Abstract
Toxin A (TcdA) and toxin B (TcdB) are major virulence factors of Clostridium difficile. These two toxins intoxicate cultured cells by similar mechanisms, and TcdB generally is more potent than TcdA in cultured cells. The exact reason for this difference is unclear. Here, we report that the cellular effects of TcdA can be substantially enhanced via an opsonizing antibody through Fc gamma receptor I (FcγRI)-mediated endocytosis. A TcdA-specific monoclonal antibody, A1H3, was found to significantly enhance the cytotoxicity of TcdA to macrophages and monocytes. The A1H3-dependent enhancement of glucosyltransferase activity, cytoskeleton disruption, and tumor necrosis factor alpha production induced by TcdA was further demonstrated using RAW 264.7 cells. Subsequent experiments indicated that the interaction of FcγRI with A1H3 underlays the antibody-dependent enhancement of the cellular effects of TcdA. While blocking FcγRII and FcγRIII with anti-CD16/32 antibodies did not affect the TcdA-mediated glucosylation of Rac1 in RAW 264.7 cells, presaturation of FcγRI with anti-CD64 antibodies in THP1 cells significantly reduced this activity. Incubation of a TcdA-A1H3 immune complex with recombinant mouse CD64 completely abrogated the A1H3-mediated enhancement of the glucosyltransferase activity of TcdA in RAW 264.7 cells. Moreover, expression of FcγRI in CHO cells strikingly enhanced the sensitivity of these cells to TcdA complexed with A1H3. We showed that the presence of A1H3 facilitated cell surface recruitment of TcdA, contributing to the antibody-dependent, FcγRI-mediated enhancement of TcdA activity. Finally, studies using chlorpromazine and endosomal acidification inhibitors revealed an important role of the endocytic pathway in the A1H3-dependent enhancement of TcdA activity.
Clostridium difficile has emerged as a leading cause of hospital-acquired enteric infections whose annual health care costs are rapidly escalating in the United States (33). The severity of C. difficile-associated infections ranges from mild diarrhea to life-threatening pseudomembranous colitis (2, 3). Several hospital outbreaks of C. difficile-associated diarrhea (CDAD) with high rates of morbidity and mortality in the past few years in North America have been attributed to the widespread use of broad-spectrum antibiotics. The emergence of more virulent C. difficile strains is also contributing to the increased incidence and severity of disease (38, 39).
Toxin A (TcdA) and toxin B (TcdB) are the two major virulence factors in pathogenic C. difficile strains. These toxins are enterotoxic, induce intestinal epithelial cell damage, and disrupt epithelium tight junctions, leading to increased mucosal permeability (46, 51, 55). Moreover, these toxins induce production of immune mediators, leading to subsequent neutrophil infiltration and severe colitis (28, 29). TcdA and TcdB are structurally homologous and putatively contain an N-terminal glucosyltransferase domain, a cysteine proteinase domain, a transmembrane domain, and a C-terminal receptor binding domain (21, 65, 66). Interaction between the C terminus and the host cell receptors is believed to initiate receptor-mediated endocytosis (11, 25, 63). Although the intracellular mode of action remains unclear, it has been proposed that the toxins undergo a conformational change at low pH in the endosomal compartment, leading to membrane insertion and channel formation (12, 15, 17, 47). A host cofactor is then required to trigger a second structural change, which is accompanied by immediate autocatalytic cleavage and release of the glucosyltransferase domain into the cytosol (44, 49, 52). Once the glucosyltransferase domain reaches the cytosol, it inactivates proteins belonging to the Rho/Rac family, leading to alterations in the cytoskeleton and ultimately cell death (23, 57).
The clinical manifestations of CDAD are highly variable and range from asymptomatic carriage to mild self-limiting diarrhea to the more severe disease pseudomembranous colitis. Systemic complications and death are increasingly common in CDAD patients (58). In life-threatening cases of CDAD, systemic complications that include cardiopulmonary arrest (22), acute respiratory distress syndrome (20), multiple organ failure (9), renal failure (6), and liver damage (53) are observed. The exact reason for these complications is unclear, but the toxin's entry into the circulation and systemic dissemination have been suggested as possible causes (16).
Protection against C. difficile appears to be conferred by antitoxin antibodies, which are present in the general population in individuals over 2 years of age; the levels of these antibodies are higher and relapse is less frequent in less severe cases (27, 30, 35, 62, 64). Disease progression and recurrence seem to be associated with different subsets of antibodies in the circulation (26), but the reason for this is unknown. In animal studies, neutralizing antibodies directed against TcdA inhibit fluid secretion in mouse intestinal loops and protect mice against systemic infection (5). Coadministration of anti-TcdA and anti-TcdB antibodies significantly reduces the mortality in a primary hamster disease model, as well as in a less stringent relapse model (1).
The mechanism of antibody-mediated protection is unclear, but it is likely that the cellular Fc receptors play some roles. Fc receptors for immunoglobulin G (IgG), which are called Fc gamma receptors (FcγRs), are widely distributed on effector cells of the immune system (including macrophages, monocytes, neutrophils, and natural killer cells) and are essential for the recognition and elimination of IgG-opsonized pathogens and immune complexes. The FcγR family consists of at least one high-affinity receptor (FcγRI or CD64) and two low-affinity receptors (FcγRIIA or CD32 and FcγRIII or CD16). Ligation of these surface receptors with the Fc portion of IgG activates the cell signaling pathways and triggers various cellular responses, such as production of reactive oxygen species, antibody-dependent cellular cytotoxicity, and release of inflammatory cytokines (7, 48).
In the course of characterizing a panel of anti-TcdA antibodies, we observed that one monoclonal antibody (MAb), MAb A1H3, greatly enhanced the killing of murine macrophages and human monocytes by TcdA. In addition, a TcdA-A1H3 immune complex was more potent than TcdA alone in inactivating Rho GTPase, disrupting the cytoskeleton, and inducing tumor necrosis factor alpha (TNF-α) production. The molecular mechanism by which A1H3 exerts this effect was explored.
MATERIALS AND METHODS
Cells and toxins.
The murine macrophage cell line RAW 264.7, the human monocyte cell line THP1, and the Chinese hamster ovary cell line CHO were obtained from the American Type Culture Collection (Manassas, VA). A CHO cell line expressing the FcγRI alpha chain, mRG1-1, was kindly provided by Daniel Conrad (Virginia Commonwealth University) (4). Cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (Invitrogen), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 1 mM sodium pyruvate. Peritoneal exudate macrophages were isolated from C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) by peritoneal lavage 3 days after intraperitoneal injection of 1 ml sterile 3% thioglycolate broth. Cells were collected by washing the peritoneal cavity with 3 ml of sterile phosphate-buffered saline, and red blood cells were lysed with RBC lysis buffer (Sigma, St. Louis, MO). Cells were incubated for 2 h at 37°C to allow adherence of macrophages. Nonadherent cells were removed subsequently by washing. Native TcdA (nTcdA) was purified from culture supernatant of toxingenic C. difficile strain VPI 10463 (kindly provided by Abraham L. Sonenshein, Tufts University School of Medicine) as previously described (24, 32, 60), with some modifications (67). The nTcdA was used to generate anti-TcdA MAbs. Full-length recombinant TcdA (rTcdA) was purified from total crude extract of Bacillus megaterium as described previously (67). The biological activity of rTcdA is essentially identical to that of nTcdA (67). Highly purified rTcdA that appeared as a single band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and was devoid of detectable Toll-like receptor 2 and Toll-like receptor 4 ligand activity as determined by bioassays was used in this study, unless otherwise specified.
Expression of rTcdA peptide fragments.
Sequences encoding truncated TcdA fragments F3 (from amino acid 1185 to amino acid 1838) and F4 (from amino acid 1839 to the carboxyl terminus) were amplified with primers F3 forward (5′-GGTTGCTGGATCCATAAGAGATTTATACCCAGGTAAATTTTACTGGAGATTCTATGC) and F3 reverse (5′-CCATGCTGAGCTCGCATTATTTATATTGATTAATCCTTTAACTAATTTACTATCTTCATCATAG) and primers F4 forward (5′- GGTTGCTGGATCCTCATTATTCTATTTTGATCCTATAGAATTTAACTTA GTAACTGGATGG) and F4 reverse (5′-CCATGCTGAGCTCGCGCCATATATCCCAGGGGCTTTTACTCCATCAAC), respectively. A BamHI site was engineered in each forward primer, and a SacI site was engineered in each reverse primer, enabling directional cloning of the PCR products into a pET32a prokaryotic expression system (EMD Biosciences, Gibbstown, NJ). The vector adds a His6 tag to the N terminus of the recombinant peptides, facilitating subsequent purification. Protein expression was induced with isopropyl-β-d-thiogalactopyranoside (IPTG) at a concentration of 0.6 mM. Recombinant peptide fragments were purified on a nickel column (GE Healthscience, Waukesha, WI).
MAb generation.
Murine hybridomas secreting anti-TcdA antibodies were generated using nTcdA as an immunogen as described elsewhere, with modifications (68). The hybridoma supernatants were screened for antigen-binding capacity by performing an enzyme-linked immunosorbent assay (ELISA) using microplates coated with 0.5 μg/ml of rTcdA. Positive hybridomas were selected and cloned. The isotype of MAbs was determined by ELISA. All antibodies were IgG isotypes, recognizing both nTcdA and rTcdA, and did not cross-react with TcdB. The reactivities of MAbs A1B1 (IgG1), A1E6 (IgG1), and A1H3 (IgG2a) were further mapped by Western blotting and ELISA using truncated TcdA peptide fragments. Mouse MAb JF1 (IgG2a; generated in our laboratory) against an irrelevant antigen was used as an isotype control.
Immunofluorescence staining.
Subconfluent cells on coverslips were treated with TcdA alone or with TcdA in the presence of MAbs (1 μg/ml) at 4°C or 37°C for 30 min. For F-actin staining, cells were incubated with toxins at 37°C for 2 h. The cells were fixed with 2% paraformaldehyde, which was followed by permeabilization in a permeabilizing buffer (phosphate-buffered saline with 1% bovine serum albumin and 0.1% Triton X-100). For F-actin staining, cells were incubated with 1 μg/ml Alexa 568-phalloidin (Invitrogen) for 30 min at room temperature. For immunocomplex or toxin staining, cells were incubated with fluorochrome-conjugated anti-mouse IgG (BD Bioscience, San Jose, CA) or polyclonal rabbit anti-TcdA serum (generated in our laboratory), followed by fluorochrome-conjugated anti-rabbit IgG (BD Bioscience). Cells were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI), and images were obtained using a confocal microscope (Leica LSM TSC SP2 AOBS; Leica, San Francisco, CA). Surface binding of A1H3 to RAW 264.7 cells was examined by flow cytometry. RAW 264.7 cells were incubated with A1H3 alone or with a TcdA-A1H3 or TcdA-A1E6 immune complex on ice for 30 min, which was followed by phycoerythrin-conjugated anti-mouse IgG staining (BD Bioscience). Cells were subsequently analyzed with a FACSCalibur flow cytometer (BD Bioscience).
TNF-α production.
RAW 264.7 cells were exposed to TcdA (50 ng/ml) or TcdA (0.4 ng/ml) with or without MAbs for 6 h. Brefeldin A (Sigma) at a concentration of 20 μM was added to block cytokine secretion. Cells were collected and permeabilized (BD PhosFlow; BD Bioscience). TNF-α production was then determined by intracellular staining using an Alexa 647-conjugated anti-mouse TNF-α antibody (BD Bioscience). Ten thousand cells were collected for flow cytometry analysis. Lipopolysaccharide (LPS) (Escherichia coli 026 strain; Sigma) at a concentration of 1 μg/ml was used as a positive control. In some experiments, cells were preincubated with pharmacologic agents known to inhibit endosome formation (chlorpromazine) or endosomal acidification (ammonium chloride and chloroquine) before addition of the TcdA-A1H3 immune complex.
Cytotoxicity assay.
Subconfluent cells were seeded in a 96-well culture plate in 100 μl of medium and exposed to TcdA with or without an MAb. A saturating dose of an MAb was used to form a TcdA-MAb immune complex before it was added to the cells. After 2 days of incubation, 10 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (5 mg/ml) was added to each well, and the plate was incubated at 37°C for 2 h. Formazan was solubilized with acidic isopropanol (0.4 N HCl in absolute isopropanol), and the absorbance at 570 nm was determined using a 96-well ELISA reader. Cell viability was expressed as a percentage that was determined by using the number of cells that survived divided by the number of cells in untreated control wells. A cytopathic change (cell rounding) was assessed using a phase-contrast microscope after 2 h of toxin treatment. The experiment was repeated three times, and triplicate wells were assessed for cytopathic changes in each experiment.
Immunodetection of Rac1.
Protein lysates of cells treated with TcdA in the presence or absence of MAbs for 4 h at 37°C were separated on a 12% Tris-glycine precast gel (Invitrogen) and transferred onto a nitrocellulose membrane. The membrane was probed with anti-Rac1 (MAb 102) (BD Bioscience) or anti-beta-actin antibodies (Sigma), followed by incubation with horseradish peroxidase-conjugated goat anti-mouse IgG (Southern Biotechnology Associates. Birmingham, AL). The protein bands were visualized using a chemiluminescent substrate (Pierce, Rockford, IL). In FcγR blocking studies, an anti-mouse CD16/32 neutralizing antibody (clone 2.4G2; BD Bioscience) or an anti-human CD64 neutralizing antibody (clone 10.1; R&D Systems, St. Paul, MN) was incubated with cells for 30 min on ice before addition of the TcdA-A1H3 immune complex. Alternatively, recombinant mouse CD64 (R&D Systems) was mixed with the TcdA-A1H3 complex before it was added to cells. In other experiments, chlorpromazine, ammonium chloride, or chloroquine was preincubated with the cells for 30 min before they were exposed to the toxin or immune complex. The blocking antibodies and the pharmacologic agents remained in the culture throughout the experiments.
RESULTS
A1H3-dependent enhancement of cytotoxicity by TcdA on macrophages and monocytes.
The anti-TcdA MAb A1H 3 recognized peptide fragment F4 (amino acid 1839 to the carboxyl terminus), whereas MAb A1E6 recognized both fragment F3 (amino acid 1185 to amino acid 1838) and fragment F4 (data not shown). We observed that A1H3 was able to enhance the cytotoxic effect of TcdA on murine macrophage RAW 264.7 and human monocyte THP1 cells. As shown in Fig. 1A, the TcdA-A1H3 immune complex was about 1,000 times more potent than TcdA alone in causing death of RAW 264.7 cells. While 100 ng/ml of TcdA was required to cause the death of approximately 40% of RAW 264.7 cells after 2 days of incubation, only 0.1 ng/ml of TcdA was needed to obtain the same effect in the presence of A1H3. The presence of other MAbs, such as A1E6 or JF1 (an irrelevant mouse IgG2a isotype MAb), did not affect the cytotoxicity of TcdA in RAW 264.7 cells. A1H3 itself did not affect cell viability (data not shown). A similar observation was made when we assessed the cytopathic effect (cell rounding) on RAW 264.7 cells (data not shown). Furthermore, A1H3 enhanced the cytotoxicity of TcdA in human monocyte THP1 cells (Fig. 1B). Antibody-dependent enhancement of cytotoxicity was not observed with intestinal epithelial cell lines, such as human HCT8 and HT29 cells or murine CT26 cells (data not shown).
FIG. 1.
A1H3-dependent enhancement of cytotoxic effects of TcdA. Cells were seeded in a 96-well plate. (A) RAW 264.7 cells were cultured with various doses of TcdA (▴) or TcdA complexed with anti-TcdA MAb A1E6 (E6), MAb A1H3 (H3), or JF1, an irrelevant antibody. (B) THP1 cells were cultured with TcdA or TcdA complexed with A1H3. After 2 days of incubation, cytotoxicity was measured by an MTT assay. Cell survival was expressed as a percentage of the value for the control group (which was defined as 100%).
Enhanced Rac1 glucosylation and cytoskeleton disruption by the TcdA-A1H3 immune complex.
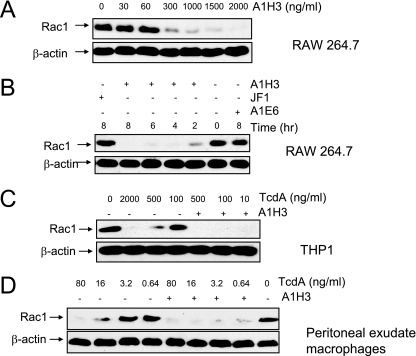
To examine whether A1H3 enhances the TcdA-induced glucosylation of the Rho GTPase Rac1, RAW 264.7 or THP1 cells were treated with low doses of TcdA with or without MAbs. Glucosylation of Rac1 was monitored by immunoblotting using anti-Rac1 (clone 102), which has a reduced affinity for the glucosylated target compared to the unmodified protein (13). After 4 h of treatment, loss of the Rac1 band was observed in cells incubated with the TcdA-A1H3 immune complex at a dose of TcdA as low as 0.4 ng/ml for RAW 264.7 cells and 10 ng/ml for THP1 cells, while TcdA alone at concentrations of 0.4 ng/ml and 100 ng/ml did not glucosylate Rac1 in RAW 264.7 and THP1 cells, respectively (Fig. 2A and 2C). Moreover, glucosylation of Rac1 by TcdA in RAW 264.7 cells exhibited an A1H3 dose-dependent pattern. Complete loss of the Rac1 band was observed when 2,000 ng/ml of A1H3 was used to form a complex with TcdA (Fig. 2A). In light of this result, 2,000 ng/ml A1H3 was considered a saturating dose and used in our subsequent experiments, unless specified otherwise. The glucosylation of Rac1 occurred in a time-dependent manner, peaking between 4 and 8 h during treatment (Fig. 2B). Other MAbs (MAbs JF1 and A1E6) did not enhance the TcdA-induced Rac1 glucosylation (Fig. 2B). To further examine whether A1H3 can enhance the TcdA-induced glucosylation of the Rho GTPase Rac1 from primary murine macrophages, peritoneal exudate macrophages were treated with TcdA in the presence or absence of A1H3. The glucosylation of Rac1 by TcdA in the primary macrophages exhibited a dose-dependent pattern (Fig. 2D). The presence of A1H3 significantly enhanced the TcdA-mediated glucosylation of Rac1 in these cells. While TcdA alone at a dose of 0.64 ng/ml failed to glucosylate Rac1, this dose in the presence of A1H3 resulted in a nearly complete loss of the Rac1 band (Fig. 2D).
FIG. 2.
A1H3-dependent enhancement of the glucosyltransferase activity of TcdA. RAW 264.7 or THP1 cells were treated with TcdA in the presence or absence MAbs. Protein lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and probed with anti-beta-actin and anti-Rac1 (MAb 102) antibodies. (A) RAW 264.7 cells were treated with TcdA (0.4 ng/ml) in the presence of the indicated doses of A1H3 for 4 h. (B) RAW 264.7 cells were incubated with TcdA (0.4 ng/ml) with or without the indicated MAbs for the times indicated. (C) THP1 cells were incubated with various doses of TcdA with or without A1H3 for 4 h. (D) Mouse peritoneal exudate macrophages were exposed to the indicated amounts of TcdA with or without A1H3 for 5 h.
We next examined whether A1H3 could enhance the disruptive effects of TcdA on the actin cytoskeleton. Actin was labeled with Alexa 568-phalloidin, and images of cells were obtained using a confocal microscope. The control RAW 264.7 monolayer exhibited an organized F-actin architecture (Fig. 3A). While exposure to TcdA at a concentration of 0.4 ng/ml (Fig. 3C) did not alter the intracellular actin architecture, disruption of the normal F-actin organization was clearly observed when TcdA (0.4 ng/ml) was complexed with A1H3 (Fig. 3D). The effect was comparable to that observed with cells treated with TcdA alone at a concentration of 50 ng/ml (Fig. 3B).
FIG. 3.
Effects of TcdA on actin organization with or without A1H3. RAW 264.7 monolayers were incubated with medium (A), TcdA (50 ng/ml) (B), TcdA (0.4 ng/ml) (C), or a TcdA (0.4 ng/ml)-A1H3 immune complex (D) for 2 h. The cells were fixed and stained for F-actin with Alexa 568-phalloidin. The F-actin distribution was examined with a confocal microscope.
A1H3-mediated enhancement of TNF-α production.
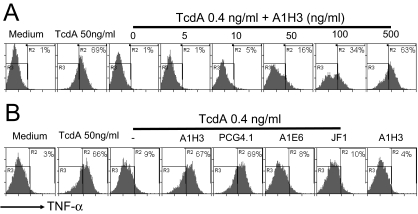
An important step in triggering the host immune response to C. difficile toxins is the release of inflammatory mediators, such as TNF-α, interleukin-1β, and interleukin-6, from macrophages and monocytes (10, 50). We have found that the TcdA-induced TNF-α production in macrophages is dependent on the glucosyltransferase activity of the toxin (60a). We examined whether the presence of A1H3 enhanced the production of TNF-α in macrophages. A1H3 was found to enhance TcdA-mediated TNF-α production in a dose-dependent manner in RAW 264.7 cells (Fig. 4A). No enhancement was detected when TcdA was complexed with MAb A1E6 or JF1 (Fig. 4B). A1H3 alone did not induce detectable TNF-α production (Fig. 4B). Since A1H3 was the only IgG2a isotype anti-TcdA MAb, we examined another commercial IgG2a isotype anti-TcdA MAb, MAb PCG4.1; when complexed with TcdA, PCG4.1 also significantly enhanced the TcdA-induced production of TNF-α in RAW 264.7 cells (Fig. 4B).
FIG. 4.
Effects of MAbs on TcdA-induced TNF-α production by macrophages. RAW 264.7 cells were treated with TcdA (50 ng/ml) or with TcdA (0.4 ng/ml) complexed with A1H3 at the indicated doses (A) or TcdA (0.4 ng/ml) complexed with 1 μg/ml MAb A1H3, A1E6, PCG4.1, or JF1 (B) for 6 h. The TNF-α production was determined by intracellular staining followed by FACS analysis as described in Materials and Methods. The R3 region shows data for TNF-α-negative cells, and the percentage of TNF-α-positive cells is indicated in the R2 region.
Role of FcγRI in antibody-dependent enhancement of toxin effects.
FcγR, which specifically recognizes the Fc portion of IgG, consists of at least FcγRI (CD64), FcγRII (CD32), and FcγRIII (CD16). To test the hypothesis that the interaction between the FcγR and A1H3 is involved in the antibody-dependent enhancement of toxin activity in macrophages, the Fc-binding sites on FcγRII and FcγRIII and those on FcγRI were presaturated with specific anti-CD16/32 and anti-CD64 antibodies, respectively. Because an antibody capable of blocking or neutralizing mouse CD64 is not available, we used anti-human CD64 antibody on THP1 cells. We examined the Rac1 glucosylation following addition of the TcdA-A1H3 immune complex. Presaturation of FcγRII and FcγRIII with anti-CD16/32 antibodies did not affect the glucosylation of Rac1 in RAW 264.7 cells (Fig. 5A). In contrast, a reduced level of Rac1 glucosylation was observed in THP1 cells treated with FcγRI-specific antibodies (anti-CD64), followed by incubation with the TcdA-A1H3 immune complex, compared with the results for untreated cultures (Fig. 5B). Moreover, preincubation of the TcdA-A1H3 complex with recombinant mouse CD64 completely abrogated the glucosyltransferase activity mediated by TcdA-A1H3 (Fig. 5C).
FIG. 5.
Role of FcγRI in A1H3-mediated enhancement of TcdA activity. Saturating doses of anti-mouse-CD16/32 or anti-human-CD64 were incubated with RAW 264.7 cells (A) or THP1 cells (B), respectively, for 30 min on ice before the addition of a TcdA-A1H3 immune complex. A mouse recombinant CD64 protein (5 μg/ml) was mixed with the TcdA-A1H3 complex before it was added to RAW 264.7 cells (C). RAW 264.7 cells (A and C), THP1 cells (B), CHO cells (D), or mRG1-1 cells (E) were treated with TcdA complexed with the indicated MAbs for 4 h. Immunodetection of Rac1 was performed as described in Materials and Methods. (F) CHO cells were treated with TcdA (CHO) or the TcdA-A1H3 complex (CHO/A1H3), or mGR1-1 cells were incubated with TcdA (mRG1) or the TcdA-A1H3 complex with (mRG1/A1H3/pAb) or without (mRG1/A1H3) rabbit anti-TcdA polyclonal antibodies for 2 days. The cytotoxic effects were then measured by an MTT assay. Cell survival was expressed as a percentage of the value for the control group (which was defined as 100%).
To further confirm that FcγRI was involved in the A1H3-dependent enhancement of the toxicity of TcdA, a murine FcγRI-expressing CHO cell line, mRG1-1, was used. CHO cells normally do not express FcγR and are relatively resistant to TcdA-mediated Rac1 glucosylation and cytotoxicity. While CHO and mRG1-1 cells responded similarly to a high dose of TcdA (500 ng/ml) in terms of Rac1 glucosylation, loss of the Rac1 band induced by TcdA at a concentration lower than 10 ng/ml was observed in mRG1-1 cells treated with the TcdA-A1H3 immune complex but not in CHO cells (Fig. 5D and 5E). No difference in Rac1 glucosylation in CHO and mRG1-1 cells was observed when A1E6 was used to form a complex with TcdA (Fig. 5D and 5E). Furthermore, A1H3 greatly augmented the cytotoxic activity of TcdA in mRG1-1 cells. As shown in Fig. 5F, the presence of A1H3 did not affect killing of the wild-type CHO cells by TcdA. In contrast, expression of the FcγRI-α chain alone strikingly enhanced the sensitivity of mRG1-1 cells to the cytotoxic effect of TcdA when it was complexed with A1H3, compared to the cells treated with TcdA alone. mRG1-1 cells were more resistant to TcdA than CHO cells, since TcdA at a concentration of 10 ng/ml did not noticeably induce death of mRG1-1 cells (Fig. 5F). A rabbit anti-TcdA serum completely blocked the cytotoxicity for mRG1-1 cells induced by the TcdA-A1H3 complex (Fig. 5F), indicating that cell death was mediated by TcdA.
Enhanced surface binding of TcdA mediated by A1H3.
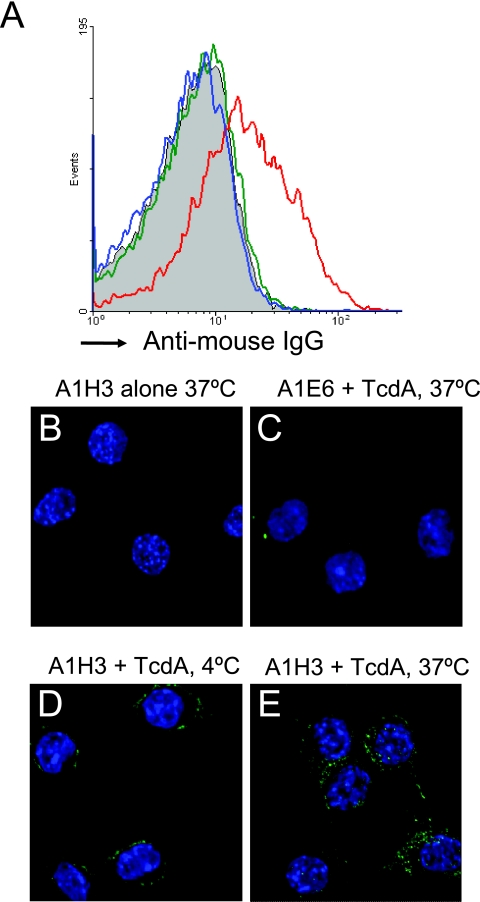
TcdA is thought to bind to a specific cellular receptor(s), which mediates its cellular uptake through endocytosis (31, 42, 45). Surface binding of TcdA-A1H3 to RAW 264.7 cells was examined using fluorochrome-conjugated anti-mouse IgG secondary antibodies. A significant shift in the mean fluorescence intensity occurred only in cells treated with the TcdA-A1H3 immune complex. In contrast, incubation with either A1H3 alone or TcdA-A1E6 did not result in a noticeable increase in fluorescence compared to the results for control cells (Fig. 6A). The high-affinity binding of the TcdA-A1H3 immune complex to RAW 264.7 cells was further demonstrated by confocal microscopy. Strong signals were observed in cells incubated with TcdA-A1H3 at both 4°C (Fig. 6D) and 37°C (Fig. 6E), temperatures that allow surface binding and subsequent internalization, respectively. No fluorescence was detected in cells incubated with A1H3 alone (Fig. 6B) or with TcdA-A1E6 (Fig. 6C).
FIG. 6.
Binding and internalization of A1H3 with RAW 264.7 cells. (A) RAW 264.7 cells were incubated with A1H3 (green), TcdA (10 ng/ml)-A1H3 (red), or TcdA-A1E6 (blue) on ice for 30 min. The binding of anti-TcdA MAbs was determined by phycoerythrin-conjugated anti-mouse Ig antibody staining and FACS analysis. Subconfluent RAW 264.7 cells on coverslips were incubated with A1H3 (B), TcdA (1 ng/ml)-A1E6 at 37°C (C), TcdA-A1H3 at 4°C (D), or TcdA-A1H3 at 37°C (E) for 30 min. Cells were fixed and stained with Alexa 488-conjugated anti-mouse Ig antibodies and DAPI. Binding and internalization of A1H3 were examined by confocal microscopy.
We assumed that A1H3 might act as a bridge, facilitating the recruitment of TcdA to the cell surface via FcγRI. We therefore incubated RAW 264.7 and mRG1-1 cells with TcdA in the presence or absence of A1H3. Cells were stained with rabbit anti-TcdA polyclonal antibodies, followed by an Alexa 488-conjugated anti-rabbit-IgG antibody, and visualized with a confocal microscope. A much brighter signal was observed in RAW 264.7 cells treated with TcdA in the presence of A1H3 (Fig. 7B) but not in RAW 264.7 cells incubated with TcdA alone (Fig. 7A). Similar results were obtained for FcγRI-expressing mRG1-1 cells (Fig. 7C and 7D). Our data suggested that the presence of A1H3 led to enhanced surface binding and internalization of TcdA, contributing to the A1H3-mediated enhancement of toxin activity.
FIG. 7.
Binding and internalization of TcdA with RAW 264.7 and mRG1-1 cells. RAW 264.7 cells (A and B) or mRG1-1 cells (C and D) grown on coverslips were incubated with 10 ng/ml of TcdA (A and C) or TcdA-A1H3 (B and D) at 37°C for 30 min. Cells were fixed and stained with polyclonal rabbit anti-TcdA antibodies, followed by Alexa 568-conjugated anti-rabbit Ig antibody. TcdA binding and internalization were examined by confocal microscopy.
Endocytosis of the TcdA-A1H3 immune complex.
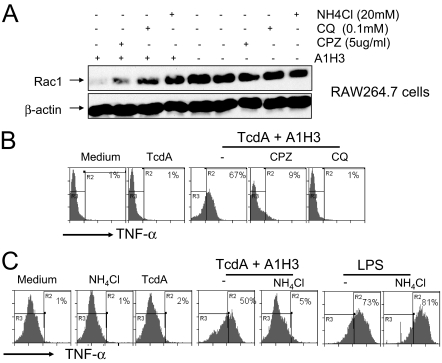
Macrophages internalize immune complexes by either endocytosis or phagocytosis. Soluble antigen-antibody immune complexes are most likely internalized via receptor-mediated endocytosis (43). To dissect the molecular mechanisms underlying the FcγRI-mediated, A1H3-dependent enhancement of TcdA toxicity, we used a panel of reagents that target various stages of the endocytic pathway. Chlorpromazine specifically inhibits the clathrin-coated pit formation at the plasma membrane. Preincubation of RAW 264.7 cells with chlorpromazine reduced the level of Rac1 glucosylation by the TcdA-A1H3 immune complex (Fig. 8A). Ammonium chloride and chloroquine, which prevent endosomal acidification, decreased the glucosytransferase activity of TcdA-A1H3 (Fig. 8A), supporting the conclusion that internalization of the TcdA-A1H3 immune complex was mediated via receptor-mediated endocytosis.
FIG. 8.
Endocytosis-dependent Rac1 glucosylation and TNF-α production. (A) RAW 264.7 cells were preincubated with chlorpromazine (CPZ), ammonium chloride (NH4Cl), or chloroquine (CQ) at the indicated doses for 30 min before addition of TcdA (0.4 ng/ml) with or without A1H3. Rac1 glucosylation was determined by Western blotting. (B) RAW 264.7 cells were preincubated with chlorpromazine (5 μg/ml) or chloroquine (0.1 mM) for 30 min before addition of a TcdA (0.4 ng/ml)-A1H3 immune complex. (C) RAW 264.7 cells were preincubated NH4Cl (20 mM) for 30 min before addition of a TcdA (0.4 ng/ml)-A1H3 immune complex or LPS (1 μg/ml). Cells treated with medium alone, TcdA (0.4 ng/ml) alone, or TcdA complexed with A1H3 served as controls. TNF-α production was determined by intracellular cytokine staining. The R3 region shows data for TNF-α-negative cells, and the percentage of TNF-α-positive cells is indicated in the R2 region.
One of the TcdA-mediated effects on macrophages is the production of TNF-α. As expected, chlorpromazine and chloroquine completely abolished TNF-α synthesis elicited by the TcdA-A1H3 immune complex in RAW 264.7 cells (Fig. 8B). While ammonium chloride did not induce TNF-α production or affect the cytokine response elicited by LPS, the presence of ammonium chloride at a concentration of 20 mM completely blocked TcdA-A1H3 complex-induced cytokine secretion (Fig. 8C). The inhibitory effects of these agents on the TNF-α production elicited by TcdA-A1H3, but not on the TNF-α production elicited by LPS, further support the view that TcdA-A1H3 enters cells via receptor-mediated endocytosis.
DISCUSSION
We report here that an anti-TcdA MAb, A1H3, enhances TcdA-mediated cellular effects in murine macrophages and human monocytes. The observed effects included (i) inducing cell rounding and death, (ii) inactivating a small Rho GTPase via glucosylation, and (iii) eliciting TNF-α production in macrophages. Antibody-dependent enhanced cytotoxic activity of TcdA was not observed in other cell types, including human intestinal epithelial HCT8 or HT29 cells or murine colonic CT26 cells (data not shown).
Experiments demonstrated an important role for FcγRI in A1H3-dependent enhancement of TcdA toxicity. First, while blocking FcγRII and FcγRIII with anti-mouse-CD16/32 antibodies did not affect the glucosylation of Rac1 in RAW 264.7 cells, presaturation of FcγRI on THP1 cells with anti-human CD64 antibodies significantly reduced the level of Rac1 glucosylation. Anti-human CD64 blocking antibodies failed to completely eliminate the enhancement of TcdA activity on THP1 cells by mouse MAb A1H3. The exact reason for this finding is unclear, but it may have been due to the inefficient interaction between mouse-derived A1H3 and human FcγR in THP1 cells. Additionally, preincubation of the TcdA-A1H3 complex with recombinant mouse CD64 completely blocked the A1H3-mediated enhancement of glucosyltransferase activity by TcdA in RAW 264.7 cells. Finally, expression of FcγRI strikingly enhanced the sensitivity of mRG1-1 cells to TcdA when it was complexed with A1H3. Similarly, the presence of A1H3 greatly enhanced the glucosyltransferase activity of TcdA in mRG1-1 cells. Neither cytotoxicity nor glucosyltransferase activity was enhanced by A1H3 in CHO cells, the parental line of mRG1-1 cells.
The FcγR family consists of at least three members, FcγRI, FcγRII, and FcγRIII (48). Murine FcγRI has a higher affinity for IgG2a than for other IgG subisotypes (14). Although FcγRI is a high-affinity receptor capable of binding an IgG monomer, noticeable surface binding of A1H3 to RAW 264.7 cells occurred only when it was complexed with TcdA (Fig. 6A, D, and E). This may be due to the relative low expression of FcγRI on these cells (data not shown). In fact, monomeric A1H3 binding was detected on mRG1-1 cells (data not shown), which were engineered to express a high level of FcγRI (4). However, the binding of TcdA with FcγRI was significantly enhanced after its association with the A1H3 antigen (Fig. 7B and D) compared with the results for cells treated with TcdA alone (Fig. 7A and C), indicating that the presence of A1H3 facilitated recruitment of TcdA to the cell surface, which consequently might contribute to the antibody-dependent enhancement of toxin activity. Although the underlying mechanism is unclear, our data showed that A1E6, an IgG1 MAb against TcdA, neither enhanced toxin binding to macrophages nor augmented toxin activity when it was complexed with TcdA. Similar to A1E6, MAb A1B1, another anti-TcdA IgG1 MAb, had no enhancing effect (data not shown). These results are in agreement with a previous report showing that only the IgG2a isotype, but not the IgG1 isotype, of anti-protective antigen antibodies of anthrax toxin enhanced the cytotoxicity of the toxin to murine macrophages (40).
Upon binding to FcγRs, the immune complexes are internalized via either phagocytosis or endocytosis. The mode of internalization is intimately linked to the size of the bound complexes (7, 41). Large opsonized particles are internalized by phagocytosis, while internalization of small soluble complexes most likely occurs via endocytosis. Mechanistically, the molecular processes underlying the FcγR-mediated phagocytosis and endocytosis differ dramatically. Endocytosis specifically requires assembly of clathrin at the site of receptor clustering (41). FcγRI-mediated phagocytosis, however, requires a signal-transducing γ chain that harbors tyrosine activation motifs (8, 19). Cells expressing the FcγRI extracellular domain (in the absence of the γ subunit) are unable to phagocytose large particles, while their endocytic functions remain intact (8). In our experiments, enhancement of TcdA activity mediated by A1H3 did not require the presence of the γ chain, since expression of the FcγRI α chain alone on mRG1-1 cells rendered the cells more susceptible to A1H3-dependent enhancement of the toxicity of TcdA (Fig. 5E and F), suggesting that the TcdA-A1H3 complex was taken up via FcγRI-mediated endocytosis. The involvement of an endocytic pathway in the uptake of TcdA-A1H3 by RAW 264.7 cells was further supported by the observation that TcdA-A1H3-mediated Rac1 glucosylation and TNF-α production were inhibited by chlorpromazine and ammonium chloride-chloroquine (Fig. 8A, B, and C), chemicals that are known to target the endocytic pathway.
Our findings may have implications for understanding the antibody response in host protection and pathogenesis of C. difficile-associated diseases. TcdA and TcdB are key virulence factors, and antibodies against these two toxins are highly protective (5, 34). Intravenous administration of Ig against TcdA and TcdB to patients with recurrent or severe CDAD resulted in resolution of symptoms (37). A higher level of anti-TcdA antibodies following colonization or primary disease has been correlated with protection from CDAD or relapse (34, 35). Finally, vaccination of long-term relapsing humans with toxoids A and B has successfully prevented additional relapses (59). However, different subsets of antibodies may have different roles in host protection and disease progression (26). While patients with recurrent CDAD do not show evidence of overall humoral immune deficiency, they do have a selectively reduced IgG2 and IgG3 response against TcdA compared to patients with single CDAD (26). Our data showed that the IgG2a subisotype of anti-TcdA actually enhanced the toxicity of TcdA on macrophages/monocytes in vitro. How these findings apply to in vivo pathogenesis of C. difficile infection remains to be determined. Antibody-dependent enhancement of viral infection has been widely described for mammalian viruses, as well as bacteriophages (36, 54, 56). Instead of neutralizing or reducing viral infectivity, the presence of virus-specific antibodies paradoxically potentiates infection of susceptible host cells, a process that is most often mediated by receptors for complement components or the Fc portion of Igs (18, 36, 54, 61). To date, no report has documented the enhancement of toxin activity in vivo by specific subsets of antibodies. Given the results of this report and a previous study (40) using in vitro cell culture models, it is likely that some toxin-specific antibodies have detrimental effects on the host mediated by enhanced toxin activity. Such effects, which have not been illustrated in humans or in animal models yet, are being investigated in our laboratory.
Acknowledgments
This work was supported by NIH grant N01AI30050 and in part by NIH grant K01DK076549 to H.F. and by grant 2007AA021702 from the National High Technology Development Program of China to J.W.
We thank Giovanni Widmer for a critical review of the manuscript, Jean Mukherjee for her help with generation of the antibodies against toxins, and Weijia Nie for technical assistance.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 23 March 2009.
REFERENCES
- 1.Babcock, G. J., T. J. Broering, H. J. Hernandez, R. B. Mandell, K. Donahue, N. Boatright, A. M. Stack, I. Lowy, R. Graziano, D. Molrine, D. M. Ambrosino, and W. D. Thomas, Jr. 2006. Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile-induced mortality in hamsters. Infect. Immun. 746339-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett, J. G. 2002. Clinical practice. Antibiotic-associated diarrhea. N. Engl. J. Med. 346334-339. [DOI] [PubMed] [Google Scholar]
- 3.Borriello, S. P. 1998. Pathogenesis of Clostridium difficile infection. J. Antimicrob. Chemother. 41(Suppl. C)13-19. [DOI] [PubMed] [Google Scholar]
- 4.Cho, S., and D. H. Conrad. 1997. A new multivalent B cell activation model—anti-IgD bound to Fc gamma RI: properties and comparison with CD40L-mediated activation. Int. Immunol. 9239-248. [DOI] [PubMed] [Google Scholar]
- 5.Corthier, G., M. C. Muller, T. D. Wilkins, D. Lyerly, and R. L'Haridon. 1991. Protection against experimental pseudomembranous colitis in gnotobiotic mice by use of monoclonal antibodies against Clostridium difficile toxin A. Infect. Immun. 591192-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunney, R. J., C. Magee, E. McNamara, E. G. Smyth, and J. Walshe. 1998. Clostridium difficile colitis associated with chronic renal failure. Nephrol. Dial. Transplant. 132842-2846. [DOI] [PubMed] [Google Scholar]
- 7.Daeron, M. 1997. Fc receptor biology. Annu. Rev. Immunol. 15203-234. [DOI] [PubMed] [Google Scholar]
- 8.Davis, W., P. T. Harrison, M. J. Hutchinson, and J. M. Allen. 1995. Two distinct regions of FC gamma RI initiate separate signalling pathways involved in endocytosis and phagocytosis. EMBO J. 14432-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobson, G., C. Hickey, and J. Trinder. 2003. Clostridium difficile colitis causing toxic megacolon, severe sepsis and multiple organ dysfunction syndrome. Intensive Care Med. 291030. [DOI] [PubMed] [Google Scholar]
- 10.Flegel, W. A., F. Muller, W. Daubener, H. G. Fischer, U. Hadding, and H. Northoff. 1991. Cytokine response by human monocytes to Clostridium difficile toxin A and toxin B. Infect. Immun. 593659-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Florin, I., and M. Thelestam. 1983. Internalization of Clostridium difficile cytotoxin into cultured human lung fibroblasts. Biochim. Biophys. Acta 763383-392. [DOI] [PubMed] [Google Scholar]
- 12.Florin, I., and M. Thelestam. 1986. Lysosomal involvement in cellular intoxication with Clostridium difficile toxin B. Microb. Pathog. 1373-385. [DOI] [PubMed] [Google Scholar]
- 13.Genth, H., J. Huelsenbeck, B. Hartmann, F. Hofmann, I. Just, and R. Gerhard. 2006. Cellular stability of Rho-GTPases glucosylated by Clostridium difficile toxin B. FEBS Lett. 5803565-3569. [DOI] [PubMed] [Google Scholar]
- 14.Gessner, J. E., H. Heiken, A. Tamm, and R. E. Schmidt. 1998. The IgG Fc receptor family. Ann. Hematol. 76231-248. [DOI] [PubMed] [Google Scholar]
- 15.Giesemann, T., T. Jank, R. Gerhard, E. Maier, I. Just, R. Benz, and K. Aktories. 2006. Cholesterol-dependent pore formation of Clostridium difficile toxin A. J. Biol. Chem. 28110808-10815. [DOI] [PubMed] [Google Scholar]
- 16.Hamm, E. E., D. E. Voth, and J. D. Ballard. 2006. Identification of Clostridium difficile toxin B cardiotoxicity using a zebrafish embryo model of intoxication. Proc. Natl. Acad. Sci. USA 10314176-14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henriques, B., I. Florin, and M. Thelestam. 1987. Cellular internalisation of Clostridium difficile toxin A. Microb. Pathog. 2455-463. [DOI] [PubMed] [Google Scholar]
- 18.Homsy, J., M. Meyer, M. Tateno, S. Clarkson, and J. A. Levy. 1989. The Fc and not CD4 receptor mediates antibody enhancement of HIV infection in human cells. Science 2441357-1360. [DOI] [PubMed] [Google Scholar]
- 19.Huang, Z. Y., D. R. Barreda, R. G. Worth, Z. K. Indik, M. K. Kim, P. Chien, and A. D. Schreiber. 2006. Differential kinase requirements in human and mouse Fc-gamma receptor phagocytosis and endocytosis. J. Leukoc. Biol. 801553-1562. [DOI] [PubMed] [Google Scholar]
- 20.Jacob, S. S., J. C. Sebastian, D. Hiorns, S. Jacob, and P. K. Mukerjee. 2004. Clostridium difficile and acute respiratory distress syndrome. Heart Lung 33265-268. [DOI] [PubMed] [Google Scholar]
- 21.Jank, T., and K. Aktories. 2008. Structure and mode of action of clostridial glucosylating toxins: the ABCD model. Trends Microbiol. 16222-229. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, S., S. A. Kent, K. J. O'Leary, M. M. Merrigan, S. P. Sambol, L. R. Peterson, and D. N. Gerding. 2001. Fatal pseudomembranous colitis associated with a variant Clostridium difficile strain not detected by toxin A immunoassay. Ann. Intern. Med. 135434-438. [DOI] [PubMed] [Google Scholar]
- 23.Just, I., J. Selzer, M. Wilm, C. von Eichel-Streiber, M. Mann, and K. Aktories. 1995. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375500-503. [DOI] [PubMed] [Google Scholar]
- 24.Kamiya, S., P. J. Reed, and S. P. Borriello. 1989. Purification and characterisation of Clostridium difficile toxin A by bovine thyroglobulin affinity chromatography and dissociation in denaturing conditions with or without reduction. J. Med. Microbiol. 3069-77. [DOI] [PubMed] [Google Scholar]
- 25.Karlsson, K. A. 1995. Microbial recognition of target-cell glycoconjugates. Curr. Opin. Struct. Biol. 5622-635. [DOI] [PubMed] [Google Scholar]
- 26.Katchar, K., C. P. Taylor, S. Tummala, X. Chen, J. Sheikh, and C. P. Kelly. 2007. Association between IgG2 and IgG3 subclass responses to toxin A and recurrent Clostridium difficile-associated disease. Clin. Gastroenterol. Hepatol. 5707-713. [DOI] [PubMed] [Google Scholar]
- 27.Kelly, C. P. 1996. Immune response to Clostridium difficile infection. Eur. J. Gastroenterol. Hepatol. 81048-1053. [DOI] [PubMed] [Google Scholar]
- 28.Kelly, C. P., S. Becker, J. K. Linevsky, M. A. Joshi, J. C. O'Keane, B. F. Dickey, J. T. LaMont, and C. Pothoulakis. 1994. Neutrophil recruitment in Clostridium difficile toxin A enteritis in the rabbit. J. Clin. Investig. 931257-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly, C. P., and J. T. LaMont. 1998. Clostridium difficile infection. Annu. Rev. Med. 49375-390. [DOI] [PubMed] [Google Scholar]
- 30.Kelly, C. P., C. Pothoulakis, F. Vavva, I. Castagliuolo, E. F. Bostwick, J. C. O'Keane, S. Keates, and J. T. LaMont. 1996. Anti-Clostridium difficile bovine immunoglobulin concentrate inhibits cytotoxicity and enterotoxicity of C. difficile toxins. Antimicrob. Agents Chemother. 40373-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krivan, H. C., G. F. Clark, D. F. Smith, and T. D. Wilkins. 1986. Cell surface binding site for Clostridium difficile enterotoxin: evidence for a glycoconjugate containing the sequence Gal alpha 1-3Gal beta 1-4GlcNAc. Infect. Immun. 53573-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krivan, H. C., and T. D. Wilkins. 1987. Purification of Clostridium difficile toxin A by affinity chromatography on immobilized thyroglobulin. Infect. Immun. 551873-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kyne, L., M. B. Hamel, R. Polavaram, and C. P. Kelly. 2002. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin. Infect. Dis. 34346-353. [DOI] [PubMed] [Google Scholar]
- 34.Kyne, L., M. Warny, A. Qamar, and C. P. Kelly. 2001. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 357189-193. [DOI] [PubMed] [Google Scholar]
- 35.Kyne, L., M. Warny, A. Qamar, and C. P. Kelly. 2000. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N. Engl. J. Med. 342390-397. [DOI] [PubMed] [Google Scholar]
- 36.Leopold, P. L., R. L. Wendland, T. Vincent, and R. G. Crystal. 2006. Neutralized adenovirus-immune complexes can mediate effective gene transfer via an Fc receptor-dependent infection pathway. J. Virol. 8010237-10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung, D. Y., C. P. Kelly, M. Boguniewicz, C. Pothoulakis, J. T. LaMont, and A. Flores. 1991. Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin. J. Pediatr. 118633-637. [DOI] [PubMed] [Google Scholar]
- 38.Loo, V. G., L. Poirier, M. A. Miller, M. Oughton, M. D. Libman, S. Michaud, A. M. Bourgault, T. Nguyen, C. Frenette, M. Kelly, A. Vibien, P. Brassard, S. Fenn, K. Dewar, T. J. Hudson, R. Horn, P. Rene, Y. Monczak, and A. Dascal. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 3532442-2449. [DOI] [PubMed] [Google Scholar]
- 39.McDonald, L. C., G. E. Killgore, A. Thompson, R. C. Owens, Jr., S. V. Kazakova, S. P. Sambol, S. Johnson, and D. N. Gerding. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 3532433-2441. [DOI] [PubMed] [Google Scholar]
- 40.Mohamed, N., J. Li, C. S. Ferreira, S. F. Little, A. M. Friedlander, G. L. Spitalny, and L. S. Casey. 2004. Enhancement of anthrax lethal toxin cytotoxicity: a subset of monoclonal antibodies against protective antigen increases lethal toxin-mediated killing of murine macrophages. Infect. Immun. 723276-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukherjee, S., R. N. Ghosh, and F. R. Maxfield. 1997. Endocytosis. Physiol. Rev. 77759-803. [DOI] [PubMed] [Google Scholar]
- 42.Na, X., H. Kim, M. P. Moyer, C. Pothoulakis, and J. T. LaMont. 2008. gp96 is a human colonocyte plasma membrane binding protein for Clostridium difficile toxin A. Infect. Immun. 762862-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norman, J. C., and J. M. Allen. 2000. Endocytosis of FcgammaRI is regulated by two distinct signalling pathways. FEBS Lett. 484179-183. [DOI] [PubMed] [Google Scholar]
- 44.Pfeifer, G., J. Schirmer, J. Leemhuis, C. Busch, D. K. Meyer, K. Aktories, and H. Barth. 2003. Cellular uptake of Clostridium difficile toxin B. Translocation of the N-terminal catalytic domain into the cytosol of eukaryotic cells. J. Biol. Chem. 27844535-44541. [DOI] [PubMed] [Google Scholar]
- 45.Pothoulakis, C., R. J. Gilbert, C. Cladaras, I. Castagliuolo, G. Semenza, Y. Hitti, J. S. Montcrief, J. Linevsky, C. P. Kelly, S. Nikulasson, H. P. Desai, T. D. Wilkins, and J. T. LaMont. 1996. Rabbit sucrase-isomaltase contains a functional intestinal receptor for Clostridium difficile toxin A. J. Clin. Investig. 98641-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pothoulakis, C., and J. T. Lamont. 2001. Microbes and microbial toxins: paradigms for microbial-mucosal interactions. II. The integrated response of the intestine to Clostridium difficile toxins. Am. J. Physiol. Gastrointest. Liver Physiol. 280G178-G183. [DOI] [PubMed] [Google Scholar]
- 47.Qa'Dan, M., L. M. Spyres, and J. D. Ballard. 2000. pH-induced conformational changes in Clostridium difficile toxin B. Infect. Immun. 682470-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ravetch, J. V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19275-290. [DOI] [PubMed] [Google Scholar]
- 49.Reineke, J., S. Tenzer, M. Rupnik, A. Koschinski, O. Hasselmayer, A. Schrattenholz, H. Schild, and C. von Eichel-Streiber. 2007. Autocatalytic cleavage of Clostridium difficile toxin B. Nature 446415-419. [DOI] [PubMed] [Google Scholar]
- 50.Ribeiro, R. A., M. V. Souza-Filho, M. H. Souza, S. H. Oliveira, C. H. Costa, F. Q. Cunha, and H. S. Ferreira. 1997. Role of resident mast cells and macrophages in the neutrophil migration induced by LTB4, fMLP and C5a des arg. Int. Arch. Allergy Immunol. 11227-35. [DOI] [PubMed] [Google Scholar]
- 51.Riegler, M., R. Sedivy, C. Pothoulakis, G. Hamilton, J. Zacherl, G. Bischof, E. Cosentini, W. Feil, R. Schiessel, J. T. LaMont, et al. 1995. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J. Clin. Investig. 952004-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rupnik, M., S. Pabst, M. Rupnik, C. von Eichel-Streiber, H. Urlaub, and H. D. Soling. 2005. Characterization of the cleavage site and function of resulting cleavage fragments after limited proteolysis of Clostridium difficile toxin B (TcdB) by host cells. Microbiology 151199-208. [DOI] [PubMed] [Google Scholar]
- 53.Sakurai, T., K. Hajiro, H. Takakuwa, A. Nishi, M. Aihara, and T. Chiba. 2001. Liver abscess caused by Clostridium difficile. Scand. J. Infect. Dis. 3369-70. [DOI] [PubMed] [Google Scholar]
- 54.Sapinoro, R., K. Volcy, W. W. Rodrigo, J. J. Schlesinger, and S. Dewhurst. 2008. Fc receptor-mediated, antibody-dependent enhancement of bacteriophage lambda-mediated gene transfer in mammalian cells. Virology 373274-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Savidge, T. C., W. H. Pan, P. Newman, M. O'Brien, P. M. Anton, and C. Pothoulakis. 2003. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology 125413-420. [DOI] [PubMed] [Google Scholar]
- 56.Schlesinger, J. J., and S. E. Chapman. 1999. Influence of the human high-affinity IgG receptor FcgammaRI (CD64) on residual infectivity of neutralized dengue virus. Virology 26084-88. [DOI] [PubMed] [Google Scholar]
- 57.Sehr, P., G. Joseph, H. Genth, I. Just, E. Pick, and K. Aktories. 1998. Glucosylation and ADP ribosylation of rho proteins: effects on nucleotide binding, GTPase activity, and effector coupling. Biochemistry. 375296-5304. [DOI] [PubMed] [Google Scholar]
- 58.Siemann, M., M. Koch-Dorfler, and G. Rabenhorst. 2000. Clostridium difficile-associated diseases. The clinical courses of 18 fatal cases. Intensive Care Med. 26416-421. [DOI] [PubMed] [Google Scholar]
- 59.Sougioultzis, S., L. Kyne, D. Drudy, S. Keates, S. Maroo, C. Pothoulakis, P. J. Giannasca, C. K. Lee, M. Warny, T. P. Monath, and C. P. Kelly. 2005. Clostridium difficile toxoid vaccine in recurrent C. difficile-associated diarrhea. Gastroenterology 128764-770. [DOI] [PubMed] [Google Scholar]
- 60.Sullivan, N. M., S. Pellett, and T. D. Wilkins. 1982. Purification and characterization of toxins A and B of Clostridium difficile. Infect. Immun. 351032-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60a.Sun, X., X. He, S. Tzipori, R. Gerhard, and H. Feng. Essential role of the glucosyltransferase activity in Clostridium difficile toxin-induced secretion of TNF-alpha by macrophages. Microb. Pathog., in press. [DOI] [PMC free article] [PubMed]
- 61.Takada, A., and Y. Kawaoka. 2003. Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev. Med. Virol. 13387-398. [DOI] [PubMed] [Google Scholar]
- 62.Torres, J. F., D. M. Lyerly, J. E. Hill, and T. P. Monath. 1995. Evaluation of formalin-inactivated Clostridium difficile vaccines administered by parenteral and mucosal routes of immunization in hamsters. Infect. Immun. 634619-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tucker, K. D., and T. D. Wilkins. 1991. Toxin A of Clostridium difficile binds to the human carbohydrate antigens I, X, and Y. Infect. Immun. 5973-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Viscidi, R., B. E. Laughon, R. Yolken, P. Bo-Linn, T. Moench, R. W. Ryder, and J. G. Bartlett. 1983. Serum antibody response to toxins A and B of Clostridium difficile. J. Infect. Dis. 14893-100. [DOI] [PubMed] [Google Scholar]
- 65.von Eichel-Streiber, C., P. Boquet, M. Sauerborn, and M. Thelestam. 1996. Large clostridial cytotoxins—a family of glycosyltransferases modifying small GTP-binding proteins. Trends Microbiol. 4375-382. [DOI] [PubMed] [Google Scholar]
- 66.Voth, D. E., and J. D. Ballard. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin. Microbiol. Rev. 18247-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang, G., B. Zhou, J. Wang, X. He, X. Sun, W. Nie, S. Tzipori, and H. Feng. 2008. Expression of recombinant Clostridium difficile toxin A and B in Bacillus megaterium. BMC Microbiol. 8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang, Q., I. Singh, A. Sheoran, X. Feng, J. Nunnari, A. Carville, and S. Tzipori. 2005. Production and characterization of monoclonal antibodies against Enterocytozoon bieneusi purified from rhesus macaques. Infect. Immun. 735166-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]