Abstract
The latency-associated nuclear antigen (LANA) of Karposi's sarcoma-associated herpesvirus has been reported to interact with glycogen synthase kinase 3β (GSK-3β) and regulate its activity, leading to inhibition of GSK-3-dependent β-catenin degradation. In this study, the interaction between LANA and GSK-3β was characterized further. LANA was found to interact with GSK-3β in vitro as well as in intact cells. However, LANA did not regulate GSK-3β kinase activity and LANA-induced upregulation of β-catenin was GSK-3β independent. LANA did not regulate the stability of β-catenin or of its reported interaction partners p53 and von Hippel-Lindau protein. Additional targets of LANA are likely to mediate its malignancy-promoting function.
Kaposi's sarcoma-associated herpesvirus (KSHV) is the causative agent of Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. The latency- associated nuclear antigen (LANA) of KSHV is one of the latent genes expressed in KSHV-infected cells. LANA is important for maintenance of latent infection and persistence of the viral episome (19). LANA also functions to increase cellular proliferation and survival by acting as a transcriptional coactivator or corepressor. In addition, LANA has been reported to regulate a number of proto-oncogene and tumor suppressors at a posttranscriptional level, including c-Myc, p53, von Hippel-Lindau protein (pVHL), hypoxia-inducible factor 1α (HIF-1α), and β-catenin (2-5, 9-11, 15).
One proposed mechanism through which LANA can stimulate cell proliferation is by upregulating β-catenin, an important transcriptional coactivator of T-cell factor (TCF)/Lef transcription factors. β-Catenin is normally subject to constitutive phosphorylation by CK1α and glycogen synthase kinase 3 (GSK-3) in the cytoplasm, resulting in an N-terminal phosphodegron which targets the β-catenin protein for SCFβ-TrCP-dependent ubiquitination and 26S proteasome-mediated degradation (1, 14). Wnt-secreted glycoproteins, upon binding to their receptors, inhibit β-catenin phosphorylation, leading to its stabilization and nuclear translocation. In cancer, β-catenin is constitutively stabilized due to mutations in the β-catenin phosphorylation sites or in the scaffold proteins Adenomatous polyposis coli and Axin, which are required for efficient β-catenin phosphorylation. LANA has been reported to stabilize β-catenin by interacting with GSK-3β and inducing its nuclear translocation, thus precluding phosphorylation of β-catenin in the cytoplasm (9, 10).
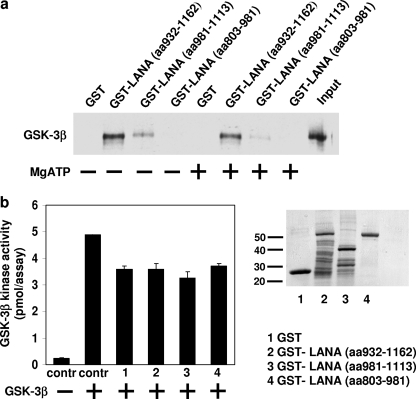
Previous studies by Fujimuro et al. (9) have shown that GSK-3β interacts with a domain comprising amino acids 1133 to 1147 in LANA. Consistent with this result, a glutathione S-transferase (GST) fusion protein containing amino acids 932 to 1162 in LANA, but not shorter fragments (amino acids 917 to 1113 or 803 to 981), was found to be able to pull down recombinant GSK-3β efficiently (Fig. 1a). Addition of Mg ATP (0.5 mM ATP plus 10 mM MgCl2) was without effect, suggesting that GSK-3β kinase activity is unlikely to regulate the interaction between the two proteins. To determine the effect of LANA on GSK-3β activity, in vitro kinase assays were performed in the presence of GST or LANA deletion-GST fusion proteins, using synthetic glycogen synthase peptide as a GSK-3 substrate. As shown in Fig. 1b, no specific effect with any of the GST-LANA fusion proteins was observed. Thus, binding of LANA(932-1162) does not regulate GSK-3β kinase activity directly, although it remains possible that full-length LANA protein has an inhibitory effect, as has been reported by Fujimuro et al. (11). It is also possible that LANA regulates GSK-3β activity in vivo in an indirect manner.
FIG. 1.
Interaction of LANA and GSK-3β in vitro. (a) GST or GST-LANA fusion proteins, as indicated, immobilized on glutathione-Sepharose, were incubated with recombinant GSK-3β in the absence or presence of MgATP (0.5 mM ATP plus 10 mM MgCl2). Subsequently, the Sepharose beads were washed with 0.3 M NaCl and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting using GSK-3β antibody. (b) In vitro kinase assays were conducted using synthetic glycogen synthase peptide substrate, essentially as described in reference 16. Briefly, GST or GST-LANA fusion proteins, immobilized on glutathione-Sepharose, were incubated with 0.05 mg/ml glycogen synthase peptide, 50 mM Tris (pH 7.5), 10 mM MgCl2, 1 mM dithiothreitol, 50 μM [γ-32P]ATP, and 16 U/ml recombinant GSK-3β for 10 min at room temperature. Aliquots of the reaction mixture were spotted onto P81 ion-exchange cellulose phosphate paper, and the filters washed with 100 mM phosphoric acid and analyzed by scintillation counting. In the left panel, GSK-3β kinase activity, expressed as percentage of that of the control (contr) (in the absence of GST or GST fusion proteins) is shown (n = 2). The right-hand panel shows a Coomassie blue-stained sodium dodecyl sulfate gel of GST and GST-LANA fusion proteins after purification with glutathione-Sepharose, which were used for the experiments in panels a and b.
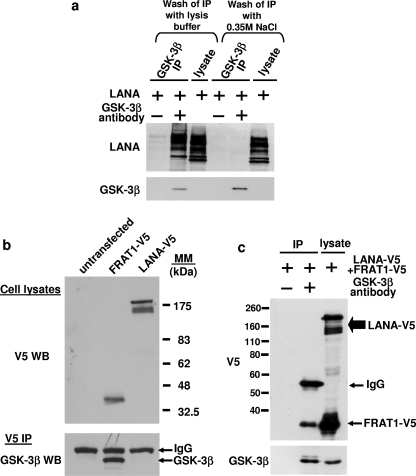
Given that LANA has been reported to inhibit GSK-3β activity in vivo by sequestering the kinase in the nucleus (9, 10), we tested the interaction of GSK-3β and LANA in intact HEK293 cells by using coimmunoprecipitation under the same conditions previously described (9-11). An antibody against GSK-3β indeed immunoprecipitated LANA when cell lysates from LANA-transfected cells were used (Fig. 2a). An interaction was also observed between cotransfected GSK-3β-V5 and LANA (data not shown). However, when the immunoprecipitates were washed under more stringent conditions with 0.35 M NaCl-containing buffer, it was noted that the interaction between endogenous GSK-3β and LANA was abolished (Fig. 2a) and the interaction between cotransfected GSK-3β-V5 and LANA was much weaker (data not shown). The experiments presented in Fig. 2b and c show that the interaction of endogenous GSK-3β with a different well-characterized GSK-3β-binding protein, FRAT1 (12, 20), was maintained under the more stringent conditions. Thus, when cells were transfected with C-terminally V5-tagged versions of LANA or FRAT1 and an immunoprecipitation was performed with V5 antibody, FRAT1, but not LANA, was found to efficiently coimmunoprecipitate endogenous GSK-3β (Fig. 2b). Similar results were obtained when a reciprocal coimmunoprecipitation experiment was performed (Fig. 2c). FRAT1 is believed to inhibit GSK-3β-dependent phosphorylation of β-catenin by competing with the scaffold protein Axin for overlapping binding sites in GSK-3 (8, 18, 21). If LANA were to regulate GSK-3β through an interaction in a similar manner or, as previously suggested, by sequestering GSK-3β and altering its subcellular localization (9, 10), an interaction with GSK-3β that is similarly robust compared to that of FRAT1 would be expected.
FIG. 2.
Interaction of LANA and GSK-3 in intact cells. (a) HEK293 cells were transfected with LANA, followed by immunoprecipitation (IP) with GSK-3β antibody, as previously described in reference 6, and Western blotting of cell lysates and immunoprecipitates with the indicated antibodies. The immunoprecipitates were washed four times with cell lysis buffer (containing 50 mM NaCl, 0.5% NP-40, 5% glycerol, 0.5 mM EDTA, 50 mM Tris [pH 7.5], 1 mM dithiothreitol) or salt wash buffer (containing 0.35 mM NaCl, 10 mM Tris [pH 7.5], 1 mM EGTA), as indicated. (b and c) Cells were transfected with FRAT1-V5 or LANA-V5, as indicated, cell lysates subjected to immunoprecipitation with V5 (b) or GSK-3β (c) antibody, and immunoprecipitates were washed with 0.35 M NaCl salt containing lysis buffer. Cell lysates and immunoprecipitates were analyzed by Western blotting with V5 and GSK-3β antibodies. IgG, immunoglobulin G; MM, molecular mass.
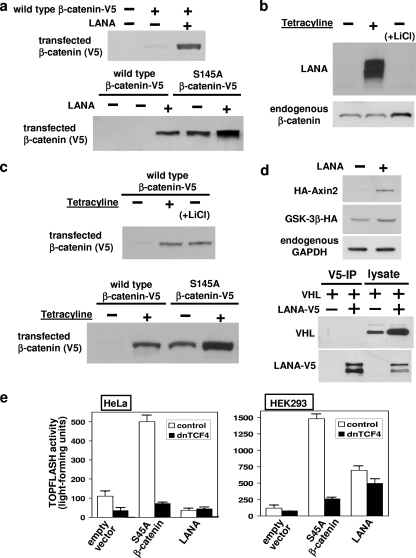
LANA has previously been shown to induce stabilization of β-catenin via inhibition of GSK-3β-dependent phosphorylation (9, 10). Consistent with these reports, cotransfection of expression plasmids for LANA with C-terminally V5-tagged wild-type β-catenin resulted in a marked increase in wild-type β-catenin protein in the presence of LANA (Fig. 3a, upper panel). GSK-3β phosphorylates a number of conserved serine and threonine residues at the N terminus of β-catenin, which requires a priming phosphorylation by CK1α at Ser45 (1, 14). Hence, GSK-3β-dependent phosphorylation and destabilization of β-catenin are prevented in the S45A mutant, resulting in much higher steady-state protein levels (Fig. 3a, lower panel, compare lanes 2 and 4). It was observed that cotransfection of LANA not only markedly increased the abundance of wild-type β-catenin but also markedly increased that of the S45A mutant (Fig. 3a, lower panel), suggesting that LANA regulates β-catenin abundance independently of GSK-3β.
FIG. 3.
LANA-induced upregulation of β-catenin is GSK-3β independent. (a) HEK293 cells were cotransfected with wild-type β-catenin-V5 or S45A mutant β-catenin-V5 (lower panel) and LANA expression plasmids, as indicated, for 2 days, followed by Western blotting of cell lysates with V5 antibody. (b) HEK293 cells stably transfected with LANA under the control of a tetracycline-inducible promoter (LANA tet-on HEK293) were treated with 1 μg/ml tetracycline for the last 24 h (to induce LANA expression) or with 30 mM LiCl for the last 5 h (to inhibit GSK-3β activity). Cytosolic endogenous β-catenin and LANA expression was analyzed as previously described (6) by Western blotting with LANA and β-catenin antibodies. (c) LANA tet-on HEK293 cells were transfected with wild-type or S45A mutant β-catenin-V5 for 2 days and treated with 1 μg/ml tetracycline for the last 24 h or with 30 mM LiCl for the last 16 h. Cytosolic concentrations of β-catenin-V5 were analyzed as described (7). (d) In the upper panel, cells were cotransfected for 2 days with LANA and hemagglutinin (HA)-Axin2 or GSK-3β-HA, as indicated, followed by Western blotting using HA or GAPDH (glyceraldehyde-3-phosphate dehydrogenase) antibodies. In the lower panel, cells were cotransfected with pVHL and LANA-V5 expression plasmids, as indicated, and cell lysates were subjected to immunoprecipitation (IP) with V5 antibody and V5 immunoprecipitates (lanes 1 and 2) or cell lysates (lanes 3 and 4) analyzed by Western blotting with VHL and V5 antibodies. (e) Topflash reporter activity was measured by cotransfecting cells grown in 12-well plates with 0.15 μg Topflash DNA (Upstate) and 0.35 μg S45A β-catenin-V5, LANA, dnTCF4, or empty vector. Two days after transfection, cells were analyzed for luciferase activity using the Steady-Glo luciferase assay system (Promega) according to the manufacturer's instructions. Repeat experiments gave similar qualitative results, but the increase in luciferase reporter activity with S45A β-catenin-V5 varied. Therefore, results from representative experiments are shown. Averages for the effect of dnTCF4 coexpression are given in the text.
In order to assess the effect of LANA on endogenous β-catenin, cell lines with stably transfected LANA under a tetracycline-inducible promoter were generated (LANA tet-on HEK293 cells), thus allowing uniform expression of LANA in all cells upon addition of tetracycline. Treatment with tetracycline resulted in strong induction of LANA protein but was without effect on the endogenous cytosolic β-catenin concentration (Fig. 3b) (even when the cell line with the highest LANA expression levels was used). In contrast, induction of endogenous β-catenin was observed upon addition of the GSK-3β inhibitor lithium chloride. Consistent with the cotransfection results in Fig. 3a, LANA induction with tetracycline markedly increased the abundance of transfected wild-type and S45A mutant β-catenin (Fig. 3c). These results suggest that LANA does not regulate endogenous β-catenin stability but upregulates the expression of transfected β-catenin in a GSK-3β-independent manner. Consistent with this, LANA induction in tet-on HEK293 cells (Fig. 4a) or lentiviral transduction of LANA into HeLa cells (data not shown) did not increase the half-life of endogenous β-catenin. These results raise the possibility that LANA increases the protein expression from transfected plasmid DNA. Indeed we observed a similar significant increase in the expression from transfected plasmids encoding a number of proteins, including Axin2 and pVHL, while other proteins showed only small increases in expression (e.g., GSK-3β) (Fig. 3d). The marked effect of LANA expression on pVHL abundance is unlikely to be due to a direct binding given that no direct interaction between the two proteins could be detected by coimmunoprecipitation assay (Fig. 3d, right panel), even when a low-stringency buffer was used to wash the immunoprecipitates. The mechanism through which LANA regulates the expression from cotransfected plasmids is currently not clear. The effect of LANA was not dependent on the cytomegalovirus promoter, given that we found a similar effect of LANA on the expression of β-catenin from an EF1 promoter-driven plasmid (data not shown). Similar to LANA, cyclic AMP protein kinase has been reported to increase the expression from cotransfected expression plasmids (17), warranting caution when measuring effects on protein concentrations in cotransfection experiments.
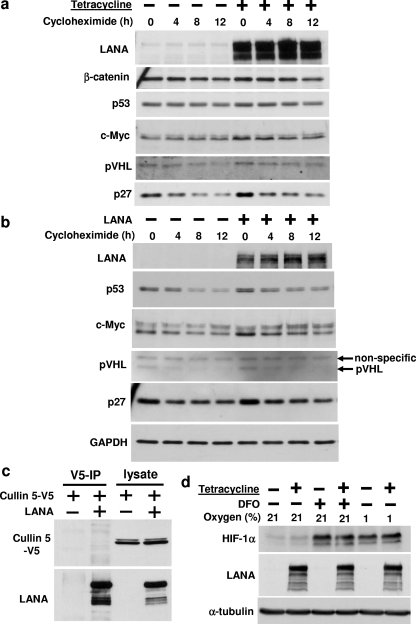
FIG. 4.
Effect of LANA on the stability and half-life of various cellular proteins. (a) LANA tet-on HEK293 cells were induced with 1 μg/ml tetracycline for 12 h, where indicated, followed by addition of 40 μM cycloheximide (t = 0 h) to inhibit new protein synthesis. Cells were lysed after 0, 4, 8, and 12 h, and cell lysates were analyzed by Western blotting using the indicated antibodies. (b) HEK293 cells were lentivirally transduced with LANA (or empty vector). Lentivirus was generated using empty or LANA-containing Puro-MaRX lentiviral expression vector in 293-gag-pol cells and pseudotyped with vesicular stomatitis virus glycoprotein. Protein turnover was estimated using cycloheximide chase as in panel a. (c) Cells were cotransfected with Cullin 5-V5 and LANA expression plasmids, as indicated, cell lysates were subjected to immunoprecipitation (IP) with LANA antibody, and LANA immunoprecipitates (lanes 1 and 2) or cell lysates (lanes 3 and 4) were analyzed by Western blotting, as indicated. (d) LANA tet-on HEK293 cells were induced with 1 μg/ml tetracycline for 24 h, where indicated, and treated with 200 μM desferrioxamine (DFO) for the last 7 h or exposed to hypoxia (1% oxygen) for the last 4 h. Cell lysates were analyzed with antibodies against HIF-1α, LANA, or α-tubulin.
The effect of LANA expression on β-catenin-dependent activation of TCF/Lef transcription factors was also tested in two cell lines using the Topflash reporter assay (Fig. 3e). In HeLa cells, LANA was without effect on Topflash activity, while S45A mutant, nondegradable β-catenin expression stimulated the reporter activity. In HEK293 cells, both S45A β-catenin and LANA stimulated Topflash reporter activity. S45A β-catenin-induced reporter activity was suppressed by 77% ± 4% (n = 3) upon expression of the dominant-negative form of TCF (dnTCF). In contrast, dnTCF suppressed LANA-induced transcriptional activation by only 35% ± 3% (n = 3), suggesting that the increase in luciferase reporter activity upon expression of LANA is largely independent of β-catenin.
LANA has been reported to interact with and induce the degradation of the p53 and pVHL proteins by forming a Cullin 5-based RING E3 ubiquitin ligase (5) and to stabilize the c-Myc protein (2, 15). We therefore used the LANA tet-on HEK293 cell line to determine the half-life of these proteins in the presence or absence of LANA. The half-life of the endogenous p53, pVHL and c-Myc proteins was determined by first inducing LANA expression with tetracycline during an overnight incubation followed by cycloheximide addition and measurement of protein abundance by Western blotting at times 0, 4, 8, and 12 h. As shown in Fig. 4a, induction of LANA with tetracycline led to a small increase in c-Myc protein expression and protein half-life, confirming previous reports (2, 15). In contrast, LANA induction did not reduce the half-life of the p53 and pVHL proteins and was also without effect on the p27 control protein. Very similar results were obtained when LANA was transduced into HEK293 cells using lentivirus (Fig. 4b). We were also unable to detect an interaction between LANA and endogenous or transfected p53 or pVHL in cells by coimmunoprecipitation (Fig. 3d; and data not shown). There was also no evidence for an interaction between LANA and Cullin 5 (Fig. 4c). Of note, an unbiased mass spectrometry-based proteomic protein-protein interaction screen also found no evidence for an interaction of LANA with endogenous p53, pVHL, Cullin 5 or GSK-3β (13).
The major cellular function of pVHL is to target the transcription factors hypoxia-inducible factor-1α (HIF-1α) and hypoxia-inducible factor-2α (HIF-2α) for ubiquitination and consequently 26S proteasome-mediated degradation. LANA has also been reported to regulate cellular HIF-1α concentrations (3, 4). We therefore determined the effect of tetracycline-induced LANA expression on endogenous HIF-1α under normoxic conditions as well as on the accumulation of HIF-1α in cells exposed to hypoxia or to the hypoxia mimic desferrioxamine. However, as shown in Fig. 4d, no effect of LANA on HIF-1α under any of these conditions was observed.
In summary, the presented results do not support a physiologically significant role of LANA in regulating cellular Wnt signaling activity via its interaction with GSK-3β or the tumor suppressor proteins p53 and pVHL. These results suggest that other cellular functions of LANA mediate its tumor-promoting function.
Acknowledgments
I gratefully acknowledge the provision of reagents, including a LANA expression plasmid, antibody, and LANA-GST fusion proteins by Chris Boshoff and Daniel Hollyman (University College London Cancer Institute); lentiviral expression vector Puro-MaRX by David Beach (Institute of Cell and Molecular Science, London); and HA-Axin2 expression plasmid by Thomas Hughes (Institute of Child Health, University College London).
Footnotes
Published ahead of print on 25 March 2009.
REFERENCES
- 1.Amit, S., A. Hatzubai, Y. Birman, J. S. Andersen, E. Ben-Shushan, M. Mann, Y. Ben-Neriah, and I. Alkalay. 2002. Axin-mediated CKI phosphorylation of β-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 161066-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bubman, D., I. Guasparri, and E. Cesarman. 2007. Deregulation of c-Myc in primary effusion lymphoma by Kaposi's sarcoma herpesvirus latency-associated nuclear antigen. Oncogene 264979-4986. [DOI] [PubMed] [Google Scholar]
- 3.Cai, Q., K. Lan, S. C. Verma, H. Si, D. Lin, and E. S. Robertson. 2006. Kaposi's sarcoma-associated herpesvirus latent protein LANA interacts with HIF-1α to upregulate RTA expression during hypoxia: latency control under low oxygen conditions. J. Virol. 807965-7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai, Q., M. Murakami, H. Si, and E. S. Robertson. 2007. A potential α-helix motif in the amino terminus of LANA encoded by Kaposi's sarcoma-associated herpesvirus is critical for nuclear accumulation of HIF-1α in normoxia. J. Virol. 8110413-10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai, Q. L., J. S. Knight, S. C. Verma, P. Zald, and E. S. Robertson. 2006. EC5S ubiquitin complex is recruited by KSHV latent antigen LANA for degradation of the VHL and p53 tumor suppressors. PLoS Pathog. 2e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chew, E. H., and T. Hagen. 2007. Substrate-mediated regulation of cullin neddylation. J. Biol. Chem. 28217032-17040. [DOI] [PubMed] [Google Scholar]
- 7.Culbert, A. A., M. J. Brown, S. Frame, T. Hagen, D. A. Cross, B. Bax, and A. D. Reith. 2001. GSK-3 inhibition by adenoviral FRAT1 overexpression is neuroprotective and induces Tau dephosphorylation and beta-catenin stabilisation without elevation of glycogen synthase activity. FEBS Lett. 507288-294. [DOI] [PubMed] [Google Scholar]
- 8.Farr, G. H., III, D. M. Ferkey, C. Yost, S. B. Pierce, C. Weaver, and D. Kimelman. 2000. Interaction among GSK-3, GBP, axin, and APC in Xenopus axis specification. J. Cell Biol. 148691-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimuro, M., F. Y. Wu, C. ApRhys, H. Kajumbula, D. B. Young, G. S. Hayward, and S. D. Hayward. 2003. A novel viral mechanism for dysregulation of beta-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nat. Med. 9300-306. [DOI] [PubMed] [Google Scholar]
- 10.Fujimuro, M., and S. D. Hayward. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus manipulates the activity of glycogen synthase kinase-3β. J. Virol. 778019-8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimuro, M., J. Liu, J. Zhu, H. Yokosawa, and S. D. Hayward. 2005. Regulation of the interaction between glycogen synthase kinase 3 and the Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen. J. Virol. 7910429-10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonkers, J., H. C. Korswagen, D. Acton, M. Breuer, and A. Berns. 1997. Activation of a novel proto-oncogene, Frat1, contributes to progression of mouse T-cell lymphomas. EMBO J. 16441-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaul, R., S. C. Verma, and E. S. Robertson. 2007. Protein complexes associated with the Kaposi's sarcoma-associated herpesvirus-encoded LANA. Virology 364317-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu, C., Y. Li, M. Semenov, C. Han, G. H. Baeg, Y. Tan, Z. Zhang, X. Lin, and X. He. 2002. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108837-847. [DOI] [PubMed] [Google Scholar]
- 15.Liu, J., H. J. Martin, G. Liao, and S. D. Hayward. 2007. The Kaposi's sarcoma-associated herpesvirus LANA protein stabilizes and activates c-Myc. J. Virol. 8110451-10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryves, W. J., L. Fryer, T. Dale, and A. J. Harwood. 1998. An assay for glycogen synthase kinase 3 (GSK-3) for use in crude cell extracts. Anal. Biochem. 264124-127. [DOI] [PubMed] [Google Scholar]
- 17.Taurin, S., N. Sandbo, Y. Qin, D. Browning, and N. O. Dulin. 2006. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J. Biol. Chem. 2819971-9976. [DOI] [PubMed] [Google Scholar]
- 18.Thomas, G. M., S. Frame, M. Goedert, I. Nathke, P. Polakis, and P. Cohen. 1999. A GSK3-binding peptide from FRAT1 selectively inhibits the GSK3-catalysed phosphorylation of axin and beta-catenin. FEBS Lett. 458247-251. [DOI] [PubMed] [Google Scholar]
- 19.Verma, S. C., K. Lan, and E. Robertson. 2007. Structure and function of latency-associated nuclear antigen. Curr. Top. Microbiol. Immunol. 312101-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yost, C., G. H. Farr III, S. B. Pierce, D. M. Ferkey, M. M. Chen, and D. Kimelman. 1998. GBP, an inhibitor of GSK-3, is implicated in Xenopus development and oncogenesis. Cell 931031-1041. [DOI] [PubMed] [Google Scholar]
- 21.Yuan, H., J. Mao, L. Li, and D. Wu. 1999. Suppression of glycogen synthase kinase activity is not sufficient for leukemia enhancer factor-1 activation. J. Biol. Chem. 27430419-30423. [DOI] [PubMed] [Google Scholar]