contact, not a cytokine, enhances NK cell anticryptococcal activity.
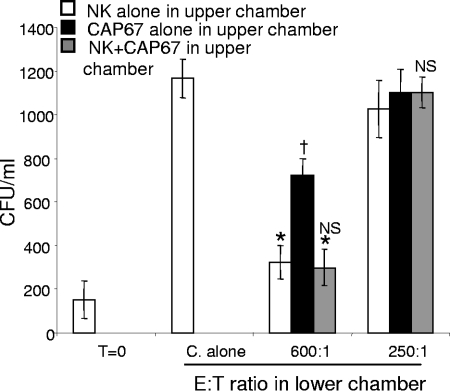
NK cells have the capacity to regulate their own activity by releasing immunity-modulating cytokines into the environment. Specifically, C. neoformans can induce the expression of cytokines in NK cells, including IFN-γ (33). To determine if a soluble factor, such as a cytokine, was released after fungal contact, resulting in enhanced NK cell anticryptococcal activity, NK cells were placed in the bottom chamber of a well separated from the top of the well by a polyethylene terephthalate membrane having 1.0-μm pores. Either NK cells alone, CAP67 cells alone, or both NK cells and CAP67 cells at an E/T ratio of 1:10 were placed in the upper chamber. This system allowed soluble factors to diffuse across the membrane and stimulate the NK cells in the bottom of the well, while preventing the NK cells and/or C. neoformans cells in the upper chamber from making contact with the cells in the lower chamber. Two E/T ratios were used, a lower ratio that allowed us to observe low levels of activity in a setting where activity is not normally present and a higher ratio that allowed us to observe augmented NK cell activity under these conditions. At the lower E/T ratio, at which unstimulated anticryptococcal activity was not observed, there was no evidence that a soluble factor initiated killing. Additionally, at the higher E/T ratio, at which anticryptococcal activity was observed, there was no evidence that killing was enhanced (Fig. 4). Moreover, when only C. neoformans was in the top chamber, not only did NK cells in the bottom chamber fail to exhibit increased anticryptococcal activity, but also they showed reduced anticryptococcal activity compared to the wells with NK cells alone in the top chamber. This suggests that C. neoformans released soluble factors that were able to cross the membrane to inhibit NK cell anticryptococcal activity (Fig. 4). This suggests that secretion of a soluble factor, such as a cytokine, is not responsible for the observed enhancement of NK cell anticryptococcal activity. Therefore, the enhanced anticryptococcal activity is most consistent with a mechanism whereby direct contact with the organism causes activation.
FIG. 4.
Enhanced NK cell anticryptococcal activity was not due to a soluble factor. Anticryptococcal activity of NK cells was assessed in the bottom chamber of a 24-well plate separated from the top chamber by a 1.0-μm membrane. NK cells alone, CAP67 cells alone, or NK cells with CAP67 at an E/T ratio of 1:10 were added to the top chamber for 48 h. The NK cells were then harvested from the bottom chamber and incubated with C. neoformans at E/T ratios of 600:1 and 250:1 for 24 h before the anticryptococcal activity was assessed. The results are representative of the results of two experiments performed in quadruplicate in which similar results were obtained. *, P < 0.05 compared to Cryptococcus alone (C. alone); NS, not significant compared to NK alone in the upper chamber for each E/T ratio; †, P < 0.05 for CAP67 alone in upper chamber compared to NK alone in upper chamber. T=0, zero time.
NK cells increase perforin transcription following fungal contact.
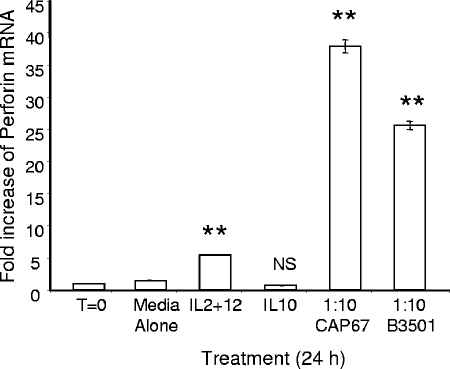
After establishing that NK cells kill in a perforin-dependent manner (37), that de novo-synthesized proteins are required for optimal killing, and that enhanced killing occurs independent of release of a soluble mediator, such as a cytokine, we hypothesized that NK cells rearmed perforin in response to fungal exposure in order to augment their anticryptococcal activity. In order to determine whether perforin is transcribed in NK cells following fungal contact, real-time PCR was performed. Two strains of fungi, strains CAP67 and B3501, were incubated with NK cells for 24 h. The perforin mRNA levels in NK cells exposed to C. neoformans were compared to the levels in NK cells in media alone (negative control), in NK cells stimulated with IL-10 (negative control), and in NK cells stimulated with IL-2 plus IL-12 (positive control), a combination that is well documented to enhance perforin production (9). Media alone and stimulation with IL-10 had no effect on NK cell perforin mRNA levels, as expected (Fig. 5). In contrast, there was a fivefold increase in NK cell perforin levels following stimulation with IL-2 plus IL-12 (Fig. 5). Surprisingly, the perforin mRNA levels increased 25- to 35-fold following incubation with C. neoformans (Fig. 5); thus, the stimulation by this treatment was far greater than the stimulation by IL-2 plus IL-12, demonstrating that C. neoformans is a potent inducer of perforin transcription.
FIG. 5.
NK cell perforin mRNA levels increased after contact with C. neoformans. NK cells were preincubated for 24 h in the presence of media alone, IL-2 plus IL-12 (10 ng/ml each), IL-10 (10 ng/ml), or CAP67 or B3501 at an E/T ratio of 1:10. The NK cell mRNA was harvested, converted to cDNA, and amplified by real-time PCR. The increases are increases compared to the perforin mRNA levels of freshly isolated primary NK cells (T=0). Perforin mRNA levels were normalized to GAPDH levels. The results are representative of the results of four experiments performed in triplicate in which similar results were obtained. **, P < 0.01 compared to NK cells in media alone; NS, not significant.
NK cell perforin labeling is reduced in the presence of the transcriptional inhibitor actinomycin D.
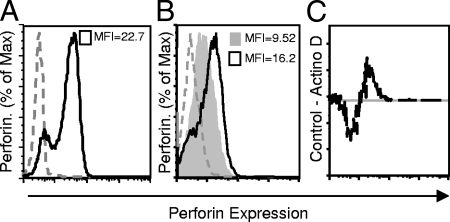
To determine if newly transcribed perforin contributes to perforin protein levels during fungal exposure, an inhibitor of transcription, actinomycin D, was used. If perforin were translated during contact with C. neoformans, we would expect greater loss of perforin labeling in the presence of the inhibitor than in its absence. NK cells were preincubated with actinomycin D at a concentration of 1 μg/ml. After 1 h, CAP67 was added to the NK cells and incubated for an additional 24 h. Intracellular perforin was labeled with anti-perforin-FITC antibodies, and the mean fluorescence intensity (MFI) of NK cells exposed to C. neoformans was compared to that of NK cells in media alone. The NK cells expressed perforin immediately following isolation (Fig. 6A). NK cells pretreated with actinomycin D during cryptococcal killing displayed lower levels of perforin labeling than NK cells incubated with C. neoformans in complete media lacking actinomycin D (Fig. 6B). Using the Flowjo population comparison software, these populations were determined to be statistically distinct with a confidence interval greater than 99.9% using the Kolmolgorov-Smirnov test. A subtraction plot for Fig. 6B is shown in Fig. 6C. The viability of the NK cells following 24 h of incubation with actinomycin D was greater than 90%. These results suggest that newly transcribed perforin is used to rearm the perforin stores of NK cells during anticryptococcal activity.
FIG. 6.
Inhibiting transcription increased the rate of perforin loss from NK cells after fungal contact. (A) Primary NK cells were freshly isolated, labeled for perforin expression (black line), and compared to the isotype control (dashed gray line) at zero time. (B) NK cells were pretreated with either actinomycin D (1 μg/ml) (shaded area) or media alone (black line) for 1 h before CAP67 was added at an E/T ratio of 1:100 and the preparations were incubated for another 24 h. The NK cells were harvested, made permeable, and labeled with anti-perforin antibody. The MFI is indicated in each panel. (C) Comparison of populations for actinomycin D and medium control performed using the Flowjo population comparison software: plot of the difference. The line is the difference plot for the two populations. The difference was determined to be statistically significant with a confidence interval greater than 99.9% using the Kolmolgorov-Smirnov test. The experiment was repeated, and similar results were obtained.
Strontium chloride treatment depletes perforin and abrogates NK cell anticryptococcal activity, but the anticryptococcal activity of NK cells can be restored by subsequent exposure to fungi.
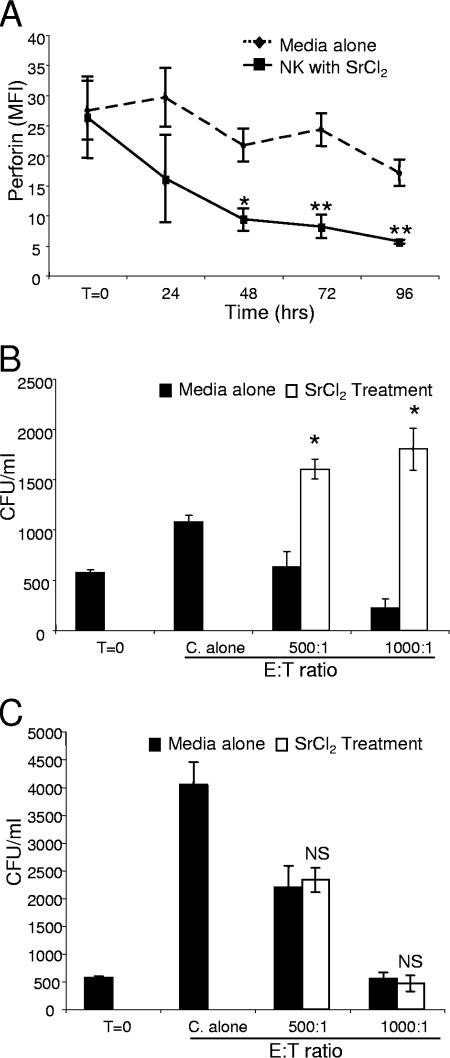
The data described above indicate that enhanced NK cell anticryptococcal activity correlates with de novo perforin production. However, it was not clear if the augmented killing was due to a direct signaling pathway induced by C. neoformans independent of degranulation or if it was simply due to direct association of the signaling involved in degranulation. To determine if NK cell degranulation can provide the signal to subsequently enhance NK cell anticryptococcal activity, 25 mM SrCl2 was used to induce general NK cell degranulation. In the presence of SrCl2, NK cell perforin labeling was significantly reduced after 48, 72, and 96 h compared to the labeling in NK cells incubated in media alone (Fig. 7A). Degranulation following treatment with SrCl2 was confirmed by LAMP-1 labeling (data not shown).
FIG. 7.
NK cells lost the ability to kill C. neoformans after strontium chloride treatment, but the ability to kill was restored after subsequent exposure to the fungus. (A) NK cells were incubated with SrCl2 or media alone for 0 to 96 h and then labeled for perforin expression with an anti-perforin-FITC antibody. The MFI was determined at various times. (B and C) Primary NK cells were preincubated with SrCl2 or in media alone for 48 h, the SrCl2 was removed, CAP67 was added, and the preparation was incubated for another 24 h (B) or 48 h (C) before the number of CFU was determined. The results are representative of the results of two to six experiments in which similar results were obtained. T=0, zero time; C. alone, incubation of C. neoformans in the absence of NK cells. *, P < 0.05 compared to media alone; **, P < 0.01 compared to media alone.
To determine the effect of degranulation on NK cell anticryptococcal activity, NK cells were treated with SrCl2 for 48 h. The NK cells were then washed and incubated with CAP67 to observe the effect on NK cell anticryptococcal activity at both 24 and 48 h. NK cells exhibited reduced anticryptococcal activity at 24 h, so the growth of C. neoformans was similar to, or even greater than, the growth in the presence of untreated NK cells (Fig. 7B), which correlated with reduced perforin labeling due to SrCl2 treatment (Fig. 7A). Thus, NK cells were unable to rearm and maintain anticryptococcal activity in response to degranulation. However, incubation of NK cells with CAP67 for 48 h following SrCl2 treatment resulted in restoration of NK cell anticryptococcal activity (Fig. 7C). These data suggest that the degranulation process alone is not sufficient to initiate the signaling that leads to the enhanced anticryptococcal activity and that C. neoformans is required to stimulate NK cell rearming.
Preincubation of NK cells with C. neoformans reduces subsequent NK cell antitumor activity.
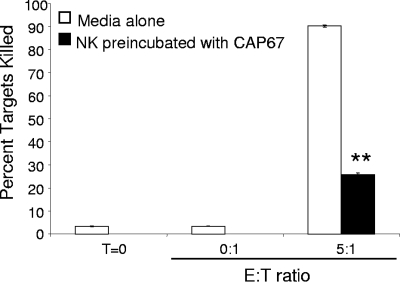
NK cells utilize perforin to kill many different types of target cells in addition to C. neoformans. One such target is the NK cell-sensitive tumor cell line K562 (16). We wondered whether the processes that lead to increased anticryptocococcal activity also augment tumor cell killing. Since CAP67 induces the greatest increase in the perforin mRNA level (Fig. 5), NK cells were preincubated with CAP67 for 48 h at an E/T ratio of 1:10. After 48 h, the cells were subjected to Ficoll-Hypaque density gradient centrifugation, which removed most of the CAP67 (there was a 100-fold reduction). The NK cells were then incubated with K562 cells labeled with CFSE to distinguish them from NK cells for an additional 4 h. Tumor killing by NK cells previously exposed to C. neoformans was compared to killing by NK cells in media alone. NK cells in media alone were able to kill K562 cells very efficiently, and over 90% of the target cells were positive for the membrane-permeable viability stain 7-AAD (Fig. 8). In contrast, NK cells that were preincubated with C. neoformans for 48 h had a drastically reduced capacity to kill K562 tumor target cells (25% of the target cells were positive for 7-AAD). This demonstrates that incubation of NK cells with C. neoformans augments subsequent killing of C. neoformans but does not enhance tumor killing. These data suggest that additional, microbe-specific mechanisms are employed in conjunction with de novo perforin production to enhance NK cell anticryptococcal activity.
FIG. 8.
NK cells lost the ability to kill K562 tumor targets after fungal stimulation. NK cells were incubated with CAP67 for 48 h at an E/T ratio of 1:10. CAP67 was removed by Ficoll-Hypaque density gradient centrifugation, and the NK cells were incubated with CFSE-labeled K562 cells for an additional 4 h. The level of viability of the CFSE-labeled K562 cells was assessed using the 7-AAD viability dye and flow cytometry. Killing of K562 cells by NK cells following preincubation with C. neoformans was compared to killing of NK cells incubated for 48 h in media alone. Each bar indicates the mean percentage of dead cells. The results are representative of the results of four experiments performed in triplicate in which similar results were obtained. **, P < 0.01 compared to media alone. T=0, zero time.
DISCUSSION
In these investigations, we made the following observations. (i) NK cells incubated with C. neoformans degranulate and loose perforin. However, despite degranulation, they exhibit enhanced anticryptococcal activity following exposure to C. neoformans. (ii) New proteins that traffic through the ER to the Golgi apparatus are required for the enhanced NK cell anticryptococcal activity. However, we were unable to demonstrate a role for the release of soluble proteins (such as cytokines) that can enhance the anticryptococcal activity, suggesting that cell contact provides the dominant signal. (iii) NK cells exhibit a marked increase in perforin transcription after exposure to C. neoformans, and inhibition of transcription results in a greater decrease in the perforin content. (iv) Exposure to C. neoformans can restore the defective anticryptococcal activity induced by nonspecific degranulation in response to SrCl2. (v) Exposure to C. neoformans enhances anticryptococcal activity but suppresses the cytotoxic activity against tumor targets.
Previous studies demonstrated that NK cells kill C. neoformans in a perforin-dependent manner (37). In this study we were interested in determining if, in addition to killing C. neoformans in a perforin-dependent manner, NK cells were capable of killing a second target and, if so, if perforin is involved in this process. Initially, we observed a decrease in NK cell perforin labeling following exposure to C. neoformans, consistent with findings suggesting that NK cells kill C. neoformans in a perforin-dependent manner (37). This loss of perforin labeling could have been a result of either degranulation of the NK cells in response to the fungus, inhibition of perforin synthesis, or enhanced degradation of perforin within the NK cells in response to the organism. LAMP-1 expression following fungal contact verified that NK cells in fact degranulated in response to the fungus.
Exposure to target cells, such as C. neoformans cells, had the potential to either activate or inhibit subsequent NK cell anticryptococcal activity. Recent studies of NK cell antitumor activity have established that NK cells become “exhausted” during prolonged exposure to tumor cells, resulting in loss of antitumor activity. This loss of NK cell lytic capacity correlated with a decrease in intracellular perforin levels, and the capacity could be restored only by addition of exogenous IL-2 (3). Furthermore, previous experiments have demonstrated that heat-killed C. neoformans cells and their products inhibit direct lymphocyte-mediated fungistasis (34). Therefore, we hypothesized that like antitumor activity, diminished NK cell perforin levels result in decreased NK cell anticryptococcal activity. Surprisingly, preincubation of NK cells with live C. neoformans had the opposite effect, enhancing subsequent anticryptococcal activity.
While this is the first time that enhanced NK cell killing has been observed with a microbial target, NK cells have previously exhibited priming for tumor cell targets. After preincubation of NK cells with a fixed tumor target, the cells were able to kill a second tumor target more efficiently than if they had not been preincubated. This enhancement was dependent on both secretion of IFN-γ and increased perforin production (8). We were therefore interested in determining if de novo protein production in general was important for the enhanced anticryptococcal activity. Both newly formed cytokines and perforin traffic through the ER to the Golgi apparatus before they are packaged into the appropriate vesicle for secretion. Therefore, experiments were performed to determine if de novo protein trafficking from the ER to the Golgi apparatus was necessary for NK cell anticryptococcal activity. To answer this question, we used BFA, a fungal metabolite that prevents the release of newly formed proteins by disrupting the Golgi apparatus, trapping proteins in the ER without inhibiting the release of preformed granules (18). We demonstrated that BFA reduced the anticryptococcal activity of NK cells, suggesting that NK cells require new protein trafficking through the ER for optimal anticryptococcal activity.
To assist in identifying the de novo proteins required for enhanced NK cell anticryptococcal activity, we were interested in determining whether the proteins were soluble, acting in an autocrine or paracrine fashion to augment NK cell anticryptococcal activity. NK cells have the ability to release many different cytokines, including IL-10, IFN-γ, granulocyte-macrophage colony-stimulating factor, tumor necrosis factor alpha, and IL-13, following target recognition (7, 36, 45). We were unable to obtain evidence that the factors responsible for the enhanced NK cell anticryptococcal activity were soluble, as supernatants from NK cells incubated with C. neoformans did not increase the activity of other NK cells. Thus, in contrast to the previous studies assessing antitumor activity, enhanced anticryptococcal activity was not dependent on secretion of a soluble factor, suggesting that the mechanism for enhanced anticryptococcal activity is a direct result of contact of the NK cells with C. neoformans.
Since NK cells are critically dependent on perforin to kill C. neoformans, we wanted to determine if the enhanced killing may be explained by increased perforin production following exposure to C. neoformans. We were surprised by the remarkable extent to which perforin mRNA levels increased in NK cells when they were stimulated by C. neoformans. In fact, the amount of perforin produced in response to C. neoformans was much greater than the amount produced in response to the well-established stimulation with IL-2 plus IL-12. In addition, the significance of new perforin mRNA production was evident, as actinomycin D-treated NK cells displayed a greater reduction in the perforin content in the presence of C. neoformans than untreated cells displayed. Overall, these data demonstrate that new perforin transcription restores the NK cell perforin pool during concurrent release in response to the fungus. Thus, the balance between de novo perforin production and perforin degranulation provides an explanation for the slow and modest reduction in perforin levels observed during cryptococcal killing. Additionally, we speculate that the source of the rearming signal is localized to the fungal cell wall. While the encapsulated strain B3501 was able to induce perforin transcription, the acapsular strain CAP67 induced greater perforin transcription. By sequestering the cell wall, the capsule may limit exposure of the NK cells to the rearming signal, resulting in reduced induction of perforin transcription by encapsulated fungal strains.
As exposure of NK cells to both C. neoformans and tumor targets enhances subsequent NK cell activity (albeit by different mechanisms), we were interested in knowing whether the process of degranulation itself could trigger enhanced NK cell anticryptococcal activity. To determine if the effect was due to degranulation, NK cells were treated with SrCl2, a chemical that induces degranulation of NK cells (47). After degranulation using SrCl2 there was a drastic reduction in the perforin content and a corresponding reduction in NK cell anticryptococcal activity at 24 h. However, in the presence of C. neoformans over the subsequent 48 h, NK cells recovered their anticryptococcal activity, suggesting that C. neoformans is required to activate the pathway involved in enhanced killing of the fungus. We acknowledge the possibility that NK cells spontaneously rearm following the removal of SrCl2; however, it is likely that C. neoformans increased the rate of rearming. NK cells induced to degranulate with C. neoformans possessed observable anticryptococcal activity 24 h after addition of the target fungus, while NK cells treated with SrCl2 did not. This indicates that there is a mechanism whereby C. neoformans induces perforin production along with degranulation, while SrCl2 induces only degranulation and the NK cells must rearm in order to have subsequent cytotoxic activity. This suggests that degranulation is not sufficient to enhance NK cell anticryptococcal activity and that C. neoformans provides an additional signal to maintain the ability to kill the fungus.
Perforin not only is utilized by NK cells to kill C. neoformans but also is employed to kill multiple types of targets. These targets include tumor targets, such as K562 cells (12, 28, 37). Due to our observation that perforin mRNA levels increased after fungal contact, we hypothesized that NK cells may also acquire enhanced tumor cytolytic activity following exposure to C. neoformans. We were disappointed to see not only that NK cells failed to show enhanced antitumor activity but also that the NK cell antitumor activity was significantly impaired. Interestingly, these data suggest that restoring the level of perforin alone is not sufficient to enhance anticryptococcal activity and that additional, C. neoformans-specific mechanisms must be involved. These data, along with the results of experiments using SrCl2, indicate that the specific NK cell interaction with C. neoformans is required to elicit enhanced anticryptococcal activity. We therefore speculate that C. neoformans interacts through recruitment of a fungus-specific receptor to the synapse, leading to receptor clustering, enhanced fungal recognition, and an increased rate of NK cell signaling and finally resulting in enhanced fungal killing. Consequently, the fungus-specific receptors may utilize receptor components essential for tumor recognition, making them unavailable for tumor-specific killing. Alternatively, it is possible that the localization of fungus-specific receptors to the microbial synapse excludes the tumor-specific receptors. The localization of one receptor to a ligation site can exclude other receptors. For example, ligation of the inhibitory CD94/NKG2A receptor complex excludes lipid rafts and consequently lipid raft-associated receptors from a synapse (52). Additionally, it is possible that the interaction with C. neoformans induces the ligand, resulting in enhanced fungal binding. Alternatively, while restoring perforin may be sufficient to maintain fungal killing, additional cytolytic molecules may have to be restored to maintain tumor killing. Perforin colocalizes with other cytolytic molecules in their lytic granules, including granzymes (26, 48, 56). Besides inducing lysis of target cells directly, perforin also aids entry of granzymes into the cell, resulting in apoptosis (5, 11, 24, 25, 38). Therefore, the initial loss of cytolytic effector molecules, accompanied by a selective rearming of perforin without rearming of other cytolytic effectors, may be insufficient for tumor killing.
The specificity of the NK cell activation and rearming may also have implications for knowledge translation to clinical practice. The data resulted in a prediction that microbicidal immunopotentiation in response to microbes is more potent than cytokine therapy as cytokines should not increase cytolytic molecule expression to the same extent as direct fungal contact. Additionally, the current results predict that NK cells directed toward an infection lose their capacity for host defense against tumors and predispose the host to tumor development. In this regard, it is interesting that infection can be a risk factor for tumor development (10, 13, 27, 57).
In conclusion, we have identified microbe-specific activation of NK cell microbicidal activity that is necessary for enhanced killing of C. neoformans. A critical element of this process is the rearming of perforin stores.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) (C.H.M.), the Canadian Foundation for AIDS research (C.H.M.), and the Jessie Bowden Lloyd Professorship in Immunology (C.H.M.) and by an equipment and infrastructure grant from the Canadian Foundation for Innovation and the Alberta Science and Research Authority. Immunofluorescence microscopy was performed in the Microscopy Core supported by a CIHR-funded grant for leukocyte trafficking with the valuable assistance of Pina Colarusso. K.J.M. is a trainee in the CIHR Training Program in Immunology, Immunopathogenesis and Inflammation, University of Calgary. C.H.M. is a Senior Scholar of the Alberta Heritage Foundation for Medical Research.
Editor: A. Casadevall
Footnotes
Published ahead of print on 23 March 2009.
REFERENCES
- 1.Abe, K., J. Kadota, Y. Ishimatsu, T. Iwashita, K. Tomono, K. Kawakami, and S. Kohno. 2000. Th1-Th2 cytokine kinetics in the bronchoalveolar lavage fluid of mice infected with Cryptococcus neoformans of different virulences. Microbiol. Immunol. 44849-855. [DOI] [PubMed] [Google Scholar]
- 2.Albright, J. W., F. M. Hatcher, and J. F. Albright. 1984. Interaction between murine natural killer cells and trypanosomes of different species. Infect. Immun. 44315-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhat, R., and C. Watzl. 2007. Serial killing of tumor cells by human natural killer cells—enhancement by therapeutic antibodies. PLoS ONE 2e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnema, J. D., L. M. Karnitz, R. A. Schoon, R. T. Abraham, and P. J. Leibson. 1994. Fc receptor stimulation of phosphatidylinositol 3-kinase in natural killer cells is associated with protein kinase C-independent granule release and cell-mediated cytotoxicity. J. Exp. Med. 1801427-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browne, K. A., E. Blink, V. R. Sutton, C. J. Froelich, D. A. Jans, and J. A. Trapani. 1999. Cytosolic delivery of granzyme B by bacterial toxins: evidence that endosomal disruption, in addition to transmembrane pore formation, is an important function of perforin. Mol. Cell. Biol. 198604-8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerboni, C., A. Gismondi, G. Palmieri, M. Piccoli, L. Frati, and A. Santoni. 1998. CD16-mediated activation of phosphatidylinositol-3 kinase (PI-3K) in human NK cells involves tyrosine phosphorylation of Cbl and its association with Grb2, Shc, pp36 and p85 PI-3K subunit. Eur. J. Immunol. 281005-1015. [DOI] [PubMed] [Google Scholar]
- 7.Cooper, M. A., T. A. Fehniger, S. C. Turner, K. S. Chen, B. A. Ghaheri, T. Ghayur, W. E. Carson, and M. A. Caligiuri. 2001. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood 973146-3151. [DOI] [PubMed] [Google Scholar]
- 8.Das, S., C. Varalakshmi, A. L. Kumari, M. Patel, and A. Khar. 2001. Target cell induced activation of NK cells in vitro: cytokine production and enhancement of cytotoxic function. Cancer Immunol. Immunother. 50428-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeBlaker-Hohe, D. F., A. Yamauchi, C. R. Yu, J. A. Horvath-Arcidiacono, and E. T. Bloom. 1995. IL-12 synergizes with IL-2 to induce lymphokine-activated cytotoxicity and perforin and granzyme gene expression in fresh human NK cells. Cell. Immunol. 16533-43. [DOI] [PubMed] [Google Scholar]
- 10.Demir, K., F. Akyuz, A. Poyanli, and A. Okten. 2006. An asymptomatic huge hepatocellular carcinoma with intra-atrial tumor thrombus in a patient with chronic hepatitis B viral infection. Turk. J. Gastroenterol. 17203-205. [PubMed] [Google Scholar]
- 11.Fellows, E., S. Gil-Parrado, D. E. Jenne, and F. C. Kurschus. 2007. Natural killer cell-derived human granzyme H induces an alternative, caspase-independent cell-death program. Blood 110544-552. [DOI] [PubMed] [Google Scholar]
- 12.Godal, R., U. Keilholz, L. Uharek, A. Letsch, A. M. Asemissen, A. Busse, I. K. Na, E. Thiel, and C. Scheibenbogen. 2006. Lymphomas are sensitive to perforin-dependent cytotoxic pathways despite expression of PI-9 and overexpression of bcl-2. Blood 1073205-3211. [DOI] [PubMed] [Google Scholar]
- 13.Goldschmidt, M. H., J. S. Kennedy, D. R. Kennedy, H. Yuan, D. E. Holt, M. L. Casal, A. M. Traas, E. A. Mauldin, P. F. Moore, P. S. Henthorn, B. J. Hartnett, K. I. Weinberg, R. Schlegel, and P. J. Felsburg. 2006. Severe papillomavirus infection progressing to metastatic squamous cell carcinoma in bone marrow-transplanted X-linked SCID dogs. J. Virol. 806621-6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hidore, M. R., and J. W. Murphy. 1989. Murine natural killer cell interactions with a fungal target, Cryptococcus neoformans. Infect. Immun. 571990-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hidore, M. R., and J. W. Murphy. 1986. Natural cellular resistance of beige mice against Cryptococcus neoformans. J. Immunol. 1373624-3631. [PubMed] [Google Scholar]
- 16.Hiserodt, J., L. Britvan, and S. Targan. 1984. Studies on the mechanism of the human natural killer cell lethal hit: a new method for rapid physical isolation of lethally hit K562 targets and inhibition of cytolysis by reduced temperature or trypsin. Cell. Immunol. 8343-51. [DOI] [PubMed] [Google Scholar]
- 17.Hoag, K. A., M. F. Lipscomb, A. A. Izzo, and N. E. Street. 1997. IL-12 and IFN-gamma are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am. J. Respir. Cell Mol. Biol. 17733-739. [DOI] [PubMed] [Google Scholar]
- 18.Isaaz, S., K. Baetz, K. Olsen, E. Podack, and G. M. Griffiths. 1995. Serial killing by cytotoxic T lymphocytes: T cell receptor triggers degranulation, re-filling of the lytic granules and secretion of lytic proteins via a non-granule pathway. Eur. J. Immunol. 251071-1079. [DOI] [PubMed] [Google Scholar]
- 19.Jewett, A., and B. Bonavida. 1995. Target-induced anergy of natural killer cytotoxic function is restricted to the NK-target conjugate subset. Cell. Immunol. 16091-97. [DOI] [PubMed] [Google Scholar]
- 20.Jewett, A., and B. Bonavida. 1996. Target-induced inactivation and cell death by apoptosis in a subset of human NK cells. J. Immunol. 156907-915. [PubMed] [Google Scholar]
- 21.Jiang, K., B. Zhong, D. L. Gilvary, B. C. Corliss, E. Hong-Geller, S. Wei, and J. Y. Djeu. 2000. Pivotal role of phosphoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nat. Immunol. 1419-425. [DOI] [PubMed] [Google Scholar]
- 22.Jimenez, B. E., and J. W. Murphy. 1984. In vitro effects of natural killer cells against Paracoccidioides brasiliensis yeast phase. Infect. Immun. 46552-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones, G. J., J. C. Wiseman, K. J. Marr, S. Wei, J. Y. Djeu, and C. H. Mody. 2009. In contrast to anti-tumor activity, YT cell and primary NK cell cytotoxicity for Cryptococcus neoformans bypasses LFA-1. Int. Immunol. 21423-432. [DOI] [PubMed] [Google Scholar]
- 24.Keefe, D., L. Shi, S. Feske, R. Massol, F. Navarro, T. Kirchhausen, and J. Lieberman. 2005. Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity 23249-262. [DOI] [PubMed] [Google Scholar]
- 25.Kelly, J. M., N. J. Waterhouse, E. Cretney, K. A. Browne, S. Ellis, J. A. Trapani, and M. J. Smyth. 2004. Granzyme M mediates a novel form of perforin-dependent cell death. J. Biol. Chem. 27922236-22242. [DOI] [PubMed] [Google Scholar]
- 26.Kojima, Y., A. Kawasaki-Koyanagi, N. Sueyoshi, A. Kanai, H. Yagita, and K. Okumura. 2002. Localization of Fas ligand in cytoplasmic granules of CD8+ cytotoxic T lymphocytes and natural killer cells: participation of Fas ligand in granule exocytosis model of cytotoxicity. Biochem. Biophys. Res. Commun. 296328-336. [DOI] [PubMed] [Google Scholar]
- 27.Lax, A. J., and W. Thomas. 2002. How bacteria could cause cancer: one step at a time. Trends Microbiol. 10293-299. [DOI] [PubMed] [Google Scholar]
- 28.Lehmann, C., M. Zeis, and L. Uharek. 2001. Activation of natural killer cells with interleukin 2 (IL-2) and IL-12 increases perforin binding and subsequent lysis of tumour cells. Br J. Haematol. 114660-665. [DOI] [PubMed] [Google Scholar]
- 29.Leon, C., D. Nandan, M. Lopez, A. Moeenrezakhanlou, and N. E. Reiner. 2006. Annexin V associates with the IFN-gamma receptor and regulates IFN-gamma signaling. J. Immunol. 1765934-5942. [DOI] [PubMed] [Google Scholar]
- 30.Levitz, S. M. 1991. Activation of human peripheral blood mononuclear cells by interleukin-2 and granulocyte-macrophage colony-stimulating factor to inhibit Cryptococcus neoformans. Infect. Immun. 593393-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levitz, S. M., and M. P. Dupont. 1993. Phenotypic and functional characterization of human lymphocytes activated by interleukin-2 to directly inhibit growth of Cryptococcus neoformans in vitro. J. Clin. Investig. 911490-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levitz, S. M., M. P. Dupont, and E. H. Smail. 1994. Direct activity of human T lymphocytes and natural killer cells against Cryptococcus neoformans. Infect. Immun. 62194-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levitz, S. M., and E. A. North. 1996. gamma Interferon gene expression and release in human lymphocytes directly activated by Cryptococcus neoformans and Candida albicans. Infect. Immun. 641595-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levitz, S. M., E. A. North, M. P. Dupont, and T. S. Harrison. 1995. Mechanisms of inhibition of Cryptococcus neoformans by human lymphocytes. Infect. Immun. 633550-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lockyer, H. M., E. Tran, and B. H. Nelson. 2007. STAT5 is essential for Akt/p70S6 kinase activity during IL-2-induced lymphocyte proliferation. J. Immunol. 1795301-5308. [DOI] [PubMed] [Google Scholar]
- 36.Loza, M. J., S. P. Peters, J. G. Zangrilli, and B. Perussia. 2002. Distinction between IL-13+ and IFN-gamma+ natural killer cells and regulation of their pool size by IL-4. Eur. J. Immunol. 32413-423. [DOI] [PubMed] [Google Scholar]
- 37.Ma, L. L., C. L. Wang, G. G. Neely, S. Epelman, A. M. Krensky, and C. H. Mody. 2004. NK cells use perforin rather than granulysin for anticryptococcal activity. J. Immunol. 1733357-3365. [DOI] [PubMed] [Google Scholar]
- 38.Metkar, S. S., B. Wang, M. Aguilar-Santelises, S. M. Raja, L. Uhlin-Hansen, E. Podack, J. A. Trapani, and C. J. Froelich. 2002. Cytotoxic cell granule-mediated apoptosis: perforin delivers granzyme B-serglycin complexes into target cells without plasma membrane pore formation. Immunity 16417-428. [DOI] [PubMed] [Google Scholar]
- 39.Mody, C. H., G. H. Chen, C. Jackson, J. L. Curtis, and G. B. Toews. 1994. In vivo depletion of murine CD8 positive T cells impairs survival during infection with a highly virulent strain of Cryptococcus neoformans. Mycopathologia 1257-17. [DOI] [PubMed] [Google Scholar]
- 40.Mody, C. H., G. B. Toews, and M. F. Lipscomb. 1989. Treatment of murine cryptococcosis with cyclosporin-A in normal and athymic mice. Am. Rev. Respir. Dis. 1398-13. [DOI] [PubMed] [Google Scholar]
- 41.Mody, C. H., C. J. Wood, R. M. Syme, and J. C. Spurrell. 1999. The cell wall and membrane of Cryptococcus neoformans possess a mitogen for human T lymphocytes. Infect. Immun. 67936-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monari, C., E. Pericolini, G. Bistoni, A. Casadevall, T. R. Kozel, and A. Vecchiarelli. 2005. Cryptococcus neoformans capsular glucuronoxylomannan induces expression of fas ligand in macrophages. J. Immunol. 1743461-3468. [DOI] [PubMed] [Google Scholar]
- 43.Murphy, J. W., M. R. Hidore, and N. Nabavi. 1991. Binding interactions of murine natural killer cells with the fungal target Cryptococcus neoformans. Infect. Immun. 591476-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy, J. W., M. R. Hidore, and S. C. Wong. 1993. Direct interactions of human lymphocytes with the yeast-like organism, Cryptococcus neoformans. J. Clin. Investig. 911553-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy, J. W., A. Zhou, and S. C. Wong. 1997. Direct interactions of human natural killer cells with Cryptococcus neoformans inhibit granulocyte-macrophage colony-stimulating factor and tumor necrosis factor alpha production. Infect. Immun. 654564-4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nabavi, N., and J. W. Murphy. 1985. In vitro binding of natural killer cells to Cryptococcus neoformans targets. Infect. Immun. 5050-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neighbour, P. A., and H. S. Huberman. 1982. Sr++-induced inhibition of human natural killer (NK) cell-mediated cytotoxicity. J. Immunol. 1281236-1240. [PubMed] [Google Scholar]
- 48.Ojcius, D. M., L. M. Zheng, E. C. Sphicas, A. Zychlinsky, and J. D. Young. 1991. Subcellular localization of perforin and serine esterase in lymphokine-activated killer cells and cytotoxic T cells by immunogold labeling. J. Immunol. 1464427-4432. [PubMed] [Google Scholar]
- 49.Petkus, A. F., and L. L. Baum. 1987. Natural killer cell inhibition of young spherules and endospores of Coccidioides immitis. J. Immunol. 1393107-3111. [PubMed] [Google Scholar]
- 50.Raffeiner, B., C. Dejaco, C. Duftner, W. Kullich, C. Goldberger, S. C. Vega, M. Keller, B. Grubeck-Loebenstein, and M. Schirmer. 2005. Between adaptive and innate immunity: TLR4-mediated perforin production by CD28null T-helper cells in ankylosing spondylitis. Arthritis Res. Ther. 7R1412-R1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandusky, M. M., B. Messmer, and C. Watzl. 2006. Regulation of 2B4 (CD244)-mediated NK cell activation by ligand-induced receptor modulation. Eur. J. Immunol. 363268-3276. [DOI] [PubMed] [Google Scholar]
- 52.Sanni, T. B., M. Masilamani, J. Kabat, J. E. Coligan, and F. Borrego. 2004. Exclusion of lipid rafts and decreased mobility of CD94/NKG2A receptors at the inhibitory NK cell synapse. Mol. Biol. Cell 153210-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stenger, S., D. A. Hanson, R. Teitelbaum, P. Dewan, K. R. Niazi, C. J. Froelich, T. Ganz, S. Thoma-Uszynski, A. Melian, C. Bogdan, S. A. Porcelli, B. R. Bloom, A. M. Krensky, and R. L. Modlin. 1998. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282121-125. [DOI] [PubMed] [Google Scholar]
- 54.Syme, R. M., T. F. Bruno, T. R. Kozel, and C. H. Mody. 1999. The capsule of Cryptococcus neoformans reduces T-lymphocyte proliferation by reducing phagocytosis, which can be restored with anticapsular antibody. Infect. Immun. 674620-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamassia, N., F. Calzetti, N. Menestrina, M. Rossato, F. Bazzoni, L. Gottin, and M. A. Cassatella. 2008. Circulating neutrophils of septic patients constitutively express IL-10R1 and are promptly responsive to IL-10. Int. Immunol. 20535-541. [DOI] [PubMed] [Google Scholar]
- 56.Tordjmann, T., A. Soulie, C. Guettier, M. Schmidt, C. Berthou, M. Beaugrand, and M. Sasportes. 1998. Perforin and granzyme B lytic protein expression during chronic viral and autoimmune hepatitis. Liver 18391-397. [DOI] [PubMed] [Google Scholar]
- 57.Travaglione, S., A. Fabbri, and C. Fiorentini. 2008. The Rho-activating CNF1 toxin from pathogenic E. coli: a risk factor for human cancer development? Infect. Agent Cancer 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trotta, R., K. A. Puorro, M. Paroli, L. Azzoni, B. Abebe, L. C. Eisenlohr, and B. Perussia. 1998. Dependence of both spontaneous and antibody-dependent, granule exocytosis-mediated NK cell cytotoxicity on extracellular signal-regulated kinases. J. Immunol. 1616648-6656. [PubMed] [Google Scholar]
- 59.Vecchiarelli, A., C. Retini, C. Monari, C. Tascini, F. Bistoni, and T. R. Kozel. 1996. Purified capsular polysaccharide of Cryptococcus neoformans induces interleukin-10 secretion by human monocytes. Infect. Immun. 642846-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, Z., E. Choice, A. Kaspar, D. Hanson, S. Okada, S. C. Lyu, A. M. Krensky, and C. Clayberger. 2000. Bactericidal and tumoricidal activities of synthetic peptides derived from granulysin. J. Immunol. 1651486-1490. [DOI] [PubMed] [Google Scholar]
- 61.Wiseman, J. C., L. L. Ma, K. J. Marr, G. J. Jones, and C. H. Mody. 2007. Perforin-dependent cryptococcal microbicidal activity in NK cells requires PI3K-dependent ERK1/2 signaling. J. Immunol. 1786456-6464. [DOI] [PubMed] [Google Scholar]
- 62.Wu, J., Y. Song, A. B. Bakker, S. Bauer, T. Spies, L. L. Lanier, and J. H. Phillips. 1999. An activating immunoreceptor complex formed by NKG2D and DAP10. Science 285730-732. [DOI] [PubMed] [Google Scholar]
- 63.Yu, C. R., J. R. Ortaldo, R. E. Curiel, H. A. Young, S. K. Anderson, and P. Gosselin. 1999. Role of a STAT binding site in the regulation of the human perforin promoter. J. Immunol. 1622785-2790. [PubMed] [Google Scholar]