Abstract
The magnitude and character of adenovirus serotype 5 (Ad5)-specific T cells were determined in volunteers with and without preexisting neutralizing antibodies (NAs) to Ad5 who received replication-defective Ad5 (rAd5)-based human immunodeficiency virus vaccines. There was no correlation between T-cell responses and NAs to Ad5. There was no increase in magnitude or activation state of Ad5-specific CD4+ T cells at time points where antibodies to Ad5 and T-cell responses to the recombinant gene products could be measured. These data indicate that rAd5-based vaccines containing deletions in the E1, E3, and E4 regions do not induce appreciable expansion of vector-specific CD4+ T cells.
Replication-defective adenoviruses (rAd) have been engineered to provide high levels of expression of foreign inserts with minimum expression of adenovirus proteins, making them excellent candidates for vaccine and gene therapy applications (3, 16). Despite promising immunogenicity, a prophylactic vaccine trial of a serotype 5 rAd (rAd5) vector expressing human immunodeficiency virus (HIV) Gag, Pol, and Nef genes (Step trial) was recently halted due to an increase in HIV infections among volunteers who had preexisting neutralizing antibodies (NAs) to Ad5 (7). This finding raises the possibility that the presence of Ad5-specific T-cell responses (specifically CD4+ T-cell responses) in subjects with preexisting Ad5 NAs could be boosted by rAd5 vaccines, thereby providing an expanded susceptible target cell population that could be more easily infected by HIV. If this mechanism were operative, it would have broad implications for the future use of rAd viruses, and indeed other virus vectors, as vaccines or therapeutic agents within HIV-susceptible populations (2, 12, 15). We therefore measured the frequency, magnitude, and activation status of rAd5-specific T cells in HIV-uninfected volunteers who had received rAd5-based HIV vaccines in the presence or absence of preexisting NAs to Ad5.
We studied 31 volunteers enrolled in two NIAID Institutional Review Board-approved phase I clinical trials of rAd5-based HIV vaccines. VRC 006 was a dose escalation study evaluating a single inoculation of a rAd5 mixture expressing EnvA, EnvB, EnvC, and fusion protein Gag/PolB at 109, 1010, and 1011 total particle units (10). VRC 008 evaluated DNA priming by needle and syringe or Biojector, followed by rAd5 boosting. Both studies enrolled healthy, HIV-uninfected adults; used the same rAd5 products; and evaluated immunogenicity on the day of and 4 weeks after rAd5 immunization. Both of these trials involved rAd5 products that contained deletions in the E1, E3, and E4 regions (8, 10).
NAs to Ad5 were determined for all volunteers as previously described (19). A 90% NA titer of 12 or more was considered positive and taken as evidence of preexisting humoral immunity to Ad5. Volunteers were chosen for assessment of Ad5-specific T-cell responses based upon the availability of peripheral blood mononuclear cell samples at key time points and the presence or absence of preexisting NAs to Ad5. Only volunteers who received the vaccine (not the placebo) were included. Table 1 lists the volunteers who were tested for Ad5-specific T-cell responses and their NA titers to Ad5 before and after rAd5 vaccination. All volunteers, except for one (volunteer 12) who had a less-than-maximum NA titer to Ad5 before vaccination, had an increase in titer by 4 weeks after vaccination, indicating the successful “take” of the rAd5-based vaccine. There was no correlation between rAd5 dose and increase in Ad5 NA titer.
TABLE 1.
Ad5 serostatus before and after vaccination
| Volunteer | Prior DNA immunization | rAd5 dose (PUa) | Ad5 NA titer
|
|
|---|---|---|---|---|
| Prevaccine | Postvaccine | |||
| 1 | No | 1011 | <12 | 739 |
| 2 | No | 1011 | <12 | 834 |
| 3 | No | 1011 | <12 | 4,787 |
| 4 | No | 1011 | <12 | 806 |
| 5 | No | 1011 | <12 | 1,033 |
| 6 | No | 1010 | <12 | 130 |
| 7 | No | 1010 | <12 | 1,354 |
| 8 | Yes | 1010 | <12 | 1,387 |
| 9 | Yes | 1010 | <12 | 575 |
| 10 | Yes | 1010 | <12 | 170 |
| 11 | Yes | 1010 | <12 | >8,748 |
| 12 | Yes | 1010 | <12 | <12 |
| 13 | No | 1011 | 30 | >8,748 |
| 14 | No | 109 | 46 | >8,748 |
| 15 | No | 109 | 70 | 328 |
| 16 | No | 1010 | 176 | >8,748 |
| 17 | No | 1010 | 478 | 6,198 |
| 18 | No | 109 | 2,472 | >8,748 |
| 19 | No | 109 | 3,502 | >8,748 |
| 20 | No | 1010 | 4,820 | >8,748 |
| 21 | No | 109 | 5,078 | >8,748 |
| 22 | No | 1011 | 6,162 | >8,748 |
| 23 | No | 109 | >8,748 | >8,748 |
| 24 | No | 1011 | >8,748 | >8,748 |
| 25 | Yes | 1010 | 643 | >8,748 |
| 26 | Yes | 1010 | 942 | >8,748 |
| 27 | Yes | 1010 | 1,510 | >8,748 |
| 28 | Yes | 1010 | 1,611 | >8,748 |
| 29 | Yes | 1010 | 2,934 | >8,748 |
| 30 | Yes | 1010 | >8,748 | >8,748 |
| 31 | Yes | 1010 | >8,748 | >8,748 |
PU, particle units.
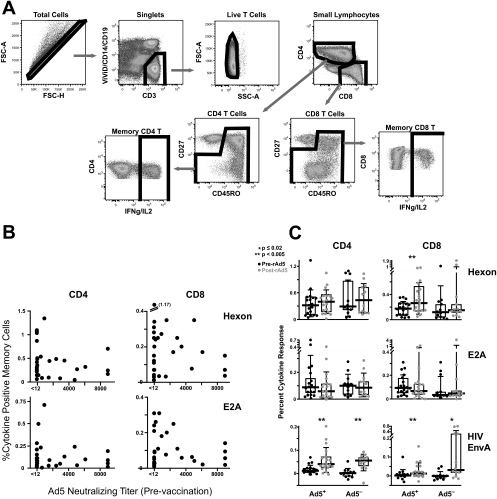
HIV-specific T-cell responses were measured by multiparameter flow cytometry after 6 h of stimulation with peptides (15-mers overlapping by 11) corresponding to the HIV EnvA protein (one of the vaccine inserts expressed in the Ad5 vectors), as previously described (13). Overlapping peptides corresponding to the major Ad5 surface protein (hexon), the Ad5 early regulatory protein (E2A), and Ad5 ORF1, -2, and -3 proteins were used to assess Ad5-specific T-cell responses, and additional markers of cell viability (ViViD), T-cell memory (CD45RO and CD27), and activation/division (CCR5, CD38, HLA-DR, and Ki67) were added to the panel for these assessments. Antibodies and fluorochromes used in this panel were CCR5-Cy7-phycoerythrin (PE), CD38-allophycocyanin, Ki67-fluorescein isothiocyanate, and CD3-Cy7-allophycocyanin, all from BD PharMingen; CD8-Cy55-PE from BD Biosciences; CD27-Cy5-PE and CD45RO-Texas Red-PE, both from Beckman Coulter; CD4-Cy5.5-PE from Caltag; CD14- and CD19-PacificBlue, CD57-QDot545, and HLA-DR-Alexa680, conjugated according to standard protocols [http://drmr.com/abcon/index.html]); gamma interferon-PE and interleukin-2-PE from BD Biosciences; and a violet amine dye from Invitrogen. Cells were analyzed on an LSRII instrument (Becton Dickinson), and data analysis was performed using FlowJo, version 8.1.1 (TreeStar). The gating strategy is shown in Fig. 1A.
FIG. 1.
CD4+ and CD8+ T-cell responses to Ad5. (A) Gating tree used to determine antigen-specific T-cell frequencies. Single CD3+ ViViD− CD14− CD19− cells were gated on CD4 or CD8 cells. Naïve CD27+ CD45RO− cells were gated out, and the frequency of cells expressing gamma interferon (IFNg) and/or interleukin-2 (IL2) was determined. FSC-A, forward scatter area; FSC-H, forward scatter height; SSC-A, side scatter area. (B) Frequencies of CD4+ and CD8+ T-cell responses after stimulation with Ad5 hexon or E2A peptides were plotted against the prevaccination Ad5 NA titer. The prevaccine T-cell response was used. (C) Frequencies of CD4+ and CD8+ T-cell responses to Ad5 hexon, E2A, and HIV EnvA before and 4 weeks after rAd5 vaccination are shown for subjects with (Ad5 NA titer of >12) and without (Ad5 NA titer of <12) preexisting NAs to Ad5. Boxed areas represent interquartile ranges, and horizontal lines represent medians.
Previously, we had found no T-cell responses to Ad5 ORF1, -2, or -3, so data from these antigen stimulations are not shown. As shown in Fig. 1B, T-cell responses to Ad5 hexon and E2A were detected, but there was no association between the NA response to Ad5 and the T-cell responses to these Ad5 proteins. Volunteers with an absence of NAs to Ad5 often had very strong CD4+ and CD8+ T-cell responses to Ad5 proteins. This probably reflects the degree of protein sequence homology between different adenovirus serotypes (11) and suggests that T-cell responses to adenoviruses may be significantly cross-reactive, while NAs are serotype specific. It also indicates that the NA response to Ad5 cannot be used as a surrogate for either a CD4+ or a CD8+ T-cell response to that adenovirus serotype.
We next asked whether Ad5-specific T-cell responses were boosted by a single rAd5 vaccination in subjects with or without preexisting NAs to Ad5. At the time point 4 weeks after vaccination, there was clear evidence of boosting of the insert-specific (EnvA) CD4+ and CD8+ T-cell responses in volunteers with and without preexisting NAs to Ad5 (Fig. 1C). The results of the Ad5-specific responses were consistent across volunteers who had received prior DNA immunization (VRC 008) and those who had not (VRC 006), so the results are combined in Fig. 1C and show no increase in Ad5 hexon- or E2A-specific CD4+ T-cell responses after rAd5 immunization irrespective of Ad5 NA status. There was evidence of an increase in the CD8+ T-cell response to Ad5 hexon (P = 0.004 by paired t test), but not that to E2A, after rAd5 vaccination. These results, while showing evidence of adenovirus-specific CD8+ T-cell boosting by rAd5 vaccination, do not indicate an expansion of Ad5-specific CD4+ T cells that could serve as a substrate for HIV infection in subjects with or without NAs to Ad5.
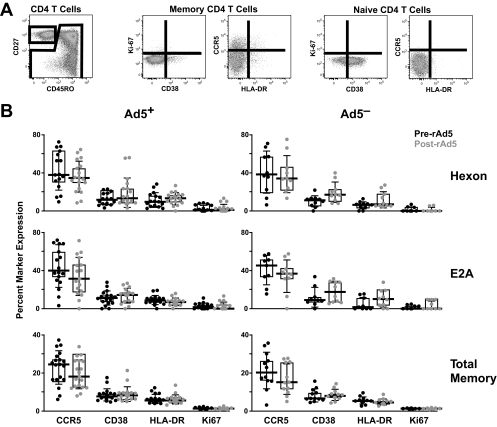
Having failed to demonstrate an expansion of Ad5-specific CD4+ T cells after vaccination, we assessed whether the activation profile of the unexpanded Ad5-specific CD4+ T cells was changed by vaccination. The gating tree is shown in Fig. 2A. Ad5 hexon- and E2A-specific CD4+ T cells expressed activation markers CCR5, CD38, and HLA-DR and a marker of recent cell division, Ki67, more frequently than did total memory CD4+ T cells (Fig. 2B). However, none of these markers were significantly increased on total or Ad5-specific CD4+ T cells after vaccination in volunteers with or without preexisting NAs to Ad5.
FIG. 2.
Vaccine-induced activation of Ad5-specific CD4+ T cells. (A) Total CD4+ memory cells or Ad5-specific CD4+ memory cells (as gated in Fig. 1A) were further defined by expression of Ki67, CD38, CCR5, and HLA-DR. (B) Percentages of Ad5 hexon-specific cells, E2A-specific cells, or total memory CD4+ T cells that express CCR5, CD38, HLA-DR, or Ki67 before and 4 weeks after rAd5 vaccination are shown for subjects with (Ad5 NA titer of >12) (left) and without (Ad5 NA titer of >12) (right) preexisting NAs to Ad5. The phenotype was assessed only for those responders for whom at least 10 cytokine-positive events were counted. None of the comparisons of pre- and postvaccination marker expression were significant at a P value of 0.02 by paired t test. Boxed areas represent interquartile ranges, and horizontal lines represent medians.
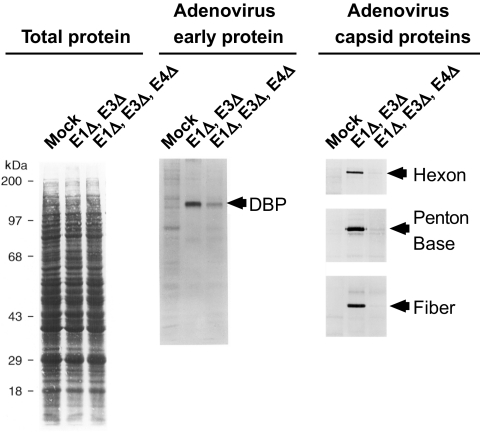
Expansion of Ad5-specific T cells after rAd5-based vaccination or gene therapy has been reported by others (14, 20, 21). Those studies evaluated Ad5-specific responses to rAd5 vectors with only the adenovirus E1 gene deleted (as used in the Step trial vaccines). The vectors used here contained deletions of the adenovirus E1, E3, and E4 genes (8, 10). While adenovirus gene deletions can render the vectors replication defective (6, 9), they do not necessarily completely shut off all adenovirus protein expression (20, 21). To demonstrate the importance of E4 deletions in limiting expression of adenovirus gene products, we measured the level of adenovirus protein synthesis in infected A549 cells as previously described (1, 4, 5). Cells were infected with adenovirus vectors with E1 and E3 deletions or with E1, E3, and E4 deletions at the same multiplicity of infection (10 focus-forming units per cell). At 24 h postinfection, [35S]methionine was added for 1 h. Levels of total and adenovirus protein synthesis in the infected and mock-infected cells were compared (Fig. 3). Adenovirus early protein single-stranded DNA binding protein, as well as late gene products hexon, penton, and fiber, was immunoprecipitated, fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and resolved by autoradiography. The results show that the amount of newly synthesized adenovirus proteins in cells infected with adenovirus with E1, E3, and E4 deletions is significantly lower than that for an adenovirus vector with E1 and E3 deletions. Therefore, our inability to detect a vaccine-induced increase in the frequency and character of the Ad5-specific T-cell response could relate to the very low levels of adenovirus proteins that were probably expressed in vivo by the rAd5 vectors with multiple deletions.
FIG. 3.
Ad5 protein expression in vitro after infection with different Ad5 vectors. A549 cells were infected with adenovirus vectors with E1 and E3 deletions or with E1, E3, and E4 deletions and [35S]methionine labeled, and levels of total and adenovirus protein synthesis in the infected and mock-infected cells were compared after sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography. Markers for the adenovirus early protein single-stranded DNA binding protein (DBP) and capsid proteins hexon, penton base, and fiber are shown.
We were therefore unable to demonstrate (i) that Ad5-specific CD4+ T cells were restricted to subjects with preexisting Ad5 NAs, (ii) that rAd5 vaccination expanded or increased the activation of Ad5-specific CD4+ T cells, or (iii) that there was a substantial effect on the magnitude or character of the Ad5-specific CD4+ T-cell response to vaccination based upon preexisting NAs to Ad5. While the kinetics of Ad5-specific T-cell responses after rAd5-based vaccination are not known, it is clear that insert-specific responses are increased at 4 weeks after vaccination and subsequently contract (10). It is therefore reasonable to assume that if Ad5-specific responses were similarly affected, they would be detected at the 4-week-postvaccination time point.
It is possible that rAd5 vaccines expand a preexisting mucosal T-cell response to Ad5 that is not reflected within the blood. While we do not have mucosal samples from our vaccine volunteers to directly address this possibility, it is likely that expansion of a mucosal response would be reflected to some degree within the blood.
The mechanism underlying the increase in HIV infections in vaccinees with NAs to Ad5 in the Step trial is yet to be determined (2, 7, 12, 15, 17). Confounding factors and alternative hypotheses have recently been proposed to account for the increased acquisition (7, 12, 15, 18). Until there is a better understanding of the processes involved, future studies of rAd5-based products should proceed with appropriate safety considerations and monitoring of adenovirus-specific responses. In addition, the use of vaccine regimens involving single injections of vectors with multiple deletions may help mitigate risk.
Acknowledgments
We thank Norman Letvin and John Mascola for their critical reviews of the manuscript and the VRC 006 and VRC 008 study teams and volunteers for their efforts and dedication.
These studies were funded by NIAID intramural funds and award 38650 from the Bill and Melinda Gates Foundation.
Footnotes
Published ahead of print on 1 April 2009.
REFERENCES
- 1.Anderson, K. P., and D. F. Klessig. 1983. Posttranscriptional block to synthesis of a human adenovirus capsid protein in abortively infected monkey cells. J. Mol. Appl. Genet. 231-43. [PubMed] [Google Scholar]
- 2.Barouch, D. H. 2008. Challenges in the development of an HIV-1 vaccine. Nature 455613-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barouch, D. H., and G. J. Nabel. 2005. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum. Gene Ther. 16149-156. [DOI] [PubMed] [Google Scholar]
- 4.Brough, D. E., V. Cleghon, and D. F. Klessig. 1992. Construction, characterization, and utilization of cell lines which inducibly express the adenovirus DNA-binding protein. Virology 190624-634. [DOI] [PubMed] [Google Scholar]
- 5.Brough, D. E., G. Droguett, M. S. Horwitz, and D. F. Klessig. 1993. Multiple functions of the adenovirus DNA-binding protein are required for efficient viral DNA synthesis. Virology 196269-281. [DOI] [PubMed] [Google Scholar]
- 6.Brough, D. E., A. Lizonova, C. Hsu, V. A. Kulesa, and I. Kovesdi. 1996. A gene transfer vector-cell line system for complete functional complementation of adenovirus early regions E1 and E4. J. Virol. 706497-6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchbinder, S. P., D. V. Mehrotra, A. Duerr, D. W. Fitzgerald, R. Mogg, D. Li, P. B. Gilbert, J. R. Lama, M. Marmor, C. Del Rio, M. J. McElrath, D. R. Casimiro, K. M. Gottesdiener, J. A. Chodakewitz, L. Corey, and M. N. Robertson. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 3721881-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butman, B. T., P. L. Gomez, R. Sheets, J. G. Gall, D. Brough, J. M. Sowers, P. Newton, V. K. Hague, and A. Lizonova. 2006. Manufacture and testing of a multi-clade adenoviral vector-based candidate vaccine against human immunodeficiency virus. BioProcess. J. 515-19. [Google Scholar]
- 9.Butman, B. T., A. Lizonova, D. E. Brough, J. M. Sowers, R. Sheets, J. Gall, P. Newton, and P. Gomez. 2006. Comprehensive characterization of the 293-ORF6 cell line. Dev. Biol. (Basel) 123225-233. [PubMed] [Google Scholar]
- 10.Catanzaro, A. T., R. A. Koup, M. Roederer, R. T. Bailer, M. E. Enama, Z. Moodie, L. Gu, J. E. Martin, L. Novik, B. K. Chakrabarti, B. T. Butman, J. G. Gall, C. R. King, C. A. Andrews, R. Sheets, P. L. Gomez, J. R. Mascola, G. J. Nabel, and B. S. Graham. 2006. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J. Infect. Dis. 1941638-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebner, K., W. Pinsker, and T. Lion. 2005. Comparative sequence analysis of the hexon gene in the entire spectrum of human adenovirus serotypes: phylogenetic, taxonomic, and clinical implications. J. Virol. 7912635-12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fauci, A. S., M. I. Johnston, C. W. Dieffenbach, D. R. Burton, S. M. Hammer, J. A. Hoxie, M. Martin, J. Overbaugh, D. I. Watkins, A. Mahmoud, and W. C. Greene. 2008. HIV vaccine research: the way forward. Science 321530-532. [DOI] [PubMed] [Google Scholar]
- 13.Graham, B. S., R. A. Koup, M. Roederer, R. T. Bailer, M. E. Enama, Z. Moodie, J. E. Martin, M. M. McCluskey, B. K. Chakrabarti, L. Lamoreaux, C. A. Andrews, P. L. Gomez, J. R. Mascola, and G. J. Nabel. 2006. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J. Infect. Dis. 1941650-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutnick, N., D. Carnathan, K. Cox, S. Dubey, D. R. Casimiro, H. C. Ertl, and M. Betts. 2009. Characterization of adenovirus-specific T cell responses in AdHu5-based vaccine recipients, abstr. 85. Abstr. 16th Conf. Retrovir. Opportun. Infect., Montreal, Quebec, Canada.
- 15.Johnston, M. I., and A. S. Fauci. 2008. An HIV vaccine—challenges and prospects. N. Engl. J. Med. 359888-890. [DOI] [PubMed] [Google Scholar]
- 16.McConnell, M. J., and M. J. Imperiale. 2004. Biology of adenovirus and its use as a vector for gene therapy. Hum. Gene Ther. 151022-1033. [DOI] [PubMed] [Google Scholar]
- 17.McElrath, M. J., S. C. De Rosa, Z. Moodie, S. Dubey, L. Kierstead, H. Janes, O. D. Defawe, D. K. Carter, J. Hural, R. Akondy, S. P. Buchbinder, M. N. Robertson, D. V. Mehrotra, S. G. Self, L. Corey, J. W. Shiver, and D. R. Casimiro. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 3721894-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perreau, M., G. Pantaleo, and E. J. Kremer. 2008. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. J. Exp. Med. 2052717-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sprangers, M. C., W. Lakhai, W. Koudstaal, M. Verhoeven, B. F. Koel, R. Vogels, J. Goudsmit, M. J. Havenga, and S. Kostense. 2003. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J. Clin. Microbiol. 415046-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang, Y., K. U. Jooss, Q. Su, H. C. Ertl, and J. M. Wilson. 1996. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 3137-144. [PubMed] [Google Scholar]
- 21.Yang, Y., F. A. Nunes, K. Berencsi, E. E. Furth, E. Gonczol, and J. M. Wilson. 1994. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. USA 914407-4411. [DOI] [PMC free article] [PubMed] [Google Scholar]