Abstract
Latent membrane antigen 1 and -2 (LMP-1/2)-specific CD8+ T cells from newly diagnosed and relapsed Hodgkin's lymphoma (HL) patients display a selective functional impairment. In contrast, CD8+ T cells specific for Epstein-Barr virus (EBV) nuclear proteins and lytic antigens retain normal T-cell function. Reversion to a dysfunctional phenotype of LMP-1/2-specific T cells is coincident with the regression of HL. To delineate the potential basis for this differential susceptibility for the loss of function, we have carried out a comprehensive functional analysis of EBV-specific T cells using ex vivo multiparametric flow cytometry in combination with assessment of antigen-driven proliferative potential. This analysis revealed that LMP-1/2-specific T cells from healthy virus carriers display a deficient polyfunctional profile compared to that of T cells specific for epitopes derived from EBV nuclear proteins and lytic antigens. Furthermore, LMP-specific T-cells are highly susceptible to galectin-1-mediated immunosuppression and are less likely to degranulate following exposure to cognate peptide epitopes and poorly recognized endogenously processed epitopes from virus-infected B cells. More importantly, ex vivo stimulation of these T cells with an adenoviral vector encoding multiple minimal CD8+ T-cell epitopes as a polyepitope, in combination with a γC cytokine, interleukin-2, restored polyfunctionality and shielded these cells from the inhibitory effects of galectin-1.
Following primary lytic infection, Epstein-Barr virus (EBV) induces a lifelong latent infection. Cytotoxic T lymphocytes (CTL) play a critical role in limiting infection during the lytic stages of infection and in controlling latently infected cells (9, 21). Although latent EBV infection is asymptomatic in most individuals, it is associated with a number of malignancies that arise in both immunocompetent and immunocompromised individuals (11). Malignancies occurring in immunocompromised individuals, such as the posttransplant lymphomas, typically display a latency type 3 pattern of gene expression, characterized by the expression of the immunodominant EBV nuclear antigens 3 to 6 (EBNA3-6) and of the less-immunogenic antigens EBNA1 and latent membrane proteins 1 and 2 (LMP-1/2). Immune escape likely occurs in this setting due to the direct suppression of lymphocyte function by immunosuppressive drugs (5, 25, 26). Conversely, malignancies occurring in immunocompetent individuals display either a latency type 1 profile, expressing only EBNA1, or a latency type 2 profile, expressing LMP-1/2, in addition to EBNA1. While latency type 1 malignancies, including Burkitt's lymphoma, display a reduction in major histocompatibility complex class I (MHC-I) surface expression and present antigen poorly to CD8+ T cells (23, 24), providing a mechanism for immune escape from CTL, latency type 2 malignancies, including Hodgkin's lymphoma (HL), retain the capacity to process and present antigen to CD8+ T cells (10, 15), suggesting that other mechanisms of escape from CTL recognition are occurring. We have recently demonstrated that during the acute stages of HL, LMP-specific T cells display a loss of T-cell function (3). Recovery of T-cell function is associated with remission. Recent studies in our laboratory have suggested a role for galectin-1 (Gal-1) in mediating immunosuppression of T cells during the acute stages of HL (4). Gal-1 expression in Hodgkin Reed-Sternberg cells was associated with a reduced CD8+ T-cell infiltrate. Furthermore, Gal-1 was shown to inhibit the proliferation of EBV-specific T cells in response to lymphoblastoid cell lines (LCL). In this study, we have now assessed the impact Gal-1 has upon the function of EBV-specific T cells with different specificities and the functional differences that exist between these T-cell populations. These studies demonstrate that LMP-1/2 and EBNA1-specific T cells are more susceptible to the immunosuppressive effects of Gal-1, which is associated with a qualitative inferiority in these T cells. However, T-cell functionality can be improved following in vitro expansion, which coincides with enhanced resistance to the suppressive effects of Gal-1.
MATERIALS AND METHODS
Healthy volunteers.
A panel of 20 EBV-seropositive healthy donors (Table 1) were recruited for this study. Each volunteer used in this study was asked to sign the consent form as outlined in the institutional ethics guidelines.
TABLE 1.
HLA types of PBMC of donors in this study
| Donor | Sexa | HLA type |
|---|---|---|
| 1 | M | A2 A11 |
| 2 | M | A24 B38 B60 |
| 3 | M | A1 A3 B7 B8 |
| 4 | M | A2 A24 B57 B62 |
| 5 | M | A3 A11 B35 B44 |
| 6 | M | A2 B57 B62 |
| 7 | F | A1 A11 B8 B35 |
| 8 | F | A2 B57 |
| 9 | F | A2 A30 B8 B45 |
| 10 | F | A1 A2 B7 B8 |
| 11 | F | A2 A32 B39 B44 |
| 12 | M | A1 A3 B7 B27 |
| 13 | F | A2 A68 B8 B15 |
| 14 | M | A23 A25 B49 B50 |
| 16 | M | A24 A26 B15 B62 |
| 17 | F | A24 B8 |
| 18 | M | A24 A29 B44 |
| 19 | M | A2 B35 B57 |
| 20 | F | A11 A24 B13 B35 |
M, male; F, female.
Expansion of LMP-/EBNA1-specific T cells.
LMP- and EBNA1-specific T cells were stimulated using the vector AdE1-LMPpoly, which has been previously described (27). Peripheral blood mononuclear cells (PBMC) were cocultured in multiwell tissue culture plates with autologous PBMC infected with AdE1-LMPpoly (multiplicity of infection of 10:1) at a responder-to-stimulator ratio of 2:1. On day 3 and every 3 to 4 days thereafter, the cultures were supplemented with growth medium containing 10 units/ml of recombinant interleukin-2. Cells were harvested for assay of T-cell function on day 14.
T-cell proliferation assay.
The proliferation of EBV-specific cells was assessed by using carboxyfluorescein succinimidyl ester (CFSE) (17). PBMC were labeled with 0.5 μM CFSE, washed thoroughly, and then preincubated with 5 μg/ml of recombinant Gal-1 or mock treated for 4 h. PBMC were then stimulated with pools of EBV-encoded CTL epitopes (Table 2), each at 1 μg/ml, or with phytohemagglutinin (PHA). Seven days later, cells were stained with peridinin chlorophyll protein-conjugated anti-CD8 and assessed for cell division on a FACSCanto.
TABLE 2.
HLA class I-restricted T-cell epitopes used in this study
| Antigen | Epitope sequence | HLA restriction |
|---|---|---|
| LMP-1/2 | PYLFWLAA | A23, A24 |
| SSCSSCPLSKI | A11 | |
| TYGPVFMCL | A24 | |
| RRRWRRLTV | B27 | |
| LLSAWILTA | A2.03 | |
| LTAGFLIFL | A2.06 | |
| CLGGLLTMV | A2.01 | |
| VMSNTLLSAW | A25 | |
| IEDPPFNSL | B40.01 | |
| YLLEMLWRL | A2 | |
| YLQQNWWTL | A2 | |
| IALYLQQNW | B57, B58 | |
| FLYALALLL | A2 | |
| CPLSKILL | B8 | |
| MGSLEMVPM | B35 | |
| EBNA1 | HPVGEADYFEY | B35 |
| RPQKRPSCI | B7 | |
| IPQCRLTPL | B7 | |
| LSRLPFGMA | B57 | |
| YNLRRGTAL | B8 | |
| VLKDAIKDL | A2.03 | |
| FVYGGSKTSL | Cw3 | |
| EBNA3-6 | FLRGRAYGL | B8 |
| RPPIFIRRL | B7 | |
| VSFIEFVGW | B58 | |
| AVLLHEESM | B35.01 | |
| YPLHEQHGM | B35.01 | |
| RRIYDLIEL | B27 | |
| EENLLDFVRF | B44 | |
| KEHVIQNAF | B44 | |
| EGGVGWRHW | B44 | |
| Lytic | GLCTLVAML | A2 |
| YVLDHLIVV | A2 | |
| ATIGTAMYK | A11 | |
| DYCNVLNFEK | A24 | |
| RAKFKQLL | B8 | |
| SENDRLRLL | B60 | |
| LPEPLPQGQLTAY | B35.08 | |
| EPLPQGQLTAY | B35.01 |
Intracellular cytokine staining.
PBMC or AdE1-LMPpoly-stimulated T cells were incubated for 12 h with peptide pools (each at 1 μg/ml), with autologous LCL in the presence of brefeldin A (BD PharMingen) at a responder-to-stimulator ratio of 20:1, or with phycoerythrin (PE)-conjugated anti-CD107a and monensin (BD Pharmingen). Cells were incubated with peridinin chlorophyll protein-conjugated anti-CD8 or with EBV-specific PE or allophycocyanin (APC)-conjugated pentamers (ProImmune, Oxford, United Kingdom) for analysis of endogenous antigen recognition prior to anti-CD8 staining. For analysis of early activation markers, cells were stained with PE-Cy7-conjugated anti-CD69 and APC-conjugated CD137. For intracellular cytokine staining, cells were then fixed and permeabilized; incubated with PE-conjugated anti-MIP1β, APC, or Alexa Fluor 700-conjugated anti-tumor necrosis factor (TNF) and Alexa Fluor 700 or fluorescein isothiocyanate-conjugated anti-gamma interferon (IFN-γ); and analyzed on a FACSCanto.
Statistical analysis.
Statistical analysis was carried out using the Mann-Whitney test. Differences were considered to be statistically significant where P values were <0.05.
RESULTS
Selective inhibition of proliferation and function of LMP-1/2- and EBNA1-specific T cells in the presence of Gal-1.
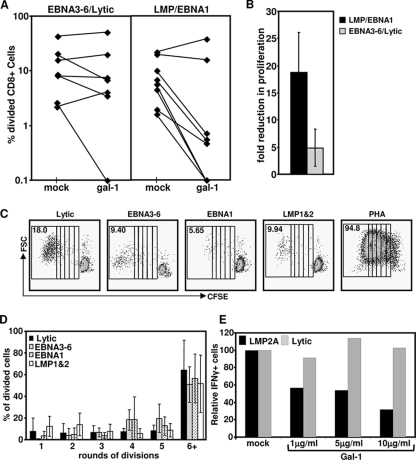
To determine the effect of Gal-1 on EBV-specific T cells, CFSE-labeled PBMC from healthy donors were stimulated with CD8+ T-cell epitopes of the EBV lytic antigens, EBNA3-6, EBNA1, or LMP-1/2 (Table 1) in the presence or absence of recombinant Gal-1 protein. The data presented in Fig. 1A show that LMP-1/2- and EBNA1-specific T cells from six of eight donors displayed dramatically reduced proliferation following treatment with Gal-1. In contrast, the proliferative capacity of the majority of lytic or EBNA3-6 antigen-specific T cells remained unaffected in the presence of Gal-1. Detailed analysis of the decrease in the number of proliferating cells confirmed a significant impact of Gal-1 on LMP-1/2- and EBNA1-specific T cells compared to its impact on EBNA3-6-/lytic antigen-specific T cells (Fig. 1B).
FIG. 1.
Effect of recombinant Gal-1 on the proliferation of EBV-specific T cells. PBMC from EBV-seropositive donors were labeled with CFSE, preincubated with Gal-1 at 5 μg/ml (or mock treated), and stimulated with pools of CTL epitopes of lytic cycle antigens, EBNA3-6, EBNA1, or LMP-1/2. Cells were harvested seven days later, stained with anti-CD8, and assessed for cell division on a FACSCanto. (A) Data represent the comparative proliferation in PBMC of individual donors with and without Gal-1 treatment. (B) Data represent the means and standard errors of the decreases in numbers of proliferating cells following treatment with Gal-1. (C) Representative data following stimulation with each EBV-specific peptide pool or PHA are shown. The numbers in the upper left of each panel represent the mean percentage of total proliferating CD8+ T cells following stimulation. Each box represents one round of cell division. FSC, forward scatter. (D) Data represent the means and standard deviations of the results of the rounds of division of CD8+ T cells following stimulation with the different pools of EBV-specific peptide epitopes. (E) PBMC from an EBV-seropositive donor were preincubated with Gal-1 at 1, 5, or 10 μg/ml (or mock treated) and stimulated with an LMP-2A epitope, CLGGLLTMV, or a BMLF1 (lytic antigen) epitope, GLCTLVAML, for 12 h in the presence of brefeldin A. IFN-γ expression by CD8+ T cells was assessed using intracellular cytokine assay. Data represent the percentages of IFN-γ-expressing cells relative to that in mock-treated cells.
To ensure that the reduced proliferative potential was not an inherent characteristic of EBNA1- and LMP-1/2-specific T cells, EBV-specific T-cell populations were assessed for the number of rounds of division following in vitro stimulation with the pooled HLA class I-restricted peptide epitopes from these antigens (Table 1). Representative data from a single stimulation with each peptide pool are shown in Fig. 1C. Due to the low number of dividing cells following stimulation with the LMP-1/2- and EBNA1-specific pools, the rounds of cell division were determined following stimulation with a mitogen, PHA. As is evident from the data in Fig. 1D, LMP-1/2- and EBNA1-specific T cells proliferated to an extent similar to the proliferation of T cells specific for EBNA3-6 and the lytic cycle antigens, indicating that these T cells are not inherently impaired in their proliferative capacity. To assess the effect of Gal-1 on the functional capacity of EBV-specific T cells, PBMC from healthy donors were stimulated with CD8+ T-cell epitopes from EBV in the presence or absence of recombinant Gal-1 protein and then assessed for IFN-γ expression. The representative data presented in Fig. 1E clearly show that pretreatment of T cells with Gal-1 dramatically impairs the ability of LMP-1/2-specific T cells to respond to cognate peptide epitopes. In contrast, the presence of Gal-1 had minimal effect on T cells specific for lytic antigens.
LMP-1/2- and EBNA1-specific T cells display a limited polyfunctional profile.
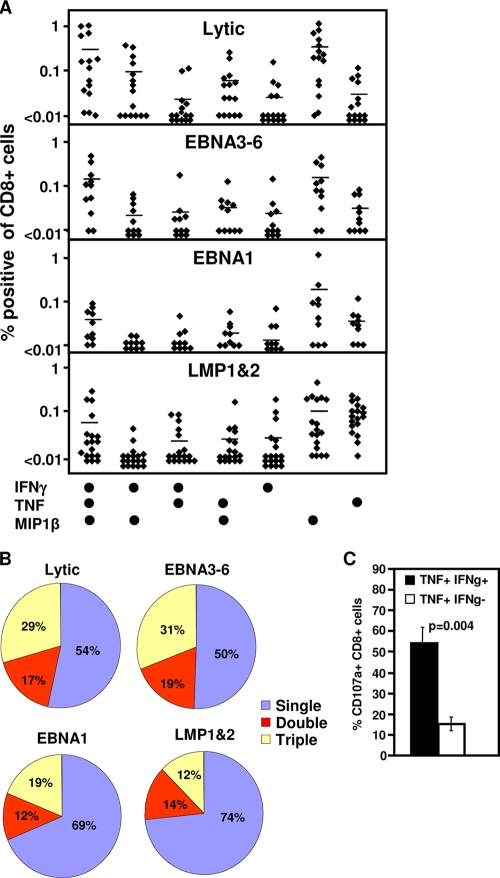
A number of recent studies have implicated a role for T-cell polyfunctionality, including the capacity to generate multiple cytokines (IFN-γ and TNF) and chemokines (MIP1β) and to rapidly degranulate (CD107a), in protection against infection (1, 6, 19). To determine if susceptibility to Gal-1-mediated inhibition of antigen-specific T-cell proliferation could be linked to differences in T-cell function, PBMC from healthy EBV-positive donors were initially assessed for the production of IFN-γ, TNF, and MIP1β by intracellular cytokine staining following stimulation with pools of lytic antigen, EBNA3-6, EBNA1, or LMP-1/2 epitopes. Responding cells were assessed for their capacity to produce either 1, 2, or 3 cytokines (Fig. 2A and B). While all T-cell populations contained a high proportion of MIP1β-positive cells, LMP-1/2-specific T cells displayed a percentage of T cells capable of producing all three cytokines that was significantly reduced in comparison to the percentage of T cells specific for EBNA3-6 and lytic cycle antigens (P = 0.046 and P = 0.007, respectively). Additionally, the LMP-1/2-specific population contained a proportion of T cells expressing TNF alone that was significantly greater than the proportion of EBNA3-6 and lytic antigen-specific T cells producing TNF alone. (P = 0.001 and P = 0.0002, respectively) (Fig. 2A and B).
FIG. 2.
Polyfunctionality of EBV-specific T-cell populations. (A and B) Cytokine profile of EBV-specific T-cell populations. PBMC from EBV-seropositive donors were stimulated with pools of lytic antigen, EBNA3-6, EBNA1, or LMP-1/2 epitopes for 12 h in the presence of brefeldin A. IFN-γ, TNF, and MIP1β expression by CD8+ T cells were assessed using intracellular cytokine assay. (A) Percentages of CD8+ T cells from individual donors producing each cytokine combination. (B) Average percentage of specific cells producing 1, 2, or 3 cytokines. (C) Degranulation of EBV-specific T cells. PBMC from EBV-seropositive donors were stimulated with the pools of lytic antigen, EBNA3-6, EBNA1, or LMP-1/2 epitopes for 12 h in the presence of monensin and anti-CD107α. IFN-γ (IFNg) and TNF expression by CD8+ T cells was assessed using intracellular cytokine assay. Data represent the means and standard errors of CD107α-positive cells that produce both TNF and IFN-γ or TNF alone.
To assess the impact of differential cytokine production on the capacity to generate immediate cytolytic function, cells producing either TNF alone or TNF and IFN-γ cells were assessed for the ability to mobilize CD107a, as a marker for degranulation, following stimulation. CD8+ T cells that produced TNF in the absence of IFN-γ demonstrated a significantly reduced capacity to degranulate upon antigen stimulation, indicating that the single-positive T cells display a reduced potential for immediate effector function (Fig. 2C).
LMP-1/2-specific CD8+ T cells display impaired capacity to recognize endogenously processed epitopes from EBV-infected B cells.
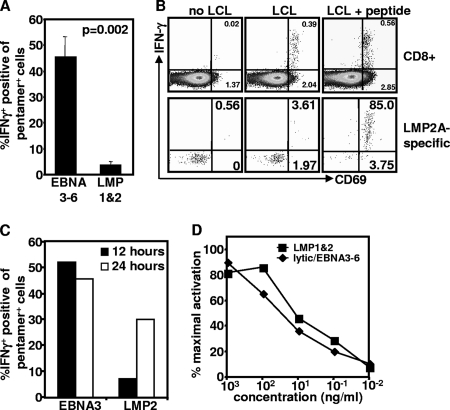
There is increasing evidence that the LMP-1/2 and EBNA1 antigens have developed strategies to limit their presentation to T cells (29-31). This reduction in recognition of endogenous antigen could potentially influence the priming of T cells against LMP-1/2 and EBNA1, inducing a quantitatively and qualitatively inferior response. To directly compare the capacity of EBNA3-6- and LMP-1/2-specific T cells to recognize antigen endogenously expressed in EBV-infected cells, PBMC were incubated with autologous EBV-transformed LCL for 12 h, stained with EBV-specific pentamers, and assessed for IFN-γ and TNF production. While EBNA3-6-specific T cells consistently generated both IFN-γ (Fig. 3A) and TNF (data not shown) in response to autologous LCL, LMP-2A-specific T cells showed a reduced capacity to generate both IFN-γ and TNF in response to autologous LCL. Similarly, LMP-2A-specific T cells also failed to upregulate CD69, an early activation marker, in response to LCL (Fig. 3B). While upregulation of CD69 in CD8+ T cells was evident following incubation with autologous LCL, upregulation in CD69 expression in LMP-2A-specific T cells was only evident following peptide coating of LCL, confirming that LMP-specific T cells do not recognize endogenous antigen as efficiently as other EBV-specific T cells. To determine if LMP-specific T cells could be activated at all following recognition of endogenous antigen, PBMC were incubated with autologous antigen for a longer time period and then assessed for IFN-γ production. The representative data in Fig. 3C demonstrate that LMP-1/2-specific T cells can be activated following incubation with autologous LCLs for 24 h.
FIG. 3.
Recognition of endogenous antigen by EBV-specific T cells. PBMC were stimulated with autologous LCL at a 20:1 responder-to-stimulator ratio in the presence of brefeldin A. (A) IFN-γ expression was assessed using intracellular cytokine assay. Data represent the means and standard errors of the results for IFN-γ-producing CD8+ T cells from PBMC of six donors in each group following incubation with autologous LCL for 12 h. (B) CD69 and IFN-γ expression by total CD8+ T cells or HLA pentamer-specific CD8+ T cells was assessed using intracellular cytokine assay. Representative data are from CD8+ T cells or LMP-2a-specific T cells from a single donor following stimulation with and without LCL, pulsed with and without an LMP-2a-specific epitope, CLGGLTMV. Data in the upper right quadrant are the percentages of CD69+ IFNY+ cells. Data in the lower right quadrant are the percentages of cells producing CD69 alone. (C) Representative data from single donors showing recognition of LCL by EBNA3-specific T cells recognizing the epitope FLRGRAYGL or LMP2a-specific T cells recognizing the epitope CLGGLLTMV after 12 or 24 h in the presence of brefeldin A for the previous 6 h. (D) EBV-specific T-cell avidity. PBMC from EBV-seropositive donors were stimulated overnight with 10-fold serial dilutions of HLA-matched EBV lytic antigen, EBNA3-6, EBNA1, or LMP-1/2 peptides. Activation of CD8+ T cells was assessed by the upregulation of the early activation markers CD137 and CD69.
A reduced/delayed recognition of endogenously expressed antigen and its potential impact upon the functionality of the LMP-specific T cells could be attributable to poor T-cell avidity (1, 13, 20). To address the issue of T-cell avidity in different T-cell populations, PBMC were incubated with 10-fold dilutions of synthetic peptide epitopes derived from lytic antigens, EBNA3-6, or LMP-1/2 proteins and analyzed for the upregulation of the early activation markers CD69 and CD137 and for the production of intracellular cytokines. No differences were detected in the avidity of lytic antigen-/EBNA3-6-specific T cells or LMP-1/2-specific T cells, either following analysis of activation markers (Fig. 3D) or intracellular cytokine assays (data not shown), suggesting that poor endogenous antigen recognition is likely due to poor processing and presentation of the LMP antigens rather than poor avidity of the T cells.
Restoration of polyfunctionality of LMP-1/2- and EBNA1-specific T cells following stimulation with recombinant polyepitope imparts Gal-1 resistance.
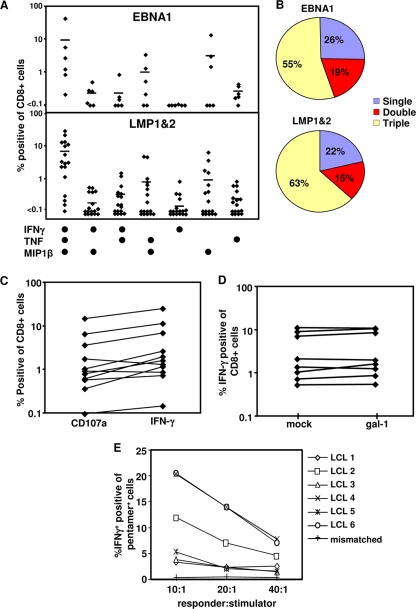
In the next set of experiments, we explored strategies to restore the polyfunctionality in LMP-1/2-specific CD8+ T cells and to shield these cells from the suppressive effects of Gal-1. PBMC from EBV-infected individuals were stimulated with a novel adenoviral vector encoding multiple CD8+ T-cell epitopes from LMP-1/2 and truncated EBNA1 in combination with a γC cytokine, interleukin-2 (27). CD8+ T cells from these in vitro-expanded populations were assessed for cytokine production and for mobilization of CD107a as a marker for cytolytic function. The data presented in Fig. 4A and B clearly show that the majority of LMP-1/2- and EBNA1-specific CD8+ T cells acquired a polyfunctional phenotype characterized by the production of IFN-γ, TNF, and MIP1β. Furthermore, the enhanced capacity to produce IFN-γ was coincident with CD107a expression following stimulation (Fig. 4C), demonstrating a dramatic improvement in the cytolytic capacity of these T cells. Most importantly, these effector cells displayed strong resistance to Gal-1-mediated inhibitory effects (Fig. 4D), suggesting that in vitro stimulation under appropriate conditions can restore their polyfunctional profile and render LMP-1/2- and EBNA1-specific T cells resistant to the suppressive effects of Gal-1. Additionally, these in vitro-expanded LMP-specific T cells expressed IFN-γ following incubation with HLA-matched EBV-transformed LCLs; however, this recognition was highly heterogeneous (Fig. 4E), which may be due to differential expression of LMP proteins or HLA A2 polymorphism.
FIG. 4.
Impact of AdE1-LMPpoly-mediated expansion on T-cell polyfunctionality. (A and B) Cytokine profiles of AdE1-LMPpoly-expanded T cells. T cells from cultures stimulated with AdE1-LMPpoly were incubated with EBNA1 or LMP-1/2 epitopes in the presence of brefeldin A. IFN-γ, TNF, and MIP1β expression by CD8+ T cells was assessed using intracellular cytokine assay. (A) Percentages of CD8+ T cells from individual donors producing each cytokine combination. (B) Mean percentages of specific cells producing 1, 2, or 3 cytokines. (C) Degranulation of AdE1-LMPpoly-stimulated T cells. Cultured T cells were incubated with EBNA1 or LMP-1/2 epitopes in the presence of monensin and anti-CD107α. Data represent the frequency of T cells that produce CD107α or IFN-γ in response to LMP or EBNA1 CTL epitopes. (D) Effect of recombinant Gal-1 on the proliferation of AdE1-LMPpoly-expanded T cells. AdE1-LMPpoly-expanded T cells were preincubated with Gal-1 at 5 μg/ml (or mock treated) and stimulated with LMP-1/2 CTL epitopes. IFN-γ expression by CD8+ T cells was assessed using intracellular cytokine assay. Data represent the comparative proliferation in PBMC from individual donors with and without Gal-1 treatment. (E) AdE1-LMPpoly-stimulated T cells were stimulated with autologous LCL at responder-to-stimulator ratios of 10:1, 20:1, and 40:1 in the presence of brefeldin A for 12 h. Data represent the percentages of IFN-γ-producing, MHC peptide pentamer-specific T cells following incubation with a panel of HLA-matched LCLs.
DISCUSSION
There is now convincing evidence from various viral infection settings demonstrating that a polyfunctional profile of CD8+ T cells, characterized by the ability to produce high levels of multiple cytokines/chemokines, is a key factor for effective immune control (1, 6, 19). Recent studies have also shown that the therapeutic/prophylactic efficacy of immune-based therapies depends on the induction/reconstitution of polyfunctional T cells (2, 19, 28). Our data extend these observations in a latent herpes virus infection setting where T cells specific for different latent proteins display differential levels of polyfunctionality. This differential ability to express cytokines/chemokines has a significant impact on the ability of these cells to resist immunosuppressive effects of factors (e.g., Gal-1) released by virus-infected malignant cells. We also provide evidence which suggests that the T cells directed toward immunodominant EBV antigens (e.g., EBNA3-6/lytic proteins) display a polyfunctional profile, while effector cells specific for subdominant antigens, such as LMP-1/2 and EBNA1, were predominantly monofunctional and lacked the ability to recognize endogenously processed epitopes from virus-infected cells. Additionally, LMP- and EBNA1-specific T cells display an enhanced susceptibility to Gal-1, providing a potential mechanism of immune escape for EBV-infected malignant cells in Hodgkin's disease. More importantly, we demonstrated that if T cells are stimulated using an efficient antigen-presenting system, these effector cells can regain pluripotency and display reduced susceptibility to Gal-1-mediated immunosuppression, offering a potential strategy for delivering effective T-cell-based therapies for EBV-positive malignancies.
It is now firmly established that the proliferation of latently infected B cells in healthy virus carriers is controlled by a population of CD8+ T cells via recognition of the latent cycle antigens, primarily EBNA3-6 (8, 9). This balance between EBV-transformed B cells, which may undergo regular rounds of proliferation characterized by a “Latency III” gene expression profile, and control by CD8+ T cells leads to life-long asymptomatic infection in most individuals. However, through a number of potential, as-yet-undefined mechanisms, this effector arm of the immune system can fail to control the differentiation of normal EBV-infected cells to malignant cells expressing a limited array of EBV latent antigens (11). Recent evidence has begun to elucidate the mechanisms by which malignant cells can evade recognition by virus-specific T cells. These include an expansion in regulatory T cells (3, 14), which function to prevent the induction of an immune response against the malignant cells and may directly suppress the function of effector CD8+ T cells (18, 34). Additionally, malignant cells are known to generate immunosuppressive molecules, such as Gal-1 in Hodgkin's disease, that directly suppress immune function, allowing immune escape (22, 33, 35). We have previously reported that high levels of expression of Gal-1 in Hodgkin's disease correlated with a reduction in the number of infiltrating CD8+ T cells at the tumor site and a specific loss in the functional capacity of LMP-specific T cells (4). We have now shown directly that LMP-1/2- and EBNA1-specific T cells with limited functional profiles are highly susceptible to the suppressive effects of Gal-1. This suggests that by limiting antigen presentation to subdominant T-cell responses that are more susceptible to direct immunosuppressive effects, EBV-infected malignant cells still receive the cell survival and proliferation signals provided by LMP-1/2 (16, 32) and are capable of maintaining the EBV episome, which requires EBNA1 (7), but can avoid immunosurveillance by CTL.
Susceptibility to Gal-1 is coincident with a reduction in the polyfunctionality of LMP-1/2- and EBNA1-specific T cells. This is potentially driven by the ability of these antigens to limit self-processing and presentation via the MHC-I pathway and subsequent poor priming following infection (29-31). Although the mechanism which rendered LMP- and EBNA1-specific T cells more susceptible to Gal-1 was not elucidated in this study, it does provide evidence that qualitative differences in T cells can be associated with differences in susceptibility to immunosuppression. However, it remains to be determined what impact improving the polyfunctionality has upon susceptibility to other mechanisms of immunosuppression that are associated with latency type 2 malignancies, including Gal-9, which is expressed in nasopharyngeal carcinoma and has recently been shown to induce apoptosis of EBV-specific CD4+ T cells (12). Nevertheless, the capacity to render these T cells resistant to the effects of Gal-1 following in vitro expansion with AdE1-LMPpoly, which is also associated with a qualitative improvement in T-cell function, provides a platform for the use of an adoptive immunotherapeutic approach to treat EBV-associated latency type 2 malignancies with in vitro-expanded CTL and for the potential use of adoptive immunotherapy or vaccination to reduce the risk of relapse by establishing a population of polyfunctional T cells that are more capable of clearing malignant cells.
Acknowledgments
This work is supported by funding from the Cancer Council of Queensland and National Health and Medical Research Council of Australia. R.K. is supported by a fellowship from NHMRC, and C.S. is supported by a fellowship from the Leukemia Foundation of Australia.
Footnotes
Published ahead of print on 8 April 2009.
REFERENCES
- 1.Almeida, J. R., D. A. Price, L. Papagno, Z. A. Arkoub, D. Sauce, E. Bornstein, T. E. Asher, A. Samri, A. Schnuriger, I. Theodorou, D. Costagliola, C. Rouzioux, H. Agut, A. G. Marcelin, D. Douek, B. Autran, and V. Appay. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 2042473-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badr, G., N. Bedard, M. S. Abdel-Hakeem, L. Trautmann, B. Willems, J. P. Villeneuve, E. K. Haddad, R. P. Sekaly, J. Bruneau, and N. H. Shoukry. 2008. Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells. J. Virol. 8210017-10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandhi, M. K., E. Lambley, J. Duraiswamy, U. Dua, C. Smith, S. Elliott, D. Gill, P. Marlton, J. Seymour, and R. Khanna. 2006. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood 1082280-2289. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi, M. K., G. Moll, C. Smith, U. Dua, E. Lambley, O. Ramuz, D. Gill, P. Marlton, J. F. Seymour, and R. Khanna. 2007. Galectin-1 mediated suppression of Epstein-Barr virus specific T-cell immunity in classic Hodgkin lymphoma. Blood 1101326-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guppy, A. E., E. Rawlings, J. A. Madrigal, P. L. Amlot, and L. D. Barber. 2007. A quantitative assay for Epstein-Barr virus-specific immunity shows interferon-gamma producing CD8+ T cells increase during immunosuppression reduction to treat posttransplant lymphoproliferative disease. Transplantation 841534-1539. [DOI] [PubMed] [Google Scholar]
- 6.Harari, A., C. Cellerai, F. B. Enders, J. Kostler, L. Codarri, G. Tapia, O. Boyman, E. Castro, S. Gaudieri, I. James, M. John, R. Wagner, S. Mallal, and G. Pantaleo. 2007. Skewed association of polyfunctional antigen-specific CD8 T cell populations with HLA-B genotype. Proc. Natl. Acad. Sci. USA 10416233-16238.17911249 [Google Scholar]
- 7.Hirai, K., and M. Shirakata. 2001. Replication licensing of the EBV oriP minichromosome. Curr. Top. Microbiol. Immunol. 25813-33. [DOI] [PubMed] [Google Scholar]
- 8.Hislop, A. D., G. S. Taylor, D. Sauce, and A. B. Rickinson. 2007. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu. Rev. Immunol. 25587-617. [DOI] [PubMed] [Google Scholar]
- 9.Khanna, R., and S. R. Burrows. 2000. Role of cytotoxic T lymphocytes in Epstein-Barr virus-associated diseases. Annu. Rev. Microbiol. 5419-48. [DOI] [PubMed] [Google Scholar]
- 10.Khanna, R., P. Busson, S. R. Burrows, C. Raffoux, D. J. Moss, J. M. Nicholls, and L. Cooper. 1998. Molecular characterization of antigen-processing function in nasopharyngeal carcinoma (NPC): evidence for efficient presentation of Epstein-Barr virus cytotoxic T-cell epitopes by NPC cells. Cancer Res. 58310-314. [PubMed] [Google Scholar]
- 11.Khanna, R., D. Moss, and M. Gandhi. 2005. Technology insight: applications of emerging immunotherapeutic strategies for Epstein-Barr virus-associated malignancies. Nat. Clin. Pract. Oncol. 2138-149. [DOI] [PubMed] [Google Scholar]
- 12.Klibi, J., T. Niki, A. Riedel, C. Pioche-Durieu, S. Souquere, E. Rubinstein, S. L. Moulec, J. Guigay, M. Hirashima, F. Guemira, D. Adhikary, J. Mautner, and P. Busson. 2009. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood 1131957-1966. [DOI] [PubMed] [Google Scholar]
- 13.La Gruta, N. L., S. J. Turner, and P. C. Doherty. 2004. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: correlation of cytokine profile and TCR avidity. J. Immunol. 1725553-5560. [DOI] [PubMed] [Google Scholar]
- 14.Lau, K. M., S. H. Cheng, K. W. Lo, S. A. Lee, J. K. Woo, C. A. van Hasselt, S. P. Lee, A. B. Rickinson, and M. H. Ng. 2007. Increase in circulating Foxp3+CD4+CD25(high) regulatory T cells in nasopharyngeal carcinoma patients. Br. J. Cancer 96617-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, S. P., C. M. Constandinou, W. A. Thomas, D. Croom-Carter, N. W. Blake, P. G. Murray, J. Crocker, and A. B. Rickinson. 1998. Antigen presenting phenotype of Hodgkin Reed-Sternberg cells: analysis of the HLA class I processing pathway and the effects of interleukin-10 on Epstein-Barr virus-specific cytotoxic T-cell recognition. Blood 921020-1030. [PubMed] [Google Scholar]
- 16.Li, H. P., and Y. S. Chang. 2003. Epstein-Barr virus latent membrane protein 1: structure and functions. J. Biomed. Sci. 10490-504. [DOI] [PubMed] [Google Scholar]
- 17.Lyons, A. B., and C. R. Parish. 1994. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171131-137. [DOI] [PubMed] [Google Scholar]
- 18.Mempel, T. R., M. J. Pittet, K. Khazaie, W. Weninger, R. Weissleder, H. von Boehmer, and U. H. von Andrian. 2006. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity 25129-141. [DOI] [PubMed] [Google Scholar]
- 19.Precopio, M. L., M. R. Betts, J. Parrino, D. A. Price, E. Gostick, D. R. Ambrozak, T. E. Asher, D. C. Douek, A. Harari, G. Pantaleo, R. Bailer, B. S. Graham, M. Roederer, and R. A. Koup. 2007. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J. Exp. Med. 2041405-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price, D. A., J. M. Brenchley, L. E. Ruff, M. R. Betts, B. J. Hill, M. Roederer, R. A. Koup, S. A. Migueles, E. Gostick, L. Wooldridge, A. K. Sewell, M. Connors, and D. C. Douek. 2005. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J. Exp. Med. 2021349-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rickinson, A. B., and D. J. Moss. 1997. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu. Rev. Immunol. 15405-431. [DOI] [PubMed] [Google Scholar]
- 22.Rodig, S. J., J. Ouyang, P. Juszczynski, T. Currie, K. Law, D. S. Neuberg, G. A. Rabinovich, M. A. Shipp, and J. L. Kutok. 2008. AP1-dependent galectin-1 expression delineates classical Hodgkin and anaplastic large cell lymphomas from other lymphoid malignancies with shared molecular features. Clin. Cancer Res. 143338-3344. [DOI] [PubMed] [Google Scholar]
- 23.Rooney, C. M., M. Rowe, L. E. Wallace, and A. B. Rickinson. 1985. Epstein-Barr virus-positive Burkitt's lymphoma cells not recognized by virus-specific T-cell surveillance. Nature 317629-631. [DOI] [PubMed] [Google Scholar]
- 24.Rowe, M., R. Khanna, C. A. Jacob, V. Argaet, A. Kelly, S. Powis, M. Belich, D. Croom-Carter, S. Lee, S. R. Burrows, et al. 1995. Restoration of endogenous antigen processing in Burkitt's lymphoma cells by Epstein-Barr virus latent membrane protein-1: coordinate up-regulation of peptide transporters and HLA-class I antigen expression. Eur. J. Immunol. 251374-1384. [DOI] [PubMed] [Google Scholar]
- 25.Sebelin-Wulf, K., T. D. Nguyen, S. Oertel, M. Papp-Vary, R. U. Trappe, A. Schulzki, A. Pezzutto, H. Riess, and M. Subklewe. 2007. Quantitative analysis of EBV-specific CD4/CD8 T cell numbers, absolute CD4/CD8 T cell numbers and EBV load in solid organ transplant recipients with PLTD. Transpl. Immunol. 17203-210. [DOI] [PubMed] [Google Scholar]
- 26.Sherritt, M. A., M. Bharadwaj, J. M. Burrows, L. E. Morrison, S. L. Elliott, J. E. Davis, L. M. Kear, R. E. Slaughter, S. C. Bell, A. J. Galbraith, R. Khanna, and D. J. Moss. 2003. Reconstitution of the latent T-lymphocyte response to Epstein-Barr virus is coincident with long-term recovery from posttransplant lymphoma after adoptive immunotherapy. Transplantation 751556-1560. [DOI] [PubMed] [Google Scholar]
- 27.Smith, C., L. Cooper, M. Burgess, M. Rist, N. Webb, E. Lambley, J. Tellam, P. Marlton, J. F. Seymour, M. Gandhi, and R. Khanna. 2006. Functional reversion of antigen-specific CD8+ T cells from patients with Hodgkin lymphoma following in vitro stimulation with recombinant polyepitope. J. Immunol. 1774897-4906. [DOI] [PubMed] [Google Scholar]
- 28.Sun, Y., S. Santra, J. E. Schmitz, M. Roederer, and N. L. Letvin. 2008. Magnitude and quality of vaccine-elicited T-cell responses in the control of immunodeficiency virus replication in rhesus monkeys. J. Virol. 828812-8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tellam, J., G. Connolly, N. Webb, J. Duraiswamy, and R. Khanna. 2003. Proteasomal targeting of a viral oncogene abrogates oncogenic phenotype and enhances immunogenicity. Blood 1024535-4540. [DOI] [PubMed] [Google Scholar]
- 30.Tellam, J., M. Rist, G. Connolly, N. Webb, C. Fazou, F. Wang, and R. Khanna. 2007. Translation efficiency of EBNA1 encoded by lymphocryptoviruses influences endogenous presentation of CD8+ T cell epitopes. Eur. J. Immunol. 37328-337. [DOI] [PubMed] [Google Scholar]
- 31.Tellam, J., C. Smith, M. Rist, N. Webb, L. Cooper, T. Vuocolo, G. Connolly, D. C. Tscharke, M. P. Devoy, and R. Khanna. 2008. Regulation of protein translation through mRNA structure influences MHC class I loading and T cell recognition. Proc. Natl. Acad. Sci. USA 1059319-9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorley-Lawson, D. A. 2001. Epstein-Barr virus: exploiting the immune system. Nat. Rev. Immunol. 175-82. [DOI] [PubMed] [Google Scholar]
- 33.van den Brule, F. A., D. Waltregny, and V. Castronovo. 2001. Increased expression of galectin-1 in carcinoma-associated stroma predicts poor outcome in prostate carcinoma patients. J. Pathol. 19380-87. [DOI] [PubMed] [Google Scholar]
- 34.Vignali, D. A., L. W. Collison, and C. J. Workman. 2008. How regulatory T cells work. Nat. Rev. Immunol. 8523-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaoka, K., K. Mishima, Y. Nagashima, A. Asai, Y. Sanai, and T. Kirino. 2000. Expression of galectin-1 mRNA correlates with the malignant potential of human gliomas and expression of antisense galectin-1 inhibits the growth of 9 glioma cells. J. Neurosci. Res. 59722-730. [DOI] [PubMed] [Google Scholar]