Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) latency is characterized by the highly regulated transcription of a few viral genes essential for genome maintenance and host cell survival. A major latency control region has been identified upstream of the divergent promoters for the multicistronic transcripts encoding LANA (ORF73), vCyclin (ORF72), and vFLIP (ORF71) and for the complementary strand transcript encoding K14 and vGPCR (ORF74). Previous studies have shown that this major latency control region is occupied by the cellular chromatin boundary factor CTCF and chromosome structural maintenance proteins SMC1, SMC3, and RAD21, which comprise the cohesin complex. Deletion of the CTCF-cohesin binding site caused an inhibition of cell growth and viral genome instability. We now show that the KSHV genes regulated by CTCF-cohesin are under cell cycle control and that mutation of the CTCF binding sites abolished cell cycle-regulated transcription. Cohesin subunits assembled at the CTCF binding sites and bound CTCF proteins in a cell cycle-dependent manner. Subcellular distribution of CTCF and colocalization with cohesins also varied across the cell cycle. Ectopic expression of Rad21 repressed CTCF-regulated transcription of KSHV lytic genes, and a Rad21-CTCF chimeric protein converted CTCF into an efficient transcriptional repressor of KSHV genes normally activated in the G2 phase. We conclude that cohesins interact with CTCF in mid-S phase and repress CTCF-regulated genes in a cell cycle-dependent manner. We propose that the CTCF-cohesin complex plays a critical role in regulating the cell cycle control of viral gene expression during latency and that failure to maintain cell cycle control of latent transcripts inhibits host cell proliferation and survival.
Cell cycle control of transcription is essential for the ordered expression of gene products that regulate cellular growth, differentiation, and division. It is generally accepted that cell cycle control is driven by the cyclin-dependent kinases and the network of molecules that are regulated by these kinases (31). In higher eukaryotes, most cell cycle-dependent transcription is regulated by the E2F family of transcription factors and their cyclin-dependent kinase-regulated interaction with the retinoblastoma (Rb) family of corepressors (4, 13, 43). However, recent studies have revealed that cell cycle control of transcription can occur through alternative mechanisms that are independent of the cononical E2F-Rb pathway (39). Interactions between transcription factors and the DNA replication or chromosome segregation machinery may provide additional mechanisms for cell cycle control of transcription.
Transcription regulation must also be coordinated with higher-order chromosome structures and chromosome dynamics during cellular division. The formation of sister chromatid junctions during cellular division is one example of a higher-order structure that is likely to have dramatic effects on transcription control mechanisms. Sister chromatid junctions are thought to be formed by the cohesin complex (17, 23, 30). Cohesin is a conserved eukaryotic protein complex that maintains sister chromatid cohesion and allows biorientation of chromosomes during mitotic segregation (34). Cohesins consist of four primary subunits that include two members of the structural maintenance of chromosome (SMC) ATPases, referred to as SMC1 and SMC3, along with Rad21 and SA1/2 (17, 29, 49). The cohesins can form a ringlike structure that can encircle the two sister chromatid DNA strands (14). Rad21 functions as the kleisin subunit that closes the circle in a cell cycle-dependent manner. Cohesins are thought to load onto chromosomes at early G1, but sister chromatid encirclement may be coupled to components of the DNA replication machinery. Proteolysis of Rad21 in anaphase allows for the segregation of sister chromatids to opposite spindle poles and the completion of mitosis. In addition to their function in sister chromatid cohesion and chromosome segregation, cohesins may have additional functions in gene regulation (8). Genetic dissection of the Drosophila Nipped-B gene and the human developmental disorder Cornelia de Lange syndrome revealed a role for cohesin components in transcription (8, 20, 36). The developmental defects are most consistent with a failure to properly regulate gene expression during development. More recent studies from our lab and others using chromatin immunoprecipitation studies have found that cohesin subunits colocalize at a high frequency with the chromatin boundary factor CTCF (33, 42, 47). This provides additional evidence that the cohesin complex may function in gene regulation and chromatin organization, independent of its role in chromosome segregation.
CTCF is an 11-zinc-finger DNA binding protein that has been implicated in chromatin boundary functioning and insulator binding (32). In Drosophila, CTCF has been shown to bind the Fab-8 insulator element, where it confers enhancer-blocking activity (27, 46). The enhancer-blocking activity of CTCF has also been well characterized in the human and mouse H19/Igf2-imprinted loci (10, 15, 18). Genome-wide chromatin immunoprecipitation (ChIP) assays have revealed a colocalization of CTCF, with histone H3 methylation on lysine 4 (K4), as well as with cohesins (2). CTCF was originally characterized as a repressor of the c-myc gene and has been mapped to several binding sites in the 5′ regulatory sequence of the myc transcription locus (11). Chromatin conformation capture studies indicate that CTCF can form higher-order structures, referred to as hubs, by linking multiple inter- and intrachromosomal locations (22, 40, 41). CTCF has also been implicated in cell cycle control of transcription, but a clear molecular mechanism for these activities has not yet been described (16).
CTCF and cohesins colocalize at a key regulatory locus in the Kaposi's sarcoma-associated herpesvirus (KSHV) genome (42). A cluster of three high-affinity CTCF sites are located within an overlapping divergent promoter that directs transcription of the major latency genes in one direction and a bicistronic lytic transcript in the opposing direction. The latency transcript encodes the viral origin binding protein LANA (ORF73), vCyclin (ORF72), and vFLIP (ORF71), as well as viral microRNAs and kaposin (K12). The lytic transcript encodes the vOX2 (K14) and vGPCR (ORF74). Both K14 and vGPCR expression are detected at low levels in latently infected cell lines and at higher levels in tumor biopsies, suggesting that regulation of these genes during KSHV latent infection is important for viral pathogenesis (5). Moreover, vGPCR has been shown to have growth transformation and angiogenic properties consistent with its essential role in the development of Kaposi's sarcoma (1). Proper regulation of these viral genes is essential for the establishment and maintenance of latency and the pathogenic progression associated with Kaposi's sarcoma. Recently, we have found that mutation of the CTCF binding sites in these regulatory regions abolishes stable latent infection and episome maintenance (42). The genetic instability of this mutation was linked to the association of CTCF with cohesins. We now show that the CTCF binding sites in the KSHV latency control region confer cell cycle regulation of transcription and provide evidence that cohesin subunits repress CTCF-directed transcription.
MATERIALS AND METHODS
Cell cultures and plasmids.
The KSHV-positive PEL cell line BCBL1 was cultured at 37C° and 5% CO2 in RPMI medium (Gibco BRL) and supplemented with 10% fetal bovine serum and penicillin-streptomycin (50 U/ml). The 293 cell line (ATCC) was maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum containing penicillin-streptomycin (50 U/ml). The 293-derived cell lines were all cultured identically to the 293 cells, except with the addition of 200 μg/ml hygromycin B for the selection of the wild-type (WT) and recombinant viral genomes. pCMV-FLAG-CTCF and pCMV-FLAG-RAD21 were constructed by PCR amplification (Platinum Taq) of CTCF and RAD21, with primers introducing a 5′ HindIII site and a 3′ XbaI site cloned in frame to N-terminal p3xFLAG-CMV-10 (Sigma). pCMV-FLAG-CTCF-RAD21 was constructed by PCR amplification, with primers introducing a 5′ HindIII site and a 3′ GlyAlaGly BamHI site for CTCF and a 5′ GAG BglII site (replaced AUG with GAG) and 3′ XbaI site for RAD21, and by cloning of both DNA fragments into N-terminal p3xFLAG-CMV-10 (Sigma). Luciferase reporter plasmids were constructed by PCR amplification of a fragment of KSHV comprising nucleotides 127298 to 127855 (KSHV 127298-127855) for the ORF73 promoter and KSHV 127298-127884 for the ORFK14 promoter, with primers introducing a 5′ NheI site and a 3′ HindIII site, and by cloning to the pGL3-Basic Vector (Promega). LoxP-substituted mutant luciferase constructs were constructed by PCR amplification from KSHV mutant BAC, as previously described (42). Site-directed mutated luciferase constructs were constructed using a QuikChange site-directed mutagenesis kit (Stratagene), according to the manufacturer's protocol. Primers used in cloning and mutagenesis are available upon request.
Centrifugal elutriation.
Centrifugal elutriation was used to separate BCBL1 and 293T into different cell cycle phases, with flow rates of 15, 18, 21, 24, 27, 30, 34, 38, 42, and 46 ml/min, as described previously (35). Fluorescence-activated cell sorter (FACS) analysis of propidium iodine (PI)-stained, fractionated cells was carried out after each centrifugal elutriation.
RT-qPCR.
Reverse transcriptase quantitative PCR (RT-qPCR) was used to determine transcription profiles of cellular and KSHV genes. Total RNAs were extracted by use of an RNeasy kit (Qiagen) and then further treated with DNase I (Ambion). A total of 2 μg of the RNA was subjected to a SuperScript II RNase H− RT (Invitrogen) reaction, with 5 μM of random decamers (Ambion), according to the manufacturers’ protocols. Diluted RT products were analyzed by real-time PCR (ABI Prism 7000 sequence detection system; Applied Biosystems). Primers used in this assay were previously described (42) and are available upon request. The level of beta-actin in each sample was used as the internal control for each qPCR. A negative control in the absence of primers was performed for each reaction. Semiquantitative RT-PCR was performed essentially as described previously (24). Starting with 2 μg of the RNA, RT products were synthesized as mentioned above, followed by DNase I treatment and purification using a QIAquick PCR purification kit (Qiagen). Equal amounts of RT products were subjected to the semiquantitative RT-PCR assay. Primers for RT-qPCR used to detect KSHV spliced transcripts were as follows: for complete latent transcript (LTc), GGT TAA AGT GGG TTT GCT GGC CTT (LTc-F2) and GCT TGG TCC GGC TGA CTT ATA AAC (LT-R1); for LT1, TCC TCG GGA AAT CTG GTC CTG TTT (LT1-F3 composed of KSHV 127297-127314 and KSHV 127812-127817) and AAG AGC AGC AGC TTG GTC CGG CTG (LT-R2); and for LT2, GGA GCG GCG ACG GTG GCT CTG TTT (LT2-F3 composed of KSHV 123758-123775 and KSHV 127812-127817). In addition, a pair of TAG AGT GGC GAG CGT ATG TG (LT2-F2) and LT-R1 primers was also used for LT2 in RT-qPCR; for beta-actin, ACC GCC GAG ACC GCG TCC GCC (forward) and TCG GGA C AGCTCAGCC ACA C (reverse) were used. To further confirm the results obtained by the RT-qPCR assay, semiquantitative PCR was performed using the following primers: for LTc, GCA TTA AGC TGC AAT ACC GCC GAT (LTc-F1) and LT-R1; for LT1, GCT CCT GCT GCT GTT GTG AAC TTT (LT1-F1) and LT-R1; and for LT2, TGA CAT TAG GGC ATC CAC GTC AGT (LT2-F1) and LT-R1.
Antibodies.
Assays such as ChIP, coimmunoprecipitation (co-IP), and Western blotting included polyclonal antibodies to immunoglobulin G (IgG) (Santa Cruz Biotechnology) and CTCF (Abcam or Santa Cruz Biotechnology). A rat monoclonal antibody for ORF73 (m-LANA) was purchased from Advanced Biotechnology, Inc. Polyclonal antibodies for SMC1, SMC3, and RAD21 were all purchased from Bethyl, Inc. A mouse monoclonal antibody for cyclin B1 was purchased from Santa Cruz Biotechnology.
Immunofluorescence and confocal microscope.
A total of 4 × 105 BCBL1 cells were cytospun onto a cover slide fastened on the glass microscope slide for 5 min at 1,000 rpm, fixed with 4% formaldehyde for 30 min for RT, and permeabilized with 1× phosphate-buffered saline/0.2% Triton X-100 for 20 min. Using the manufacturer's protocol, RAD21 was detected using mouse monoclonal antibody to RAD21 (Santa Cruz Biotechnology) and Alexa Fluor 488 (green)-conjugated goat anti-mouse (Molecular Probes) as a secondary antibody. CTCF was detected using rabbit polyclonal antibody to CTCF (Abcam) and Alexa Fluor 594 (red)-conjugated goat anti-mouse (Molecular Probes) as a secondary antibody. Images were recorded using a Leica TCS SP2 confocal microscope equipped with argon (488 nm) and HeNe (594 nm) lasers.
The electrophoretic mobility shift assay (EMSA), ChIP assay, and luciferase assay have been described previously (7, 42). Additional protocols and oligonucleotide sequences can be made available upon request.
RESULTS
Cell cycle transcription of KSHV genes regulated by CTCF-cohesin complex.
To examine the potential cell cycle regulation of KSHV latency control genes, we used centrifugal elutriation to fractionate latently infected KSHV BCBL1 cells at different stages in the cell cycle (Fig. 1A). BCBL1 cells were elutriated at different flow rates, stained with PI, and analyzed for DNA content by FACS analysis. G0/G1 phases were represented by fractions elutriated with a 15- to 21-ml/min flow rate. S phase was represented by 24- to 30-ml/min-rated fractions, and the G2/M phases were represented by 34- to 46-ml/min-rated fractions (Fig. 1A). Cell cycle positions were validated using Western blotting (Fig. 1B) with antibodies to cyclin B1 whose protein levels are known to increase at the G2/M phase of the cell cycle (19). We observed that the CTCF and cohesin subunits RAD21 and SMC1 increased abundance throughout the cell cycle. Interestingly, KSHV-encoded LANA accumulated late in the cell cycle, with an expression profile similar to that of cyclin B. Actin levels remained constant throughout the cell cycle. These findings suggest that the expression of CTCF, cohesin, and LANA is subject to cell cycle control.
FIG. 1.
Cell cycle regulation of KSHV genes in latently infected BCBL1 cells. (A) Cell cycle fractionation was monitored by FACS analysis of PI-stained BCBL1 cells after centrifugal elutriation. (B) Western blot analysis of the elutriated BCBL1 cells, with antibodies specific to beta-actin, cyclin B1, SMC1, CTCF, Rad21, and LANA. (C to F) Elutriated BCBL1 cells were analyzed by RT-qPCR for KSHV latency-associated transcripts ORF73, ORF72, and ORF71 (C); KSHV lytic-associated transcripts ORF74, K14, and ORF50 (D); or cellular transcripts for cyclin A2 (Cyc-A2) and B (Cyc-B) (E) or cyclin E1 (Cyc-E1) and cMyc P2 (Myc-P2) transcripts (F). All RT-qPCRs were normalized to cellular actin.
To determine if transcription of KSHV latency control genes was subject to cell cycle regulation, we first examined the mRNA expression profiles of KSHV genes in BCBL1 cells using RT-qPCR relative to cellular actin. Latency-associated transcripts for LANA (ORF73) and vCyclin (ORF72) were readily detected and accumulated throughout the cell cycle, with the highest level occurring in late G2 (fractions of 36 to 46 ml/min). The mRNA expression level for the lytic-associated transcripts K14 and vGPCR (ORF74) was much lower than those of LANA and vCyclin but increased dramatically in the late G2 phase of the cell cycle (Fig. 1D). We did not detect significant levels of immediate-early gene Rta (ORF50) at any stage of the cell cycle, suggesting that these cells were not undergoing lytic cycle reactivation, nor was any viral replication detected by changes in viral genome copy number (data not shown). These results indicate that K14 and vGPCR are expressed during the G2 phase of the cell cycle in latently infected BCBL1 cells. To further validate that elutriated cells expressed the appropriate cell cycle-regulated cellular genes, we examined transcripts of cyclin E, A2, and B, which were expressed in the stage of the cell cycle expected based on FACS profiles (Fig. 1E and F). We also examined c-myc transcripts from the P2 promoter, which was also subject to cell cycle changes, with an accumulation in late G2 (Fig. 1F). These data indicate that KSHV genes in the latency control locus are subject to cell cycle regulation similar to that of cyclin B and myc P2.
RNA splicing of latency-associated transcripts is not altered by the cell cycle.
Since KSHV gene transcripts located in a latency transcriptional unit are produced by complex splicing, it was reasonable to test if the splicing mechanism was subject to cell cycle regulation. The alternative splicing of latent ORF73/ORF72/ORF71 leads to the production of a 5.3-kb tricistronic RNA (encoding LANA, vCyclin, and vFLIP, called LT1), a 1.7-kb bicistronic RNA (encoding vCyclin and vFLIP, called LT2), and a 3.3-kb monocistronic RNA (encoding only LANA-1) (38, 44). Primers used in RT-qPCR were specifically designed to detect LTc (no spliced latent transcript; LTc-F2 and LT-R2), LT1 (LT1-F3 and LT-R2), and LT2 (LT2-F2 and LT-R2) (Fig. 2A). The RT-qPCR assay revealed that production of LTc, LT1, and LT2 increased in later phases of the cell cycle, similar to each transcription pattern of vFLIP, vCyclin, and LANA (Fig. 2B to D). According to these patterns, relative abundance of LT2 or LT1 to LTc was consistent throughout the cell cycle of BCBL1 cells (LT1/LTc) (Fig. 2E and data not shown). The RNA splicing patterns were further confirmed by semiquantitative RT-PCR using different primer sets and analyzed by agarose gel electrophoresis (data not shown). These results suggest that transcription initiation, and not alternative splicing, is the primary control of cell cycle transcription for the leftward latency transcripts. However, we cannot exclude that some splicing events may be subject to cell cycle control.
FIG. 2.
RNA splicing of KSHV latency transcripts does not vary across the cell cycle. (A) Diagram of KSHV latency transcriptional unit. The arrangement and orientation of KSHV genes refer to nucleotide positions designated by Russo et al. (37). Short or long spliced regions were denoted in blue. Pairs of RT-qPCR primers were denoted below each latent transcript; LTc-F2 and LT-R2 for LTc, LT1-F3 and LT-R2 for LT1, and LT2-F2 and LT-R2 for LT2. LT1-F3 is a junction primer that binds to a part of one spliced stretch (red box on the left) and the first six bases of the other spliced region (red box on the right). (B to D) RT-qPCR analysis of latency transcripts for LTc (B), LT1 (C), and LT2 (D) are quantified relative to cellular actin for various stages of the cell cycle, indicated by ml/min of elutriated fractions. (E) The relative abundance of LT2 to LTc is quantified at various stages across the cell cycle and revealed little change in splice products, as a function of the cell cycle.
Cell cycle enrichment of cohesins and CTCF at the KSHV latency control region.
We previously found that cohesins colocalize with CTCF at the KSHV latency control region (42). We therefore asked whether the CTCF or cohesin subunits may bind to this region in a cell cycle-dependent manner. To this end, we performed ChIP assays with BCBL1 cells fractionated throughout the cell cycle by centrifugal elutriation (Fig. 3). ChIP DNA was analyzed by real-time PCR and primers designed to detect enrichment of the CTCF-cohesin binding site in the KSHV latency control region (KSHV 127556-127631) and cellular cMyc locus (promoter 2 located in exon 1), along with an experimental negative control region (KSHV 54721-55080). The control ChIP assay with anti-IgG antibodies showed no specificity to the enrichment of CTCF or cohesin subunits at any of these DNA regions. However, the ChIP assay with either anti-CTCF or anti-cohesin antibodies demonstrated that these proteins bound with high specificity to the KSHV latency control region, and to cMyc P2, but not to the negative control region (KSHV 54721-55080) (Fig. 3). The binding activities of CTCF were relatively high in the late G1 phase (fraction, 18 ml/min), peaked at approximately mid-S phase (fraction, 30 ml/min), and then decreased in the G2/M phase (fraction, 46 ml/min). The cohesin subunits followed a pattern similar to that of CTCF but showed a large peak of enrichment in mid-S phase. RAD21 and SMC3 demonstrated peak binding in mid-S phase (fraction, 30 ml/min), while SMC3 appeared to bind slightly earlier in S phase for both the KSHV and cMyc control regions. The cell cycle pattern of binding was strikingly similar at the KSHV latency control region and the cMyc P2 binding sites. These results indicate that the CTCF and cohesin chromatin binding is cell cycle regulated. These results also suggest that CTCF-cohesin binding may contribute to cell cycle-regulated transcription at these loci.
FIG. 3.
Cell cycle-dependent association of CTCF and cohesins with the KSHV latency control region. BCBL1 cells elutriated over the cell cycle were assayed by ChIP, with antibodies specific to RAD21, SMC1, SMC3, CTCF, or control IgG. Resultant ChIP products were analyzed by real-time PCR, with primers for the KSHV CTCF binding site (ORF73/72/71 control region; KSHV 127556-127631), a negative control region (KSHV 54721-55080), and a CTCF binding site in the human c-myc P2 promoter (42). Both CTCF and cohesins colocalized at CTCF binding sites and were most enriched at mid-S phase of the BCBL1 cell cycle.
CTCF binding sites are required for cell cycle-regulated transcription at the latency control region.
To determine if the CTCF-cohesin binding contributes to the cell cycle regulation of KSHV gene transcription, we examined the transcription profile for a KSHV bacterial artificial chromosome (BAC) lacking all three CTCF binding sites in the latency control region (42). Stable 293T cell pools containing either KSHV WT BAC or mutant BAC were fractionated by centrifugal elutriation and prepared for qPCR analysis of viral and cellular mRNA. The cell cycle profile of each elutriated fraction was determined by PI staining and FACS analysis (Fig. 4A). Transcripts for cyclin E, A, and B were monitored and found to be enriched at the appropriate stages of the cell cycle (data not shown). In KSHV WT BAC-transfected 293 cells, we found that RNA levels for KSHV latency-associated transcripts (K14, ORF74, ORF73, ORF72) increased throughout the cell cycle and peaked in G2 (Fig. 4B and C). A similar pattern of cell cycle control was observed in the latently infected BCBL1 lymphoma cell line, although the amplitude of change was greater in the BCBL1 cells (Fig. 1D). In contrast, the KSHV mutant BAC expressed a different pattern of latency-associated transcription, with little change across the cell cycle, and a small peak occurring in mid-S phase (Fig. 4B and C). These results suggested that intact CTCF-cohesin binding sites are required for the proper cell cycle regulation of viral gene transcription.
FIG. 4.
Mutation of CTCF binding sites in the KSHV latency control region abolishes cell cycle transcription regulation. 293T cells were transfected with either KSHV WT BAC or CTCF deletion mutant BAC, followed by centrifugal elutriation to analyze cell cycle profiles of KSHV latency and lytic gene transcription. (A) FACS analysis of PI-stained 293T cells that were transfected with WT or mutant BACs and fractionated by centrifugal elutriation. (B) RT-qPCR was used to analyze WT (left series of bars) and mutant (right series of bars) KSHV BAC RNA for K14 (top) or ORF74 (bottom) at various stages of the cell cycle, as indicated by elutriated fraction number. (C) Same analysis as in panel B, except that KSHV genes for ORF72 (top) and ORF73 (lower) were analyzed. All RT-qPCR was quantified relative to the GFP transcripts encoded by the BAC vector for each sample.
CTCF binding sites are required to suppress K14-ORF74 transcription.
To further examine the functional role of CTCF binding sites at the KSHV latency control region, we assayed the effects of CTCF deletion or substitution mutations on the ability to regulate latent or lytic gene expression in a reporter plasmid. The three CTCF binding sites have been identified previously by DNase I footprinting (42). Site-directed mutations (SDMs) in each of the three binding sites were introduced and assayed for their ability to disrupt CTCF binding in vitro using an EMSA (Fig. 5A and B). We found that substitution mutation of at least six nucleotides was required for complete disruption of CTCF binding in vitro (data not shown). To assay the effects of CTCF binding site mutations on transcription control, we generated a series of pGL3-based reporter gene constructs that were fused with DNA regions flanking either the rightward K14 promoter (positions −298 to +209) (KSHV 127596-127647) or the leftward ORF73 promoter (positions −13 to +623) (KSHV 127925-127871) (Fig. 5C). The pair of reporter gene constructs either contains a WT CTCF-cohesin binding site (WT construct) or SDMs in all three CTCF-cohesin binding sites (Fig. 5A) or contains a deletion mutation generated by Lox P substitution (LoxP), as described previously (42). Mutation of the CTCF-cohesin binding site in either the LoxP deletion or site-directed mutant of the pGL3-K14 constructs increased luciferase activity by approximately three or five times, respectively, relative to the luciferase activity of the WT pGL-K14 construct (Fig. 5D). In contrast, the CTCF deletion or substitution mutant had little effect on the transcription levels of the latency-associated ORF73 promoter pGL3-73. These results indicate that the CTCF-cohesin complex functions to repress transcription of the K14-ORF74 promoter but has little effect on the ORF73 promoter, at least in the context of transient transfection of reporter plasmids.
FIG. 5.
CTCF binding sites suppress transcription of the K14 promoter. (A) Site-directed mutagenesis of three CTCF binding sites is indicated. Mutated nucleotides are marked in red. (B) DNA binding was monitored by EMSA for CTCF at differential concentrations of CTCF protein. Radiolabeled oligonucleotide probes used were a negative control (Cntrl), the WT CTCF binding site 3 (WT-BS3), and the mutated CTCF binding site 3 (SDM-BS3), as indicated. (C) Schematic description of luciferase reporter constructs containing either KSHV ORFK14 (pGL3-K14) or ORF73 (pGL3-73) promoter regions. (D) Relative luciferase assays with pGL3-K14- or pGL3-73-derived luciferase constructs. 293T cells were transfected with 1 μg of the indicated luciferase constructs per 1 million cells. A total of 100 ng of the plasmid pGL4.74 (Renilla internal luciferase control vector) was used to monitor transfection efficiency. Firefly luciferase activity was subsequently normalized with Renilla luciferase activity.
CTCF and cohesin interactions and subcellular localizations change across the cell cycle.
To explore the molecular basis for the cell cycle regulation of transcription, we examined the cell cycle dependence of the protein-protein interaction between CTCF and cohesins. Co-IP between CTCF and cohesin subunits (SMC3 and RAD21) could be readily demonstrated in asynchronous BCBL1 cell extracts (Fig. 6A). The cell cycle dependence of this interaction was investigated using whole-cell extracts of BCBL1 cells that were cell cycle fractionated by centrifugal elutriation (Fig. 6B). Immunoprecipitations (IPs) with antibodies to SMC1 (Fig 6C, top) and SMC3 (Fig. 6C, bottom) were probed by Western blotting with anti-CTCF. We detected elevated levels of CTCF in the G1 and S phase fractions (18 to 30 ml/min) and reduced levels at later phases of the cell cycle (38 to 46 ml/min) (Fig. 6C). In contrast, the interaction intensity between SMC1 and SMC3 (top) and SMC3 and RAD21 (bottom) was not reduced at the later phase of the cell cycle (Fig. 6C). These IP experiments suggest that the CTCF and cohesin interaction occurs in G1/S and dissociates in G2/M.
FIG. 6.
Cell cycle regulation of CTCF-cohesin interaction and subcellular colocalization. (A) Asynchronous BCBL1 cell extracts were subjected to IP with antibodies to RAD21 (left) or SMC3 (right) or control IgG and then assayed by immunoblotting (IB) with CTCF-specific antibody. (B) FACS analysis of PI-stained BCBL1 cells fractionated by centrifugal elutriation. (C) Cell cycle fractions were analyzed by IP, with antibody to SMC1 (top), followed by IB with SMC1, CTCF, or SMC3 antibodies (as indicated). IPs with antibody to SMC3 (bottom) were analyzed by IP, with antibodies to SMC1, CTCF, and RAD21 (as indicated). (D and E) Cell cycle-fractionated BCBL1 cells were assayed by indirect IF with anti-RAD21 monoclonal antibody (green) and anti-CTCF polyclonal antibody (red). Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole) (data not shown). (D to F) The images were obtained at various increasing magnifications.
To further investigate potential cell cycle changes in CTCF-cohesin interactions, we performed an immunofluorescence (IF) assay to test whether in vivo colocalization of CTCF and cohesin is dependent on the cell cycle. BCBL1 cells were fractionated by centrifugal elutriation and subjected to IF with anti-RAD21 monoclonal mouse antibody (green) and anti-CTCF polyclonal rabbit antibody (red) (Fig. 6D to F). Confocal analysis of CTCF and RAD21 colocalization revealed that in vivo colocalization of the two proteins was moderate at the G1 phase, strongest at S phase, and then weakest at G2/M phase. Interestingly, endogenous CTCF appeared to traffic from the nuclear periphery to the nuclear core in a cell cycle-dependent manner. We detected most CTCF as diffusely nuclear in G1, accumulating in the central nuclear compartment at the S phase, and enriched at the nuclear periphery at the G2/M phase. In contrast, endogenous RAD21 was dispersed in the nucleus throughout the cell cycle, even though a small portion of RAD21 was observed trafficking to the nuclear periphery with CTCF. These findings suggest that the protein-protein interaction between CTCF and cohesins may be regulated in part by the cell cycle-dependent changes in the subcellular distribution and trafficking of CTCF.
Rad21 represses transcription of CTCF-regulated KSHV lytic genes.
To investigate whether the interaction of cohesins with CTCF played an important role in the cell cycle regulation of transcription, we generated expression vectors for FLAG-tagged RAD21 and CTCF. We also generated a fusion protein that covalently links CTCF to RAD21 through a flexible glycine-alanine peptide linker (Fig. 7A). Flag-tagged CTCF, RAD21, and CTCF-RAD21 were first assayed for protein expression levels in transient transfected 293T cells (Fig. 7A, bottom). Western blotting revealed that all Flag-tagged constructs expressed each corresponding protein with the expected molecular weight. We first tested the effect of CTCF and RAD21 expression vectors alone and in combination on the pGL3-K14 or pGL3-73 reporter constructs (Fig. 7B). We found that ectopic expression of CTCF enhanced pGL3-K14 by approximately sixfold, while the pGL3-73 promoter was activated by less than twofold. Addition of RAD21 by itself inhibited pGL3-K14 by twofold but had no effect on pGL3-73. Combining RAD21 with CTCF inhibited CTCF activation of pGL3-K14 by twofold but enhanced CTCF activation of pGL3-73 by twofold (Fig. 7B). The RAD21 inhibition of CTCF activation of pGL3-73 was also shown to be dose dependent on RAD21 expression (data not shown). The RAD21 inhibition of CTCF activation of pGL3-K14 was also shown to be dose dependent on RAD21 expression (data not shown). To further explore the potential function of RAD21 on CTCF transcription properties, we next examined the properties of the CTCF-RAD21 fusion construct on these reporter plasmids (Fig. 7C). As shown in Fig. 7B, CTCF by itself activated transcription of pGL3-K14 but had little effect on pGL3-73. In contrast, expression of CTCF-RAD21 fusion protein, like RAD21 alone, had no significant effect on the reporter constructs. Since the CTCF-RAD21 construct expresses at levels similar to those of CTCF alone, these data suggest that the RAD21 fusion blocks the transcription activation function of CTCF at the pGL3-K14 promoter.
FIG. 7.
CTCF-RAD21 interaction represses KSHV transcription. (A) Schematic of Flag-tagged RAD21, CTCF, and CTCF-RAD21 (C-R) fusion protein constructs. FLAG-tagged constructs were transfected into 293T cells, and their expression was determined by Western blotting, with antibody to Flag. (B) Luciferase assays with pGL3-K14 or pGL3-73 luciferase constructs in the presence of ectopic expression of FLAG-CTCF, FLAG-RAD21, or a combination of the two, as indicated. (C) Same assays as in panel B, except FLAG-CTCF-RAD21 fusion protein was included. (D) BCBL1 cells were transfected with FLAG-RAD21, FLAG-CTCF, or FLAG-CTCF-RAD21, along with mGFP expression vector. GFP-positive BCBL1 cells were sorted, and KSHV gene transcription for K14, ORF74, ORF73, or ORF72 was quantified by RT-qPCR relative to cotransfected mGFP.
To further investigate the ability of RAD21 to regulate CTCF transcription function, we assayed the ability of CTCF, RAD21, or CTCF-RAD21 fusion protein to affect the expression of endogenous KSHV genes (Fig. 7D). Flag-tagged CTCF, Rad21, and CTCF-Rad21 fusion constructs were nucleofected to BCBL1 cells, along with pmaxGFP plasmids, and cells were sorted for green fluorescent protein (GFP) expression. RT-qPCR assays were conducted to determine cellular and KSHV gene transcription profiles. We found that Flag-tagged CTCF and RAD21 constructs did not cause a significant effect on the endogenous KSHV genes for K14, ORF74, ORF73, or ORF72. However, when CTCF-RAD21 fusion constructs were introduced, all KSHV genes assayed were significantly repressed (Fig. 7D). These findings demonstrate that the forced interaction of CTCF with RAD21, as occurs in the CTCF-RAD21 fusion protein, constitutively represses transcription of KSHV genes in a latently infected lymphoma cell line.
DISCUSSION
CTCF and cohesins colocalize at several key regulatory sites in the human and mouse genomes, including at the cMyc promoter and the Igf2/H19-imprinted loci. In this work, we have further characterized the CTCF-cohesin interaction at the latency control region of the KSHV genome. We found that KSHV latency genes are subject to cell cycle regulation (Fig. 1). Moreover, we found that the cohesin subunits SMC1, SMC3, and RAD21 dissociate from the CTCF binding sites in the G2 and M phases (Fig. 3). Deletion of CTCF binding sites altered the normal cell cycle control of transcription for KSHV latency-associated genes, including LANA (ORF73), vCyclin (ORF72), and vGPCR (ORF74) (Fig. 4). Co-IP revealed that the CTCF-cohesin interaction is diminished in the G2/M phase of the cell cycle (Fig. 5). Furthermore, the subnuclear distribution of CTCF changes across the cell cycle, suggesting that CTCF trafficking from the nuclear center to the nuclear periphery may be partly responsible for the changes in transcription regulation (Fig. 6D to F). Finally, we show that a fusion protein that enforces an association between CTCF and RAD21 functions as a potent transcription repressor of KSHV latency-associated genes. We conclude that cohesin interaction with CTCF is involved in the cell cycle regulation of transcription and that cohesins alter the transcription properties of CTCF.
CTCF is known to have complex transcription regulatory properties, including both activator and repressorlike features. We found that ectopic expression of CTCF activated K14 transcription, as was observed on a reporter plasmid in transient transfections (Fig. 7C). However, deletion of the CTCF binding site in the same reporter gene led to an increase in gene expression (Fig. 5D). This suggests that CTCF can function as an activator when supplied ectopically but that endogenous levels of CTCF function as a repressor. This could be explained by the existence of a limiting corepressor associated with endogenous CTCF or by a posttranslational modification of endogenous CTCF that mediates repression activity. Evidence for both of these mechanisms exists. Recently, sumoylation of CTCF has been shown to confer transcription repression function to CTCF (25). Earlier studies have implicated casein kinase II phosphorylation of CTCF in the activation function (9) and a poly-ADP ribosylation requirement for enhancer-promoter interaction function (50). Potential corepressors of CTCF include PC2, a polycomb protein and SUMO E3 ligase (25), and nucleophosmin, a nucleolar histone chaperone (51). Additionally, noncoding RNAs can evict CTCF from overlapping chromosome binding sites and alter transcription control at that locus (21). Remarkably, all of these mechanisms may occur at the KSHV latency control region, where CTCF sites overlap the 5′ untranslated region of the LANA-vCyclin latency transcript. In addition to these CTCF modulatory mechanisms, our studies provide evidence that cell cycle-dependent association with cohesins can also modulate CTCF transcription regulatory function.
Cohesins are known to function by forming a ringlike structure that provides the sister chromatid cohesion necessary for chromosome segregation (29, 34). Cohesin assembly and disassembly on chromatin are known to be cell cycle regulated. At centromeres, cohesin dissociation is triggered by the cell cycle-regulated proteolysis of RAD21 through the separase pathway (48), while loss of cohesin at the chromosome arms is mediated through posttranslational modifications, including phosphorylation (28). In addition to its function in sister chromatid cohesion, cohesins have been implicated in various gene regulatory functions, including heterochromatin formation in Saccharomyces cerevisiae (6), enhancer-promoter interactions in Drosophila (36), and insulator function at the human and mouse H19/Igf2-imprinted loci (47). Our studies suggest that cohesins can also confer a cell cycle-dependent gene regulatory activity at the KSHV latency control region. Cohesin subunit binding was most enriched at mid-S phase, when sister chromatid cohesion is likely to be formed. Sister chromatid cohesion may produce topological constraints on RNA polymerase II transcription. After the S phase, cohesins dissociate from the latency control region and fail to co-IP with CTCF (Fig. 3 and 6). The loss of cohesin binding correlates well with a loss of transcription repression, especially at the K14-ORF74 promoter. Prevention of the dissociation of cohesin from CTCF by expression of the CTCF-RAD21 fusion protein caused the constitutive repression of KSHV genes K14 and ORF74, as well as ORF73 and ORF72 (Fig. 7D). Since cohesin subunits dissociate from the CTCF protein and CTCF chromatin binding sites in late S phase, we propose that cohesins provide a cell cycle-dependent transcription repressor function. CTCF may serve as a scaffold that represses transcription when bound to cohesins (S phase) but allows active transcription when cohesins have dissociated (G2/M).
Changes in CTCF subcellular localization may also contribute to cell cycle control of gene expression. We observed that CTCF subcellular localization altered significantly at different stages of the cell cycle (Fig. 6D to F). Similar changes in CTCF subnuclear localization have been reported. In one study, CTCF localized to the nucleoli through an interaction with nucleophosmin (51). This interaction and subcellular localization correlated with the insulator function of CTCF. In a second study, the nucleolar localization of CTCF was dependent on poly-ADP ribosylation (45). Our studies indicate that CTCF accumulates in the nuclear periphery at the late G2/M and early G1 phases and enters the nuclear interior in the late G1 and S phases. The different subnuclear localization patterns of CTCF may be explained by the different cell types and cell cycle isolation procedures used in the different studies. In our study, the colocalization with cohesins was largely restricted to the nuclear interior and reduced at the nuclear periphery. The nuclear periphery has been associated with transcription inactivity and heterochromatin (12). Although we have not determined whether KSHV genomes similarly relocalize in the cell cycle during latency, similar viral genomes have been shown to alter the subnuclear position during different stages of their life cycle, including latency and productive infection (3, 26). We propose that the interaction with cohesins in the S phase initiates a change in the function and subcellular localization of CTCF (Fig. 8). We also suggest that DNA replication and the formation of sister chromatid junctions by cohesins that are associated with CTCF are also likely to alter CTCF functional properties. For KSHV, we find that these interactions are essential for maintaining the proper transcription control of the latency genes. This may partly explain why viral genomes that lack CTCF-cohesin binding sites are genetically unstable (42). The cell cycle-dependent interaction of CTCF with cohesins may also help explain the complex properties of CTCF at other chromosomal loci, including at the c-myc promoter and the H19-imprinted locus.
FIG. 8.
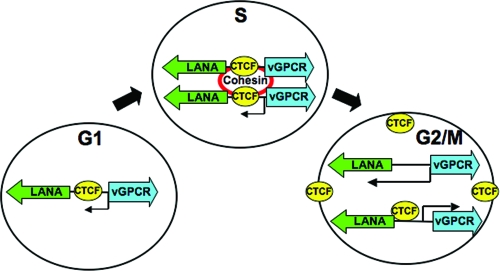
Cell cycle regulation of cohesin interaction with CTCF regulates KSHV latent cycle gene expression. Speculative model depicting the G1-, S-, and G2-dependent changes in KSHV gene expression, as indicated by the thickness of each arrow emerging from the left (ORF73/72/71 transcript) and right (K14-ORF74) promoters. CTCF interaction with cohesins peaks in mid-S phase, when cellular and KSHV genomes have divided. Cohesins are envisioned as maintaining viral sister chromatid cohesion through the S phase. Loss of cohesin binding in the G2 phase leads to a derepression of KSHV promoters, with a most notable increase in the rightward transcript for K14-ORF74. During late G2, CTCF binding to KSHV genome decreases, and CTCF protein relocalizes to the nuclear periphery.
Acknowledgments
We thank William Stedman for initiating this study and for valuable suggestions. We also thank Andreas Wiedmer, Jayaraju Dheekollu, and Andrew Rennekamp for advice and technical support with centrifugal elutriation. We thank Jamie Hayden and the Wistar Microscopy Facility for technical support with confocal microscopy. We thank Jeffery Faust for help with flow cytometry and cell cycle analysis.
We are indebted to the Wistar Institute Cancer Biology Training Grant and to the NIH grant CA117830 for support.
Footnotes
Published ahead of print on 15 April 2009.
REFERENCES
- 1.Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. G. Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gerhengorn, and E. A. Mesri. 1998. G-protein coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 39186-89. [DOI] [PubMed] [Google Scholar]
- 2.Barski, A., S. Cuddapah, K. Cui, T. Y. Roh, D. E. Schones, Z. Wang, G. Wei, I. Chepelev, and K. Zhao. 2007. High-resolution profiling of histone methylations in the human genome. Cell 129823-837. [DOI] [PubMed] [Google Scholar]
- 3.Bell, P., P. M. Lieberman, and G. G. Maul. 2000. Lytic but not latent replication of Epstein-Barr virus is associated with PML and induces sequential release of nuclear domain 10 proteins. J. Virol. 7411800-11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blais, A., and B. D. Dynlacht. 2004. Hitting their targets: an emerging picture of E2F and cell cycle control. Curr. Opin. Genet. Dev. 14527-532. [DOI] [PubMed] [Google Scholar]
- 5.Cesarman, E., R. G. Nador, F. Bai, R. A. Bohenzky, J. J. Russo, P. S. Moore, Y. Chang, and D. M. Knowles. 1996. Kaposi's sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J. Virol. 708218-8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, C. R., C. S. Wu, Y. Hom, and M. R. Gartenberg. 2005. Targeting of cohesin by transcriptionally silent chromatin. Genes Dev. 193031-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chau, C. M., X. Y. Zhang, S. B. McMahon, and P. M. Lieberman. 2006. Regulation of Epstein-Barr virus latency type by the chromatin boundary factor CTCF. J. Virol. 805723-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorsett, D. 2007. Roles of the sister chromatid cohesion apparatus in gene expression, development, and human syndromes. Chromosoma 1161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Kady, A., and E. Klenova. 2005. Regulation of the transcription factor, CTCF, by phosphorylation with protein kinase CK2. FEBS Lett. 5791424-1434. [DOI] [PubMed] [Google Scholar]
- 10.Fedoriw, A. M., P. Stein, P. Svoboda, R. M. Schultz, and M. S. Bartolomei. 2004. Transgenic RNAi reveals essential function for CTCF in H19 gene imprinting. Science 303238-240. [DOI] [PubMed] [Google Scholar]
- 11.Filippova, G. N., A. Lindblom, L. J. Meincke, E. M. Klenova, P. E. Neiman, S. J. Collins, N. A. Doggett, and V. V. Lobanenkov. 1998. A widely expressed transcription factor with multiple DNA sequence specificity, CTCF, is localized at chromosome segment 16q22.1 within one of the smallest regions of overlap for common deletions in breast and prostate cancers. Genes Chromosomes Cancer 2226-36. [PubMed] [Google Scholar]
- 12.Finlan, L. E., D. Sproul, I. Thomson, S. Boyle, E. Kerr, P. Perry, B. Ylstra, J. R. Chubb, and W. A. Bickmore. 2008. Recruitment to the nuclear periphery can alter expression of genes in human cells. PLoS Genet. 4e1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giacinti, C., and A. Giordano. 2006. RB and cell cycle progression. Oncogene 255220-5227. [DOI] [PubMed] [Google Scholar]
- 14.Haering, C. H., A. M. Farcas, P. Arumugam, J. Metson, and K. Nasmyth. 2008. The cohesin ring concatenates sister DNA molecules. Nature 454297-301. [DOI] [PubMed] [Google Scholar]
- 15.Hark, A. T., C. J. Schoenherr, D. J. Katz, R. S. Ingram, J. M. Levorse, and S. M. Tilghman. 2000. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature 405486-489. [DOI] [PubMed] [Google Scholar]
- 16.Heath, H., C. R. de Almeida, F. Sleutels, G. Dingjan, S. van de Nobelen, I. Jonkers, K. W. Ling, J. Gribnau, R. Renkawitz, F. Grosveld, R. W. Hendriks, and N. Galjart. 2008. CTCF regulates cell cycle progression of alphabeta T cells in the thymus. EMBO J. 272839-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirano, T. 2006. At the heart of the chromosome: SMC proteins in action. Nat. Rev. Mol. Cell Biol. 7311-322. [DOI] [PubMed] [Google Scholar]
- 18.Ishihara, K., M. Oshimura, and M. Nakao. 2006. CTCF-dependent chromatin insulator is linked to epigenetic remodeling. Mol. Cell 23733-742. [DOI] [PubMed] [Google Scholar]
- 19.Jackman, M., M. Firth, and J. Pines. 1995. Human cyclins B1 and B2 are localized to strikingly different structures: B1 to microtubules, B2 primarily to the Golgi apparatus. EMBO J. 141646-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krantz, I. D., J. McCallum, C. DeScipio, M. Kaur, L. A. Gillis, D. Yaeger, L. Jukofsky, N. Wasserman, A. Bottani, C. A. Morris, M. J. Nowaczyk, H. Toriello, M. J. Bamshad, J. C. Carey, E. Rappaport, S. Kawauchi, A. D. Lander, A. L. Calof, H. H. Li, M. Devoto, and L. G. Jackson. 2004. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat. Genet. 36631-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefevre, P., J. Witham, C. E. Lacroix, P. N. Cockerill, and C. Bonifer. 2008. The LPS-induced transcriptional upregulation of the chicken lysozyme locus involves CTCF eviction and noncoding RNA transcription. Mol. Cell 32129-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling, J. Q., T. Li, J. F. Hu, T. H. Vu, H. L. Chen, X. W. Qiu, A. M. Cherry, and A. R. Hoffman. 2006. CTCF mediates interchromosomal colocalization between Igf2/H19 and Wsb1/Nf1. Science 312269-272. [DOI] [PubMed] [Google Scholar]
- 23.Losada, A. 2007. Cohesin regulation: fashionable ways to wear a ring. Chromosoma 116321-329. [DOI] [PubMed] [Google Scholar]
- 24.Lu, F., L. Day, S. J. Gao, and P. M. Lieberman. 2006. Acetylation of the latency-associated nuclear antigen regulates repression of Kaposi's sarcoma-associated herpesvirus lytic transcription. J. Virol. 805273-5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacPherson, M. J., L. G. Beatty, W. Zhou, M. Du, and P. D. Sadowski. 2009. The CTCF insulator protein is posttranslationally modified by SUMO. Mol. Cell. Biol. 29714-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maul, G. G. 2008. Initiation of cytomegalovirus infection at ND10. Curr. Top. Microbiol. Immunol. 325117-132. [DOI] [PubMed] [Google Scholar]
- 27.Moon, H., G. Filippova, D. Loukinov, E. Pugacheva, Q. Chen, S. T. Smith, A. Munhall, B. Grewe, M. Bartkuhn, R. Arnold, L. J. Burke, R. Renkawitz-Pohl, R. Ohlsson, J. Zhou, R. Renkawitz, and V. Lobanenkov. 2005. CTCF is conserved from Drosophila to humans and confers enhancer blocking of the Fab-8 insulator. EMBO Rep. 6165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakajima, M., K. Kumada, K. Hatakeyama, T. Noda, J. M. Peters, and T. Hirota. 2007. The complete removal of cohesin from chromosome arms depends on separase. J. Cell Sci. 1204188-4196. [DOI] [PubMed] [Google Scholar]
- 29.Nasmyth, K., and C. H. Haering. 2005. The structure and function of SMC and kleisin complexes. Annu. Rev. Biochem. 74595-648. [DOI] [PubMed] [Google Scholar]
- 30.Nasmyth, K., and A. Schleiffer. 2004. From a single double helix to paired double helices and back. Philos. Trans. R. Soc. Lond. B 35999-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nurse, P. 2002. Cyclin dependent kinases and cell cycle control (Nobel lecture). Chembiochem 3596-603. [DOI] [PubMed] [Google Scholar]
- 32.Ohlsson, R., R. Renkawitz, and V. Lobanenkov. 2001. CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends Genet. 17520-527. [DOI] [PubMed] [Google Scholar]
- 33.Parelho, V., S. Hadjur, M. Spivakov, M. Leleu, S. Sauer, H. C. Gregson, A. Jarmuz, C. Canzonetta, Z. Webster, T. Nesterova, B. S. Cobb, K. Yokomori, N. Dillon, L. Aragon, A. G. Fisher, and M. Merkenschlager. 2008. Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell 132422-433. [DOI] [PubMed] [Google Scholar]
- 34.Peters, J. M., A. Tedeschi, and J. Schmitz. 2008. The cohesin complex and its roles in chromosome biology. Genes Dev. 223089-3114. [DOI] [PubMed] [Google Scholar]
- 35.Ritzi, M., K. Tillack, J. Gerhardt, E. Ott, S. Humme, E. Kremmer, W. Hammerschmidt, and A. Schepers. 2003. Complex protein-DNA dynamics at the latent origin of DNA replication of Epstein-Barr virus. J. Cell Sci. 1163971-3984. [DOI] [PubMed] [Google Scholar]
- 36.Rollins, R. A., M. Korom, N. Aulner, A. Martens, and D. Dorsett. 2004. Drosophila Nipped-B protein supports sister chromatid cohesion and opposes the stromalin/Scc3 cohesion factor to facilitate long-range activation of the cut gene. Mol. Cell. Biol. 243100-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 9314862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarid, R., J. S. Wiezorek, P. S. Moore, and Y. Chang. 1999. Characterization and cell cycle regulation of the major Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) latent genes and their promoter. J. Virol. 731438-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simmons Kovacs, L. A., D. A. Orlando, and S. B. Haase. 2008. Transcription networks and cyclin/CDKs: the yin and yang of cell cycle oscillators. Cell Cycle 72626-2629. [DOI] [PubMed] [Google Scholar]
- 40.Simonis, M., P. Klous, E. Splinter, Y. Moshkin, R. Willemsen, E. de Wit, B. van Steensel, and W. de Laat. 2006. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C). Nat. Genet. 381348-1354. [DOI] [PubMed] [Google Scholar]
- 41.Splinter, E., H. Heath, J. Kooren, R. J. Palstra, P. Klous, F. Grosveld, N. Galjart, and W. de Laat. 2006. CTCF mediates long-range chromatin looping and local histone modification in the beta-globin locus. Genes Dev. 202349-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stedman, W., H. Kang, S. Lin, J. L. Kissil, M. S. Bartolomei, and P. M. Lieberman. 2008. Cohesins localize with CTCF at the KSHV latency control region and at cellular c-myc and H19/Igf2 insulators. EMBO J. 27654-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens, C., and N. B. La Thangue. 2003. E2F and cell cycle control: a double-edged sword. Arch. Biochem. Biophys. 412157-169. [DOI] [PubMed] [Google Scholar]
- 44.Talbot, S. J., R. A. Weiss, P. Kellam, and C. Boshoff. 1999. Transcriptional analysis of human herpesvirus-8 open reading frames 71, 72, 73, K14, and 74 in a primary effusion lymphoma cell line. Virology 25784-94. [DOI] [PubMed] [Google Scholar]
- 45.Torrano, V., J. Navascues, F. Docquier, R. Zhang, L. J. Burke, I. Chernukhin, D. Farrar, J. Leon, M. T. Berciano, R. Renkawitz, E. Klenova, M. Lafarga, and M. D. Delgado. 2006. Targeting of CTCF to the nucleolus inhibits nucleolar transcription through a poly(ADP-ribosyl)ation-dependent mechanism. J. Cell Sci. 1191746-1759. [DOI] [PubMed] [Google Scholar]
- 46.Wallace, J. A., and G. Felsenfeld. 2007. We gather together: insulators and genome organization. Curr. Opin. Genet. Dev. 17400-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wendt, K. S., K. Yoshida, T. Itoh, M. Bando, B. Koch, E. Schirghuber, S. Tsutsumi, G. Nagae, K. Ishihara, T. Mishiro, K. Yahata, F. Imamoto, H. Aburatani, M. Nakao, N. Imamoto, K. Maeshima, K. Shirahige, and J. M. Peters. 2008. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature 451796-801. [DOI] [PubMed] [Google Scholar]
- 48.Wirth, K. G., G. Wutz, N. R. Kudo, C. Desdouets, A. Zetterberg, S. Taghybeeglu, J. Seznec, G. M. Ducos, R. Ricci, N. Firnberg, J. M. Peters, and K. Nasmyth. 2006. Separase: a universal trigger for sister chromatid disjunction but not chromosome cycle progression. J. Cell Biol. 172847-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yokomori, K. 2003. SMC protein complexes and the maintenance of chromosome integrity. Curr. Top. Microbiol. Immunol. 27479-112. [DOI] [PubMed] [Google Scholar]
- 50.Yu, W., V. Ginjala, V. Pant, I. Chernukhin, J. Whitehead, F. Docquier, D. Farrar, G. Tavoosidana, R. Mukhopadhyay, C. Kanduri, M. Oshimura, A. P. Feinberg, V. Lobanenkov, E. Klenova, and R. Ohlsson. 2004. Poly(ADP-ribosyl)ation regulates CTCF-dependent chromatin insulation. Nat. Genet. 361105-1110. [DOI] [PubMed] [Google Scholar]
- 51.Yusufzai, T. M., H. Tagami, Y. Nakatani, and G. Felsenfeld. 2004. CTCF tethers an insulator to subnuclear sites, suggesting shared insulator mechanisms across species. Mol. Cell 13291-298. [DOI] [PubMed] [Google Scholar]