Abstract
Foot-and-mouth disease virus (FMDV) can use a number of different integrins (αvβ1, αvβ3, αvβ6, and αvβ8) as receptors to initiate infection. Infection mediated by αvβ6 is known to occur by clathrin-mediated endocytosis and is dependent on the acidic pH within endosomes. On internalization, virus is detected rapidly in early endosomes (EE) and subsequently in perinuclear recycling endosomes (PNRE), but not in late endosomal compartments. Due to the extreme sensitivity of FMDV to acidic pH, it is thought that EE can provide a pH low enough for infection to occur; however, definitive proof that infection takes place from within these compartments is still lacking. Here we have investigated the intracellular transport steps required for FMDV infection of IBRS-2 cells, which express αvβ8 as their FMDV receptor. These experiments confirmed that FMDV infection mediated by αvβ8 is also dependent on clathrin-mediate endocytosis and an acidic pH within endosomes. Also, the effect on FMDV infection of dominant-negative (DN) mutants of cellular rab proteins that regulate endosomal traffic was examined. Expression of DN rab5 reduced the number of FMDV-infected cells by 80%, while expression of DN rab4 or DN rab7 had virtually no effect on infection. Expression of DN rab11 inhibited infection by FMDV, albeit to a small extent (∼35%). These results demonstrate that FMDV infection takes place predominantly from within EE and does not require virus trafficking to the late endosomal compartments. However, our results suggest that infection may not be exclusive to EE and that a small amount of infection could occur from within PNRE.
Foot-and-mouth disease virus (FMDV) is a member of the Aphthovirus genus of the family Picornaviridae and the etiological agent responsible for FMD, an economically important and severe vesicular condition of cloven-hoofed animals, including cattle, pigs, sheep, and goats (2). The mature virus particle consists of a positive-sense single-stranded RNA genome (vRNA) enclosed within a nonenveloped icosahedral capsid formed from 60 copies each of four virus-encoded proteins, VP1 to VP4 (1).
The initial stage of FMDV infection is virus binding to cell surface integrins via a highly conserved RGD motif located on the GH loop of VP1. A number of different species of RGD-binding integrins (αvβ1, αvβ3, αvβ6, and αvβ8) have been reported to serve as receptors for FMDV (5, 23-26). Using pharmacological and dominant-negative (DN) inhibitors of specific endocytic pathways in combination with immunofluorescence confocal microscopy, the cell entry pathway used by FMDV has been determined for αvβ6-expressing cells (6, 36). These studies established that infection occurs by clathrin-mediated endocytosis and is dependent on the acidic pH within endosomes, which serves as the trigger for capsid disassembly and translocation of the vRNA across the endosomal membrane into the cytosol. Internalized virus was detected rapidly in early endosomes (EE) and subsequently in perinuclear recycling endosomes (PNRE), but not in late endosomes (LE) or lysosomes (Lys) (the late endosomal compartments). Due to the extreme sensitivity of FMDV to acidic pH (15), it is thought that EE can provide a pH low enough for virus disassembly to occur; however, definitive proof that infection takes place from within EE is still lacking. For example, the possibility cannot be excluded that a productive infection requires virus transport to late endosomal compartments, where, following capsid disassembly and viral genome transfer into the cytosol, the capsid proteins are rapidly degraded.
rab proteins control multiple membrane trafficking events in the cell. They are members of the ras superfamily of small GTP-binding proteins and cycle between active GTP- and inactive GDP-bound states (22, 38, 39, 47, 50). Conversion between these states is regulated by guanine nucleotide exchange factors, which stimulate the binding of GTP, and GTPase-activating proteins that which accelerate GTP hydrolysis. Activated rab proteins are recruited onto membrane-bounded compartments where they regulate many steps of vesicle trafficking, including vesicle budding, movement, tethering, and fusion (35, 61). Each rab is recruited to a specific compartment and functions through interactions with specific effectors that mediate the downstream rab-associated functions (39). In mammalian cells, at least 12 rab proteins that regulate trafficking through the endosomal pathway have been identified (27). Of these, rab4, rab5, rab7, and rab11 play major roles in endocytic vesicle trafficking. rab5 is present on EE and regulates transport of incoming endocytic vesicles from the plasma membrane (PM) to EE and homotypic EE fusion events (3, 8, 10, 20, 30, 44, 52). Both rab4 and rab11 are regulators of receptor recycling from EE back to the PM (34); rab4 is localized primarily to EE and regulates rapid recycling directly back to the PM (16, 45, 48, 51, 56), and rab11 is localized primarily to the PNRE and regulates a slower recycling pathway through these compartments (21, 43, 54, 60). In addition rab11 also regulates membrane traffic from endocytic recycling compartments to the trans-Golgi network (55). rab7 is located primarily on LE and regulates traffic from EE to LE and between LE and Lys (7, 9, 18, 32, 40, 58, 59). The unique targeting of rab proteins to distinct cellular compartments and their specificity as regulators of vesicular trafficking has made them important tools for studying endocytosis. For example, expression of DN or constitutively active mutants of rab proteins that regulate endosomal traffic has been used to identify the intracellular transport steps that are required for infection by a number of different viruses (13, 14, 28, 31, 41, 42, 49, 53, 57, 59).
Here we have investigated the intracellular transport steps required for FMDV infection using porcine IBRS-2 cells, which are derived from a natural host of FMDV. IBRS-2 cells use αvβ8, and not αvβ6, as the major FMDV receptor (11). Our initial experiments confirmed that FMDV infection mediated by αvβ8 is dependent on clathrin-mediated endocytosis and on an acidic pH within endosomes. The effect on FMDV infection within IBRS-2 cells of DN mutants of cellular rab proteins that regulate endosomal traffic was examined. These experiments show that rab5 is needed for FMDV infection, as expression of DN rab5 reduced the number of FMDV-infected cells by ∼80%. In contrast, expression of either DN rab4 or DN rab7 had virtually no effect on infection. Expression of DN rab11 inhibited infection by FMDV, albeit to a small extent (∼35%). These results demonstrate that FMDV infection takes place predominantly from within EE and does not require virus trafficking to the late endosomal compartments. However, our results suggest that infection may not be exclusive to EE and that a small amount of infection could occur from within PNRE.
MATERIALS AND METHODS
Cells and viruses.
IBRS-2 cells were cultivated in Glasgow's modified Eagle's medium supplemented with 10% adult bovine serum, 20 mM glutamine, penicillin (100 SI units/ml), and streptomycin (100 μg/ml). Working stocks of FMDV O1Kcad2 were prepared using primary bovine thyroid cells, and the multiplicity of infection (MOI) was based on the virus titer on IBRS-2 cells as described previously (11).
Antibodies and reagents.
The αvβ8 monoclonal antibody (MAb) 14E5 was a gift from Stephen Nishimura (UCSF). The anti-α-tubulin MAb (DM1A) was from Sigma (Poole, United Kingdom). The anti-green fluorescent protein (anti-GFP) antibody was from Abcam (Cambridge, United Kingdom). The antibody to c-myc (9E10) was from the Developmental Studies Hybridoma Bank (University of Iowa). The rabbit polyclonal serum to the capsid proteins of type O FMDV was from the FMD World Reference laboratory (IAH Pirbright). MAb 2C2 was a gift from Emiliana Brocchi (IZS, Brescia, Italy) and recognizes the FMDV 3A protein (17). The Alexa-fluor-conjugated goat anti-rabbit and goat anti-mouse secondary antibodies and Alexa-labeled transferrin (TN) and Dil low-density lipoprotein (LDL) were all from Invitrogen-Molecular Probes (Paisley, United Kingdom). Stock solutions of concanamycin A (Fluka/Sigma Poole, United Kingdom) and nocodazole (Sigma, Poole, United Kingdom) were prepared in dimethyl sulfoxide (DMSO) at 10 mg/ml. Working stocks of these reagents were diluted in Dulbecco modified Eagle's medium. Where appropriate, an equivalent dilution of DMSO was included as the mock treatment.
Plasmids.
Mammalian expression plasmids (pEGFP-C2) for enhanced GFP (EGFP)-wild-type (wt) rab5, EGFP-DN rab5S34N, EGFP-wt rab4, EGFP-DN rab4N121I, EGFP-wt rab11, EGFP-DN rab11S25N, and EGFP-wt rab7 were generous gifts from Stephen Ferguson (Robarts Research Institute, Canada). pEGFP-DN rab7T22N was a generous gift from María Isabel Colombo (IHEM Argentina). pCMV-MYC containing AP180C fused to the c-myc tag was a gift from Harvey McMahon (MRC, Cambridge, United Kingdom).
Enzyme-linked immunospot assay.
The enzyme-linked immunospot assay used to quantify infection has been described in detail previously (6). Briefly, cells in 96-well plates were infected with FMDV (MOI = 1) for 1 h at 37°C. The cells were washed twice to remove unattached virus and incubation continued for a further 4 h at 37°C. Cells were fixed in 4% paraformaldehyde (PFM) in phosphate-buffered saline (PBS) for 40 min, washed, and treated with 0.1% Triton X-100 in PBS for 15 min. The wells were then incubated with block buffer (10 mM Tris-HCl [pH 7.5], 140 mM NaCl, 1 mM CaCl2, 0.5 mM MgCl2, 10% normal goat serum, 1% fish gelatin) for 0.5 h. The cells were then incubated sequentially with MAb 2C2 (which recognizes the FMDV nonstructural 3A protein) and goat anti-mouse immunoglobulin G2a (IgG2a)-specific biotin conjugate (Southern Biotechnology Associates/Cambridge BioScience, United Kingdom) with washing between the antibody treatments. Finally, the cells were incubated with streptavidin-conjugated alkaline phosphatase. In the presence of enzyme substrate (alkaline phosphatase conjugate substrate kit; Bio-Rad), the infected cells turned dark blue and were counted using an ELIspot apparatus (Carl Zeiss). For experiments with concanamycin A, the cells were pretreated with the drug for 0.5 h and then infected with FMDV for 1 h also in the presence of the drug. The cells were then washed and incubated at 37°C in the absence of drug. To control for the effects of concanamycin A on intracellular virus replication, additional cell cultures were infected for 1 h (as described above) in the absence of the drug and then drug treated for 1.5 h after the virus inocula had been removed. For experiments with nocodazole, the cells were pretreated with the drug for 0.5 h prior to infection (as described above), and the drug remained present throughout the assay.
Transfection and infection of transfected cells.
Cells on glass coverslips were transfected with expression plasmids (1 μg) using 1 μl Lipofectamine 2000 (Invitrogen, Paisley, United Kingdom). At 6 h posttransfection, the cells were infected with FMDV O1Kcad2 (MOI, ∼0.5) for 3 h at 37°C. The cells were fixed with 4% PFM in PBS for 40 min and treated for 0.5 h with 0.1% Triton X-100 in PBS. The cells were washed with PBS and incubated in block buffer (10 mM Tris, 150 mM NaCl, 1 mM CaCl2, 0.5 mM MgCl2, 10% normal goat serum, and 1% gelatin) for 0.5 h. The cells were then incubated with primary antibodies in block buffer for 1 h, washed extensively, and incubated with Alexa-fluor-conjugated secondary antibodies in block buffer for 1 h. After washing, the coverslips were mounted onto microscope slides using Vectashield mounting medium with DAPI (4′,6′-diamidino-2-phenylindole) (Vector Labs, Peterborough, United Kingdom) and sealed with clear nail varnish. Cells expressing wt or DN rab proteins were identified using the EGFP tag fused to the N terminus of the rab protein. Cells expressing AP180C were identified using antibody 9E10, which recognizes the c-myc tag fused to AP180C, followed by a goat anti-mouse IgG1 Alexa-488-labeled secondary antibody. Infected cells were identified using a rabbit polyclonal serum specific to type O FMDV capsids in combination with an Alexa-568-conjugated goat anti-rabbit IgG secondary antibody. To eliminate cross talk between the fluorescent dyes, all data were collected sequentially using a Leica SP2 scanning laser confocal microscope. For experiments that involved dual antibody labeling of virus and AP180C, the specificity of the secondary antibodies was confirmed by showing a lack of cross-reactivity between the goat anti-mouse-IgG for the primary rabbit antiserum and the goat anti-rabbit antibody for the murine MAb 9E10.
Detection of cell surface-exposed αvβ8.
Cells, prepared on coverslips as described above, were cooled on ice and incubated sequentially with primary (MAb 14E5) and secondary (Alexa-568-conjugated goat anti-mouse IgG) antibodies with washing steps between incubations and without permeabilization. The cells were then fixed with cold 4% PFM in PBS for 40 min and mounted as described above.
RESULTS
Integrin αvβ8-mediated FMDV infection of IBRS-2 cells requires clathrin-dependent endocytosis and low endosomal pH.
Previously we and others have shown that FMDV infection of human SW480 cells transfected to express αvβ6 occurs via clathrin-mediated endocytosis and requires an acidic pH within endosomes (6, 36). Here we have investigated the postentry, endosomal transport steps that are required for FMDV infection using porcine IBRS-2 cells, which are derived from a natural host of FMDV. IBRS-2 cells use αvβ8, and not αvβ6, as the major FMDV receptor (11). In our initial experiments we investigated whether αvβ8-mediated infection is also dependent on clathrin-mediated endocytosis and the acidic pH within endosomes.
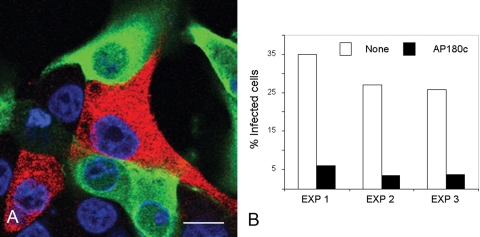
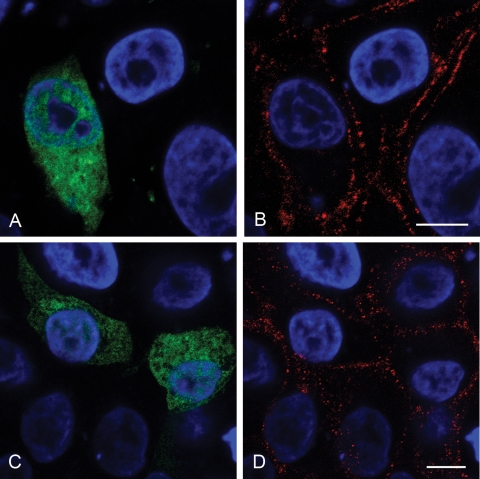
The clathrin coat assembly protein AP180 is vital for clathrin cage assembly, and expression of its C-terminal region (AP180C) acts as a DN inhibitor of clathrin-mediated endocytosis (19) and inhibits FMDV infection mediated by αvβ6 (6). IBRS-2 cells were transfected to express AP180C as a c-myc-tagged fusion protein. By 6 h posttransfection, ∼30% of the cells expressed detectable levels of AP180C (the expressing population). These cells were identified using antibody 9E10 (which recognizes the c-myc tag on AP180C) and a goat anti-mouse IgG1 Alexa-488-labeled secondary-antibody and are shown in green in Fig. 1. At 6 h posttransfection, the cells were infected with FMDV at a low MOI (∼0.5) for 3 h before fixation and processing for confocal microscopy. Infected cells were identified using a rabbit polyclonal serum specific to type O FMDV capsids in combination with an Alexa-568-conjugated goat anti-rabbit IgG secondary antibody and appear red. Figure 1 shows that expression of AP180C inhibited FMDV infection of IBRS-2 cells. Figure 1A shows representative cells from one experiment of three and that the majority of the AP180C-expressing cells were not infected. Figure 1B shows quantification of the data for the three experiments. For each transfection experiment, both the AP180C-expressing cell population (green) and the nonexpressing cell population (the cells that did not express AP180C) were scored for infection (red); only 3 to 6% of the AP180C-expressing cells were infected, compared to 25 to 35% of the nonexpressing cells.
FIG. 1.
Expression of AP180C inhibits FMDV infection of IBRS-2 cells. IBRS-2 cells were transfected to express AP180C and infected with FMDV. (A) Representative cells from one experiment. The cells expressing AP180C (green) were identified via the c-myc fusion tag. FMDV-infected cells (red) were identified using a rabbit anti-FMDV polyclonal antiserum. The cell nuclei are shown in blue. Bar, 10 μm. (B) For each transfection, the AP180C-expressing and nonexpressing cell populations were scored for infection. The data are shown as the percentage of infected cells for each of these populations from three independent experiments.
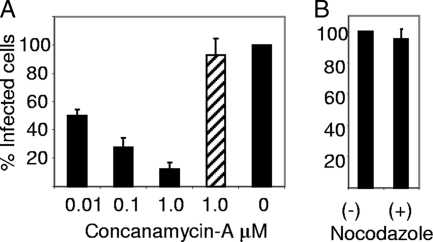
FMDV infection is inhibited by reagents that raise endosomal pH (4, 6). We confirmed that infection of IBRS-2 cells also requires active endosomal acidification. The cells were infected in the presence or absence of concanamycin A, a potent and specific inhibitor of the vacuolar proton ATPase, and infection was quantified using an enzyme-linked immunospot assay (see Material and Methods) which enables individually infected cells to be identified (6). Cells were treated with the drug at the indicated concentrations (Fig. 2A) for 0.5 h prior to infection with FMDV (MOI = 1) for 1 h also in the presence of the drug. The cells were then washed and incubated at 37°C for a further 4 h in the absence of drug. Under these conditions, the number of infected cells was reduced by up to 85% compared with the mock-treated cells (i.e., no drug treatment). To confirm that the inhibitory effects of the drug were restricted to cell entry of the virus, concanamycin A (1 μM) was added to additional cultures after the virus inocula had been removed (i.e., after the 1-h virus internalization step). Under these conditions the number of infected cells was similar to that for the mock-treated cell monolayer, showing that an acidic pH inside endosomes is needed for cell entry and not for postentry intracellular virus replication. Taken together, these experiments confirmed that αvβ8-mediated infection of IBRS-2 cells occurs via clathrin-mediated endocytosis and that virus cell entry requires an acidic pH within endosomes.
FIG. 2.
FMDV infection of IBRS-2 requires active endosomal acidification but not an intact microtubule network. (A) IBRS-2 cells were pretreated with the indicated concentration of concanamycin A for 0.5 h and then infected with FMDV for 1 h also in the presence of the drug. The 0 indicates infection in the absence of the drug. The cells were then washed and incubated at 37°C in the absence of drug for a further 4 h and infection quantified using an enzyme-linked immunospot assay. The hatched bar shows the replication control where the drug was added after the virus inocula had been removed, i.e., after the virus internalization step. (B) Cells were pretreated with or without nocodazole (10 μM) for 0.5 h prior to infection with FMDV as described above. For the drug-treated cells, nocodazole remained present throughout the assay. −, cells infected in the absence of nocodazole; +, cells infected in the presence of nocodazole. Infection was quantified using an enzyme-linked immunospot assay. For each drug (concanamycin A and nocodazole), results from one representative experiment of two are shown; each gave similar results. For each experiment, the results shown are the means ± standard deviations for triplicate measurements. For panels A and B, the data are shown as the number of infected cells in the drug-treated wells normalized to the number of infected cells in the absence of drug.
FMDV infection of IBRS-2 cells does not require microtubules.
Nocodazole disrupts microtubules and inhibits vesicular trafficking, including transport between EE and LE. Previously we have shown that αvβ6-mediated infection of SW480 cells is resistant to treatment of cells with nocodazole (6, 33), suggesting that infection takes place from within EE or PNRE and not from within LE. To determine if αvβ8-mediated infection is also insensitive to microtubule disruption, we investigated the effect of nocodazole on FMDV infection of IBRS-2 cells. Initially we confirmed that 0.5 h of treatment with nocodazole (10 μM) disrupted the microtubules by visualizing tubulin in mock- and nocodazole-treated cells under a confocal microscope using the antitubulin antibody (data not shown). Cell monolayers were treated with nocodazole for 0.5 h prior to infection (as described above for concanamycin), and the drug remained present throughout the assay. The number of infected cells was then quantified using an enzyme-linked immunospot assay (see above). The results of this experiment are shown in Fig. 2B, which shows that FMDV infection of IBRS-2 cells does not require an intact microtubule network, since nocodazole did not affect infection.
Regulation of FMDV infection by cellular rab proteins.
The above results with nocodazole suggest that for a productive infection, FMDV does not need to travel to late endosomal compartments. Virus trafficking through endocytic pathways can be investigated more precisely using DN versions of rab proteins (see the introduction). Therefore, IBRS-2 cells were transfected to express either wt or DN mutants of rab4, rab5, rab7, or rab11 and the effect on infection determined using confocal microscopy. The cells expressing rab proteins (the expressing populations) were visualized by an EGFP tag (shown as green in the figures) fused to the N terminus of each rab protein. For each transfection ∼35 to 40% of the cells expressed detectable levels of the rab protein at 6 h posttransfection. Western blot analysis using an anti-GFP antibody confirmed that the majority of the EGFP expressed in the transfected cells remained fused to the rab protein (data not shown). At 6 h posttransfection, the cells were infected with FMDV at a low MOI (0.5) for 3 h. The cells were then fixed with PFM and the infected cells identified as described for Fig. 1. For each wt/DN rab, cells were transfected in parallel, and the rab-expressing cell populations (green) were scored for FMDV infection (red). The number of DN rab-expressing cells that were infected was normalized to the number of wt rab-expressing cells that were infected.
(i) rab5.
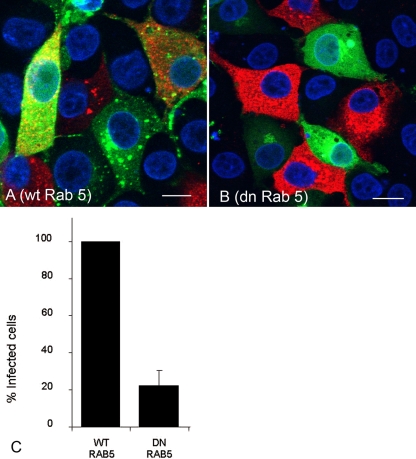
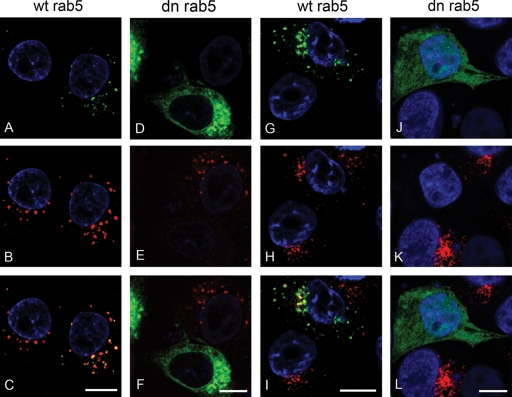
Initially we investigated the effect of DN rab5 on FMDV infection. rab5 regulates both the fusion of incoming endocytic vesicles with EE and homotypic EE fusions. Figure 3 shows the results of these experiments. Figure 3A and B show representative cells from one experiment of three. A larger proportion of cells expressing wt rab5 (Fig. 3A) were infected by FMDV (appearing yellow in the figure) compared to the cells expressing DN rab5 (Fig. 3B). Figure 3C shows the normalized data for all three experiments and that expression of DN rab5 resulted in an ∼80% reduction in the number of infected cells compared to the cell population expressing the wt rab. These results are consistent with the known roles of rab5 in regulating clathrin-mediated endocytosis and the inhibition of FMDV infection caused by the expression of AP180C (Fig. 1). To further characterize the inhibitory effect of DN rab5 on FMDV infection, both the integrity of EE (using an antibody to EEA1, a marker for EE) and clathrin-mediated endocytosis (using uptake of Alexa-labeled TN, a marker for clathrin-mediated endocytosis) were investigated in cells expressing DN rab5. Cells expressing wt rab5 (green) (Fig. 4A) had EE (red) (Fig. 4B) similar to the cells not expressing the rab protein. In contrast, virtually all of the cells (∼90%) expressing DN rab5 (green) (Fig. 4D) had fewer and smaller EE (red) (Fig. 4E) than the nonexpressing cells. Consistent with these observations, cells expressing wt rab5 (green) (Fig. 4G) internalized Alexa-labeled TN (red) (Fig. 4H) to a similar extent as the nonexpressing cells, whereas virtually all of the cells expressing DN rab5 (green) (Fig. 4J) internalized much less Alexa-488-labeled TN (red) (Fig. 4K), indicating that clathrin-mediated endocytosis had been inhibited. These data show that the inhibitory effect of DN rab5 on infection most likely results from a failure of incoming virus to reach acidic compartments (see Discussion). However, DN rab5 could also inhibit receptor recycling, thereby reducing expression of cell surface αvβ8, virus-cell attachment, and hence infection. To eliminate this possibility, we investigated αvβ8 surface expression in DN rab5-expressing cells by confocal microscopy. Figure 5A and B show that αvβ8 expression appeared normal in cells expressing DN rab5 compared with cells in the nonexpressing population, showing that the inhibitory effect of DN rab5 does not results from a depletion of αvβ8 from the cell surface.
FIG. 3.
DN rab5 inhibits FMDV infection of IBRS-2 cells. (A and B) IBRS-2 cells were transfected to express wt rab5 (A) or DN rab5 (B) and infected with FMDV. Panels A and B show representative cells from one experiment. Cells expressing the rab protein (green) were identified by the EGFP tag. FMDV-infected cells (red) were identified as for Fig. 1. Yellow indicates rab-expressing cells that are infected. The cell nuclei are shown in blue. Bars, 10 μm. (C) Quantitative data for the experiments. Cells expressing either wt or DN rab5 were scored for infection. The data are shown as the percentage of the DN rab5-expressing cell population that was infected normalized to the level of infection of cells expressing the wt rab protein. The results are the means ± standard deviations for three independent experiments.
FIG. 4.
Effect of DN rab5 on EE integrity and clathrin-mediated endocytosis. IBRS-2 cells were transfected to express wt rab5 (A and G) or DN rab5 (D and J). Cells expressing the rab protein (green) were identified by the EGFP tag. Panels B and E show the same cells as in panels A and D labeled for EE (EEA-1; red). Panels C and F show overlays of the panels immediately above. Areas of yellow indicate regions of colocalization. Panels H and K show internalization of Alexa-568-conjugated TN (red) for the same cells as shown in panels G and J, respectively. Panels I and L show overlays of the panels immediately above. The cell nuclei are shown in blue. Bars, 10 μm.
FIG. 5.
Expression of DN rab5 or DN rab11 does not inhibit surface expression of αvβ8. (A and C) IBRS-2 cells were transfected to express either DN rab5 (A) or DN rab11 (C). Cells expressing the rab protein (green) were identified by the EGFP tag. (B and D) Same cells as in panels A and C, respectively, surface labeled for the FMDV receptor, integrin αvβ8 (red) with MAb 14E5. The cell nuclei are shown in blue. Bars, 10 μm.
(ii) rab7.
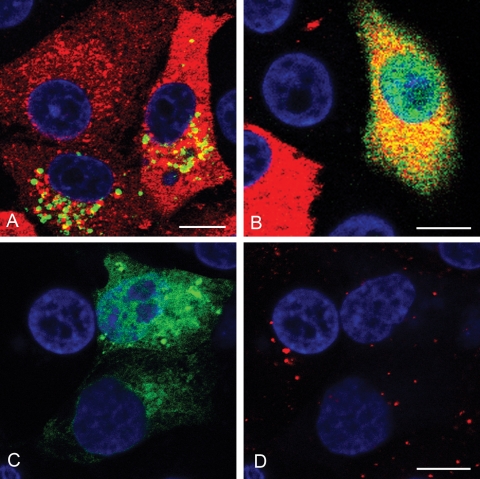
rab7 is the major rab that regulates traffic from EE to LE and from LE to Lys. Expression of DN rab7 did not appear to inhibit FMDV infection (Fig. 6A and B), as the proportion of DN rab7-expressing cells that were infected was similar to that of the cells expressing the wt rab (Fig. 7). To verify that expression of DN rab7 inhibited vesicular transport to LE we investigated the effect of DN rab7 on intracellular trafficking of Dil LDL. Normally LDL enters cells by clathrin-mediated endocytosis and trafficks to LE and Lys via EE. In cells of the nonexpressing cell population, internalized LDL accumulated in vesicles in the perinuclear region of the cell with an appearance characteristic of LE (Fig. 6C and D). In contrast, in virtually all of the cells expressing DN rab7, internalized LDL remained in smaller, more peripheral vesicles characteristic of EE and did not accumulate in perinuclear vesicles. Together these results show that when trafficking between EE and LE is inhibited, FMDV infection proceeds normally, confirming that FMDV does not need to traffic to late endosomal compartments for infection.
FIG. 6.
Effects of DN rab7 on LDL trafficking and FMDV infection. (A and B) IBRS-2 cells were transfected to express wt rab7 (A) or DN rab7 (B) and infected with FMDV. Panels A and B show representative cells from one experiment. Cells expressing the rab protein (green) were identified by the EGFP tag. FMDV-infected cells (red) were identified as for Fig. 1. Cells expressing either wt or DN rab7 were scored for infection as for Fig. 3. (Quantification of these data is shown in Fig. 7.) (C) IBRS-2 cells were transfected to express DN rab7. (D) Uptake of Alexa-568-labeled LDL for the same cells as shown in panel C. In the nonexpressing cells, LDL (red) accumulated in larger perinuclear vesicles characteristic of LE, whereas in the cells expressing DN-rab7, LDL labeling was confined to smaller, more peripheral vesicles. The cell nuclei are shown in blue. Bars, 10 μm.
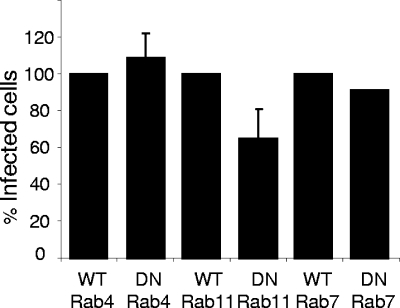
FIG. 7.
Effects of DN mutants of rab4, rab7, and rab11 on FMDV infection of IBRS-2 cells. IBRS-2 cells were transfected to express wt or DN mutants of rab4, rab7, and rab11 and infected with FMDV. Cells expressing the rab protein were identified by the EGFP tag. FMDV-infected cells were identified as for Fig. 1. Cells expressing either the wt or DN rab were scored for infection. The data are shown as the percentage of the DN rab-expressing cell population that were infected normalized to the level of infection of cells expressing the corresponding wt rab protein. For rab4 and rab11 the results are the means ± standard deviations for three independent experiments. For rab7 the results are shown as the means for two independent experiments, each giving near-identical results.
(iii) rab4 and rab11.
rab4 and rab11 are the major rab proteins involved in regulating receptor recycling from EE back to the PM. rab4 is predominantly found on EE and regulates a rapid recycling pathway from EE directly to the PM, whereas rab11 is located predominantly on PNRE and is the major rab that regulates a slower recycling pathway via these compartments (see the introduction). Expression of either DN rab4 or DN rab11 did not noticeably change the number, size, or cellular distribution of EE (i.e., EEA-1-positive compartments), indicating that these organelles remain intact in cells expressing these mutant rab proteins (data not shown). The data in Fig. 7 show that expression of DN rab4 did not significantly reduce the proportion of infected cells compared to cells expressing wt rab4. Figure 7 also indicates that expression of DN rab11 reduced the number of infected cells by ∼35% (note that a decrease was observed in each of three separate experiments but the effect was not statistically significant [P = 0.1]) compared to cells expressing wt rab11, and hence we cannot rule out the possibility that infection may not be exclusive to EE and that a small amount of infection could occur from within PNRE.
Integrins are known to recycle through rab11-regulated PNRE, and therefore it was possible that the inhibitory effect of DN rab11 on infection could result from a depletion of αvβ8 from the cell surface. Figure 5C and D show that this explanation is unlikely, as, similar to the case for DN rab5-expressing cells, αvβ8 expression on the surfaces of DN rab11-expressing cells appeared normal compared to that on the cells in the nonexpressing population, suggesting that the small inhibitory effect of DN rab11 may be the result of a proportion of the input virus being transported to PNRE for infection (see Discussion).
DISCUSSION
Previously, we and others (6, 36) have shown that FMDV infection mediated by the integrin αvβ6 requires clathrin-mediated endocytosis and the low pH within endosomes. Here we have established that clathrin-mediated endocytosis and the acidic pH within endosomes are also required for infection mediated by αvβ8; however, the precise endosomal compartment from which infection (i.e., viral RNA membrane penetration) occurs had not been identified previously. Our goal in the current study was to provide an insight into the site of infection.
The unique targeting of rab proteins to distinct cellular membranes/organelles and their specificity as regulators of vesicular trafficking have made them important tools to study endocytosis, and DN versions of rab proteins have been used to identify the endocytic uptake and intracellular transport steps required for infection by a number of viruses (13, 14, 28, 31, 41, 42, 49, 53, 57, 59). Here we have used expression of DN rab proteins to identify the intracellular transport steps required for FMDV infection. Our data have identified EE as critical organelles for this infection. Expression of DN rab5 disrupted EE integrity, impaired clathrin-mediated endocytosis, and inhibited FMDV infection, indicating that a productive infection needs a rab5-dependent step. The inhibitory effect of DN rab5 on infection most likely resulted from inhibiting the key role played by rab5 in delivering incoming clathrin-dependent endocytic vesicles to EE (3, 8, 20). However, alternative explanations are possible. First, inhibiting rab5 function could impair receptor recycling, thereby depleting FMDV receptors from the cell surface and reducing virus-cell attachment and hence virus endocytosis and infection. Here we have shown (Fig. 5) that expression of DN rab5 did not noticeably reduce cell surface expression of αvβ8 (the FMDV receptor on IBRS-2 cells), showing that the inhibitory effect of DN rab5 was unlikely to result from reduced expression of cell surface FMDV receptors. Second, in addition to regulating clathrin-mediated endocytosis, rab5 has been reported to play roles in clathrin-independent endocytic pathways, including the delivery of internalized caveolae to EE (37), and regulation of macropinocytosis (29, 46). Furthermore, apart from roles in endocytosis, rab5 could be required for intracellular virus replication, as rab5 has been implicated in virus-induced host cell membrane rearrangements and/or viral genome replication of hepatitis C virus (53). Our observation that FMDV infection is inhibited by AP180C, a potent and specific inhibitor of clathrin-mediated endocytosis (Fig. 1), strongly supports the conclusion that the inhibitory effect of DN rab5 on FMDV infection of IBRS-2 cells results from a failure of internalized virus to reach EE and hence the lower-pH environment needed to trigger infection.
These results show that virus entry into EE is critical for infection; however, this observation alone does not confirm EE as the site of infection, as the disruptive effect of DN rab5 on EE integrity would be expected to impair delivery of virus not only to EE but also to downstream acidic compartments such as PNRE, LE, and Lys. Therefore, we also investigated the effects of DN versions of rab proteins that regulate vesicular trafficking away from EE. rab7 is a key regulator of transport from EE to LE and between LE and Lys. Under conditions where LDL trafficking to LE (and Lys) was impaired by DN rab7, FMDV infection was not inhibited, implying that a productive infection does not require virus transport from EE to LE and Lys. This conclusion is supported by our observation that nocodazole, which disrupts microtubules and hence traffic to LE, did not inhibit infection either (Fig. 2). Receptor recycling pathways also originate from EE, and rab4 and rab11 are important regulators of these pathways. rab4 regulates a rapid recycling from EE directly to the PM, whereas rab11 is the major rab that regulates the slower recycling through acidic PNRE. Previously, we have shown that FMDV is rapidly internalized and delivered to EE. Subsequently, viral capsid proteins (derived from the input virus) accumulate in PNRE (6), consistent with the temporal transit of virus through EE to PNRE. Here we have shown that expression of DN rab4 did not inhibit infection, suggesting that the rab4 recycling pathway is not needed for FMDV infection. Expression of DN rab11 appeared to inhibit FMDV infection by a relatively small amount (∼35%); however, this effect was not statistically significant (from three independent transfections). The apparent inhibitory effect of DN rab11 could be explained by a slowing of αvβ8 recycling, thereby reducing the amount of available receptor at the cell surfaces for virus binding. However, our data (Fig. 5) suggest that receptor availability at the cell surface is normal in the presence of DN rab11. The rate of capsid disassembly within endosomes is not known, and it is possible that a small proportion of internalized virus reaches PNRE before capsid disassembly and viral RNA translocation to the cytosol are triggered. This scenario does not necessarily imply a direct role for rab11 in infection, as receptor-bound virus that is slow to disassemble in EE could be delivered to PNRE in a rab11-dependent manner; however, once within PNRE, rab11 may not be directly involved in viral genome transfer. If this was the case, then the effect of DN rab11 on infection could result from redirecting a small proportion of the internalized virus to LE and Lys, resulting in virus degradation rather than a productive infection. In summary, our results with DN versions of rab4, rab7, and rab11 show that virus transport away from EE is not required for infection.
In conclusion, our results identify EE as the critical organelle for FMDV infection and are consistent with the majority of viral genome transfer to the cytoplasm taking place from within this compartment. Furthermore, our results show that for a productive infection to occur, virus does not need to traffic beyond EE. Integrins are known to recycle through EE and PNRE during processes such as cell migration and cancer invasion (12). This raises the possibility that by evolving to use a recycling receptor for virus internalization, FMDV could evade the hostile, degradative environment of LE and Lys, thereby increasing the chance of a productive infection.
Acknowledgments
We thank Sheila Wilsden for pBTYs and IBRS-2 cells and Emiliana Brocchi and Stephen Nishimura for MAbs 2C2 and 14E5, respectively. We also thank Stephen Ferguson, María Isabel Colombo, and Harvey McMahon for plasmids.
H.J. was supported by the BBSRC. This work was also supported by DEFRA.
Footnotes
Published ahead of print on 8 April 2009.
REFERENCES
- 1.Acharya, R., E. Fry, D. Stuart, G. Fox, D. Rowlands, and F. Brown. 1989. The three-dimensional structure of foot-and-mouth disease virus at 2.9 A resolution. Nature 337709-716. [DOI] [PubMed] [Google Scholar]
- 2.Alexandersen, S., Z. Zhang, A. I. Donaldson, and A. J. Garland. 2003. The pathogenesis and diagnosis of foot-and-mouth disease. J. Comp. Pathol. 1291-36. [DOI] [PubMed] [Google Scholar]
- 3.Barbieri, M. A., R. L. Roberts, A. Mukhopadhyay, and P. D. Stahl. 1996. Rab5 regulates the dynamics of early endosome fusion. Biocell 20331-338. [PubMed] [Google Scholar]
- 4.Baxt, B. 1987. Effect of lysosomotropic compounds on early events in foot-and-mouth disease virus replication. Virus Res. 7257-271. [DOI] [PubMed] [Google Scholar]
- 5.Berinstein, A., M. Roivainen, T. Hovi, P. W. Mason, and B. Baxt. 1995. Antibodies to the vitronectin receptor (integrin αVβ3) inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J. Virol. 692664-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berryman, S., S. Clark, P. Monaghan, and T. Jackson. 2005. Early events in integrin αvβ6-mediated cell entry of foot-and-mouth disease virus. J. Virol. 798519-8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottger, G., B. Nagelkerken, and P. van der Sluijs. 1996. Rab4 and Rab7 define distinct nonoverlapping endosomal compartments. J. Biol. Chem. 27129191-29197. [DOI] [PubMed] [Google Scholar]
- 8.Bucci, C., R. G. Parton, I. H. Mather, H. Stunnenberg, K. Simons, B. Hoflack, and M. Zerial. 1992. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70715-728. [DOI] [PubMed] [Google Scholar]
- 9.Bucci, C., P. Thomsen, P. Nicoziani, J. McCarthy, and B. van Deurs. 2000. Rab7: a key to lysosome biogenesis. Mol. Biol. Cell 11467-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bucci, C., A. Wandinger-Ness, A. Lutcke, M. Chiariello, C. B. Bruni, and M. Zerial. 1994. Rab5a is a common component of the apical and basolateral endocytic machinery in polarized epithelial cells. Proc. Natl. Acad. Sci. USA 915061-5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burman, A., S. Clark, N. G. Abrescia, E. E. Fry, D. I. Stuart, and T. Jackson. 2006. Specificity of the VP1 GH loop of foot-and-mouth disease virus for αv integrins. J. Virol. 809798-9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caswell, P. T., and J. C. Norman. 2006. Integrin trafficking and the control of cell migration. Traffic 714-21. [DOI] [PubMed] [Google Scholar]
- 13.Colpitts, T. M., A. C. Moore, A. A. Kolokoltsov, and R. A. Davey. 2007. Venezuelan equine encephalitis virus infection of mosquito cells requires acidification as well as mosquito homologs of the endocytic proteins Rab5 and Rab7. Virology 36978-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coyne, C. B., L. Shen, J. R. Turner, and J. M. Bergelson. 2007. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbes 2181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curry, S., C. C. Abrams, E. Fry, J. C. Crowther, G. J. Belsham, D. I. Stuart, and A. M. King. 1995. Viral RNA modulates the acid sensitivity of foot-and-mouth disease virus capsids. J. Virol. 69430-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daro, E., P. van der Sluijs, T. Galli, and I. Mellman. 1996. Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc. Natl. Acad. Sci. USA 939559-9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Diego, M., E. Brocchi, D. Mackay, and F. De Simone. 1997. The non-structural polyprotein 3ABC of foot-and-mouth disease virus as a diagnostic antigen in ELISA to differentiate infected from vaccinated cattle. Arch. Virol. 1422021-2033. [DOI] [PubMed] [Google Scholar]
- 18.Feng, Y., B. Press, and A. Wandinger-Ness. 1995. Rab 7: an important regulator of late endocytic membrane traffic. J. Cell Biol. 1311435-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford, M. G. J., B. M. F. Pearse, M. K. Higgins, Y. Vallis, D. J. Owen, A. Gibson, C. R. Hopkins, P. R. Evans, and H. T. McMahon. 2001. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 2911051-1055. [DOI] [PubMed] [Google Scholar]
- 20.Gorvel, J. P., P. Chavrier, M. Zerial, and J. Gruenberg. 1991. rab5 controls early endosome fusion in vitro. Cell 64915-925. [DOI] [PubMed] [Google Scholar]
- 21.Green, E. G., E. Ramm, N. M. Riley, D. J. Spiro, J. R. Goldenring, and M. Wessling-Resnick. 1997. Rab11 is associated with transferrin-containing recycling compartments in K562 cells. Biochem. Biophys. Res. Commun. 239612-616. [DOI] [PubMed] [Google Scholar]
- 22.Grosshans, B. L., D. Ortiz, and P. Novick. 2006. Rabs and their effectors: achieving specificity in membrane traffic. Proc. Natl. Acad. Sci. USA 10311821-11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson, T., S. Clark, S. Berryman, A. Burman, S. Cambier, D. Mu, S. Nishimura, and A. M. King. 2004. Integrin αvβ8 functions as a receptor for foot-and-mouth disease virus: role of the beta-chain cytodomain in integrin-mediated infection. J. Virol. 784533-4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson, T., A. M. King, D. I. Stuart, and E. Fry. 2003. Structure and receptor binding. Virus Res. 9133-46. [DOI] [PubMed] [Google Scholar]
- 25.Jackson, T., A. P. Mould, D. Sheppard, and A. M. King. 2002. Integrin αvβ1 is a receptor for foot-and-mouth disease virus. J. Virol. 76935-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson, T., D. Sheppard, M. Denyer, W. Blakemore, and A. M. King. 2000. The epithelial integrin αvβ6 is a receptor for foot-and-mouth disease virus. J. Virol. 744949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordens, I., M. Marsman, C. Kuijl, and J. Neefjes. 2005. Rab proteins, connecting transport and vesicle fusion. Traffic 61070-1077. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan, M. N., B. Sukumaran, U. Pal, H. Agaisse, J. L. Murray, T. W. Hodge, and E. Fikrig. 2007. Rab 5 is required for the cellular entry of dengue and West Nile viruses. J. Virol. 814881-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanzetti, L., A. Palamidessi, L. Areces, G. Scita, and P. P. Di Fiore. 2004. Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature 429309-314. [DOI] [PubMed] [Google Scholar]
- 30.McLauchlan, H., J. Newell, N. Morrice, A. Osborne, M. West, and E. Smythe. 1998. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr. Biol. 834-45. [DOI] [PubMed] [Google Scholar]
- 31.Meertens, L., C. Bertaux, and T. Dragic. 2006. Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles. J. Virol. 8011571-11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meresse, S., J. P. Gorvel, and P. Chavrier. 1995. The rab7 GTPase resides on a vesicular compartment connected to lysosomes. J. Cell Sci. 1083349-3358. [DOI] [PubMed] [Google Scholar]
- 33.Miller, L. C., W. Blakemore, D. Sheppard, A. Atakilit, A. M. King, and T. Jackson. 2001. Role of the cytoplasmic domain of the beta-subunit of integrin αvβ6 in infection by foot-and-mouth disease virus. J. Virol. 754158-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohrmann, K., and P. van der Sluijs. 1999. Regulation of membrane transport through the endocytic pathway by rabGTPases. Mol. Membr. Biol. 1681-87. [DOI] [PubMed] [Google Scholar]
- 35.Novick, P., and M. Zerial. 1997. The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 9496-504. [DOI] [PubMed] [Google Scholar]
- 36.O'Donnell, V., M. LaRocco, H. Duque, and B. Baxt. 2005. Analysis of foot-and-mouth disease virus internalization events in cultured cells. J. Virol. 798506-8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelkmans, L., T. Burli, M. Zerial, and A. Helenius. 2004. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 118767-780. [DOI] [PubMed] [Google Scholar]
- 38.Pfeffer, S. R. 2001. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11487-491. [DOI] [PubMed] [Google Scholar]
- 39.Pfeffer, S. R. 2005. Structural clues to Rab GTPase functional diversity. J. Biol. Chem. 28015485-15488. [DOI] [PubMed] [Google Scholar]
- 40.Press, B., Y. Feng, B. Hoflack, and A. Wandinger-Ness. 1998. Mutant Rab7 causes the accumulation of cathepsin D and cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J. Cell Biol. 1401075-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quirin, K., B. Eschli, I. Scheu, L. Poort, J. Kartenbeck, and A. Helenius. 2008. Lymphocytic choriomeningitis virus uses a novel endocytic pathway for infectious entry via late endosomes. Virology 37821-33. [DOI] [PubMed] [Google Scholar]
- 42.Rauma, T., J. Tuukkanen, J. M. Bergelson, G. Denning, and T. Hautala. 1999. rab5 GTPase regulates adenovirus endocytosis. J. Virol. 739664-9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren, M., G. Xu, J. Zeng, C. De Lemos-Chiarandini, M. Adesnik, and D. D. Sabatini. 1998. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc. Natl. Acad. Sci. USA 956187-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rink, J., E. Ghigo, Y. Kalaidzidis, and M. Zerial. 2005. Rab conversion as a mechanism of progression from early to late endosomes. Cell 122735-749. [DOI] [PubMed] [Google Scholar]
- 45.Roberts, M., S. Barry, A. Woods, P. van der Sluijs, and J. Norman. 2001. PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr. Biol. 111392-1402. [DOI] [PubMed] [Google Scholar]
- 46.Schnatwinkel, C., S. Christoforidis, M. R. Lindsay, S. Uttenweiler-Joseph, M. Wilm, R. G. Parton, and M. Zerial. 2004. The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms. PLoS Biol. 2E261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segev, N. 2001. Ypt and Rab GTPases: insight into functions through novel interactions. Curr. Opin. Cell Biol. 13500-511. [DOI] [PubMed] [Google Scholar]
- 48.Sheff, D. R., E. A. Daro, M. Hull, and I. Mellman. 1999. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 145123-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sieczkarski, S. B., and G. R. Whittaker. 2003. Differential requirements of Rab5 and Rab7 for endocytosis of influenza and other enveloped viruses. Traffic 4333-343. [DOI] [PubMed] [Google Scholar]
- 50.Somsel Rodman, J., and A. Wandinger-Ness. 2000. Rab GTPases coordinate endocytosis. J. Cell Sci. 113183-192. [DOI] [PubMed] [Google Scholar]
- 51.Sonnichsen, B., S. De Renzis, E. Nielsen, J. Rietdorf, and M. Zerial. 2000. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 149901-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stenmark, H., R. G. Parton, O. Steele-Mortimer, A. Lutcke, J. Gruenberg, and M. Zerial. 1994. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 131287-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stone, M., S. Jia, W. D. Heo, T. Meyer, and K. V. Konan. 2007. Participation of rab5, an early endosome protein, in hepatitis C virus RNA replication machinery. J. Virol. 814551-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ullrich, O., S. Reinsch, S. Urbe, M. Zerial, and R. G. Parton. 1996. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 135913-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urbe, S., L. A. Huber, M. Zerial, S. A. Tooze, and R. G. Parton. 1993. Rab11, a small GTPase associated with both constitutive and regulated secretory pathways in PC12 cells. FEBS Lett. 334175-182. [DOI] [PubMed] [Google Scholar]
- 56.van der Sluijs, P., M. Hull, P. Webster, P. Male, B. Goud, and I. Mellman. 1992. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell 70729-740. [DOI] [PubMed] [Google Scholar]
- 57.Vidricaire, G., and M. J. Tremblay. 2005. Rab5 and Rab7, but not ARF6, govern the early events of HIV-1 infection in polarized human placental cells. J. Immunol. 1756517-6530. [DOI] [PubMed] [Google Scholar]
- 58.Vitelli, R., M. Santillo, D. Lattero, M. Chiariello, M. Bifulco, C. B. Bruni, and C. Bucci. 1997. Role of the small GTPase Rab7 in the late endocytic pathway. J. Biol. Chem. 2724391-4397. [DOI] [PubMed] [Google Scholar]
- 59.Vonderheit, A., and A. Helenius. 2005. Rab7 associates with early endosomes to mediate sorting and transport of Semliki forest virus to late endosomes. PLoS Biol. 3e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilcke, M., L. Johannes, T. Galli, V. Mayau, B. Goud, and J. Salamero. 2000. Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-Golgi network. J. Cell Biol. 1511207-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2107-117. [DOI] [PubMed] [Google Scholar]