Abstract
The ubiquitin-like ISG15 protein, as well as its conjugating enzymes, is induced by type I interferons (IFNs). Experiments using ISG15 knockout (ISG15−/−) mice established that ISG15 and/or its conjugation inhibits the replication of influenza A virus. However, in contrast to the virus inhibition results for mice, the rates of virus replication in ISG15+/+ and ISG15−/− mouse embryo fibroblasts in tissue culture were similar. Here we focus on human tissue culture cells and on the effect of ISG15 and/or its conjugation on influenza A virus gene expression and replication in such cells. We demonstrate that IFN-induced antiviral activity against influenza A virus in human cells is significantly alleviated by inhibiting ISG15 conjugation using small interfering RNAs directed against ISG15-conjugating enzymes. IFN-induced antiviral activity against influenza A virus protein synthesis was reduced 5- to 20-fold by suppressing ISG15 conjugation. The amounts of the viral proteins that were restored by these siRNA treatments were approximately 40 to 50% of the amounts produced in cells that were not pretreated with IFN. Further, we show that ISG15 conjugation inhibits influenza A virus replication 10- to 20-fold at early times after infection in human cells. These results show that ISG15 conjugation plays a substantial role in the antiviral state induced by IFN in human cells. In contrast, we show that in mouse embryo fibroblasts ISG15 conjugation not only does not affect influenza A virus replication but also does not contribute to the IFN-induced antiviral activity against influenza A virus gene expression.
Virus infection activates the synthesis of type I interferons (IFN-α and IFN-β), which induce the synthesis of a large array of proteins, many of which play crucial roles in the antiviral response (1). One of the most strongly induced proteins is ISG15, a 15-kDa ubiquitin-like protein that becomes conjugated to many cellular proteins (6, 8, 9, 12, 18, 22, 26, 30). Three of the human enzymes that catalyze this conjugation, the UbE1L E1 enzyme, the UbcH8 E2 enzyme, and the Herc5 E3 enzyme, are also induced by IFN-β (4, 10, 26, 27, 29). Although it had been reported that UbcH8 functions in both ISG15 and ubiquitin conjugation (3, 10, 13, 25, 28, 29), a recent study demonstrated that UbcH8 is unlikely to function in ubiquitin conjugation in vivo for two reasons: Km measurements revealed that the E1 ubiquitin-activating enzyme, unlike UbE1L, exhibits very low affinity for UbcH8, and UbcH8 is poorly, if not at all, expressed in the absence of IFN treatment, indicating that UbcH8 functions only during the IFN response (5). A large number of human proteins that are targets for ISG15 conjugation have been identified (22, 26, 30). Most of these targets are constitutively expressed proteins that function in diverse cellular pathways, but several of the targets are IFN-α/-β-induced antiviral proteins.
Because the NS1 protein of influenza B virus (NS1B) was shown to bind ISG15 and inhibit its conjugation to target proteins, it was proposed that ISG15 and/or its conjugation is inhibitory to the replication of influenza B virus (27). Subsequently, experiments using ISG15 knockout (ISG15−/−) mice established that ISG15 and/or its conjugation inhibits the replication of not only influenza B virus but also influenza A virus (16). For example, at one of the inoculum levels employed for influenza A virus, 52% of the ISG15−/− mice died, whereas a significantly smaller percentage, 23%, of the ISG15+/+ mice died. However, the effect of ISG15 and/or its conjugation on influenza A virus replication was not detected in mouse embryo fibroblasts (MEFs) in tissue culture. MEFs supported only very limited replication of influenza A virus, and there was no significant difference in virus replication between ISG15+/+ and ISG15−/− MEFs (16). These investigators postulated that influenza A virus replication was probably selectively spared in other cell types of the ISG15−/− mouse. A subsequent study showed that ISG15 conjugation exerts its antiviral action against influenza B virus (and presumably against influenza A virus) in radioresistant stromal cells of the mouse (14). However, an antiviral effect of ISG15 conjugation against influenza A virus has not yet been demonstrated in mouse cells in tissue culture.
In the present study we focus on human tissue culture cells and on the effect of ISG15 and/or its conjugation on the replication of influenza A virus in such cells. We show that IFN-induced antiviral activity against influenza A virus in human cells is significantly alleviated by inhibiting ISG15 conjugation using small interfering RNAs (siRNAs) against ISG15-conjugating enzymes. Our results show that both the synthesis of viral proteins and the early rate of virus replication are inhibited by ISG15 conjugation. In contrast, we show that in MEFs ISG15 conjugation not only does not affect influenza A virus replication but also does not contribute to IFN-induced antiviral activity against influenza A virus gene expression.
MATERIALS AND METHODS
Cells and viruses.
A549 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), and Calu3 cells (23, 24) were maintained in Advanced MEM (Invitrogen) containing 10% FBS. ISG15 wild-type (ISG15+/+) and ISG15−/− MEFs were kindly provided by Deborah J. Lenschow (Washington University, St. Louis, MO) (16). MEFs were grown in DMEM supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. Influenza A/Udorn/72 (Ud) virus stocks were grown in 10-day-fertilized eggs, and virus titers were determined by plaque assays of Madin-Darby canine kidney (MDCK) cells. For single-cycle virus infections, human cells (A549 or Calu3 cells) or MEFs were infected with the Ud virus at a high multiplicity of infection (MOI) of 5 PFU/cell. Infected cells were incubated in DMEM containing 2% fetal calf serum (A549 cells and MEFs) or OptiMEM (Invitrogen) containing 2% fetal calf serum (Calu3 cells).
siRNA knockdowns.
All siRNA duplexes were purchased from Invitrogen and resuspended in diethyl pyrocarbonate-treated water to a final storage concentration of 20 μM. The siRNA for UbcH8 has been described previously (29). Three different siRNAs for ISG15 and UbE1L were purchased. Because all three were effective, we will specify only one for each of the two targets: ISG15, AAUCUUCUGGGUGAUCUGCGCCUUC; UbE1L, UAGUGCUGGCGUCUCAGUUUCUCCU. siRNA transfection was performed on 80% confluent A459 or Calu3 cells. The control siRNA, Stealth RNA interference negative control (medium G/C), was obtained from Invitrogen. The cells were trypsinized, washed, and resuspended in serum- and antibiotic-free OptiMEM at 3 × 105 to 6 × 105 cells/ml. Approximately 106 cells were seeded into one well of a six-well tissue culture plate immediately before siRNA transfection. The Xtreme siRNA transfection reagent (Roche) was used for all the siRNA transfections, which were performed in accordance with the manufacturer's protocol. Final concentration of each siRNA was 20 nM, except for the ISG15 and UbE1L double knockdown, where 40 nM of the UbE1L siRNA and 60 nM of the ISG15 siRNA were used. siRNA-transfected cells were incubated in serum- and antibiotic-free OptiMEM for 24 h at 37°C. The culture medium was replaced with DMEM supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin for A549 cells or Advanced MEM with 10% FBS and penicillin-streptomycin-glutamine for Calu3 cells. Human IFN-β (1,000 units/ml) (Betatheron; Berlix Co.) was added where indicated. Cells were mock or IFN-β treated for 24 h prior to influenza A virus infection.
Quantitative immunoblots.
Collected cells were extracted in radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 1% NP-40; 0.5% sodium deoxycholate; 0.1% sodium dodecyl sulfate) supplemented with Complete protease inhibitor (Roche). Proteins (30 μg/sample) were separated on 12% polyacrylamide-sodium dodecyl sulfate gels and transferred onto an Immobilon-FL polyvinylidene difluoride membrane (Millipore) by a semidry transfer method at 0.8 mA/cm2. The resulting blots were incubated with 5% nonfat dried milk in phosphate-buffered saline (PBS) at room temperature for 1 h to minimize nonspecific binding of antibodies. Primary antibodies were diluted in 5% milk in PBS plus 0.2% Tween 20 (PBST solution). The following primary antibodies were used: rabbit anti-human ISG15, which was prepared by Cocalico Biologics using recombinant glutathione S-transferase-human ISG15 as the antigen; goat anti-Ud NS1A protein; rabbit antibody against the major structural proteins of the Ud virus, which detects the hemagglutinin (HA), NP, and M1 (matrix) proteins, provided by Robert A. Lamb (2); rabbit antibody against UbcH8 (Abgent); mouse antitubulin antibody; and rabbit antibody against mouse ISG15, provided by Ivan Horak and Klaus-Peter Knobeloch (21). Blots were incubated with the primary antibody at room temperature for 1 h or at 4°C overnight. Two procedures were used to quantitate the levels of individual proteins. In one procedure, the blots were washed four times with PBST at room temperature and were then incubated in 5% milk (PBST solution) at room temperature for an hour with a fluorescent-dye-conjugated secondary antibody: Alexa Fluor 680 goat anti-rabbit immunoglobulin G (IgG) (Invitrogen), Dylight 800 goat anti-mouse IgG (Rockland Immunochemical), or Dylight 680 donkey anti-goat IgG (Rockland Immunochemical). After being washed four times with PBST and two times with PBS, the membranes were analyzed with a Odyssey scanner and Li-Cor software. The same protein samples were also subjected to a standard Western blot analysis using enhanced chemiluminescence reagents. Quantitation was carried out by scanning the films and analyzing the scans using ImageJ software (NIH). Tubulin levels were used to correct for gel loading differences. In fact, tubulin levels varied by at most 10% in each analysis. The same results were obtained by the two quantitation procedures. For the quantitation of the level of ISG15 conjugates, it was possible to use only the second procedure.
Northern blots.
Cells were collected, and total RNA was extracted with Trizol reagent (Invitrogen). RNA (10 μg) was resolved on 1.2% agarose gel and was then transferred and UV cross-linked onto a nylon membrane (Nytran; Whatman Schleicher & Schuell). To prepare the 32P-labeled probe for NS1A mRNA, the PCR product of the full-length NS gene was used as the template. Random primers were produced by the Klenow fragment of DNA polymerase I in the presence of [α-32P]dCTP (11). After hybridization, signal strength was determined by scanning the activated phosphor imaging screen (Bio-Rad) using Typhoon Trio (GE Healthcare) and analyzed by ImageQuant software.
RESULTS
siRNA knockdown of ISG15 conjugation alleviates the IFN-induced inhibition of influenza A virus gene expression.
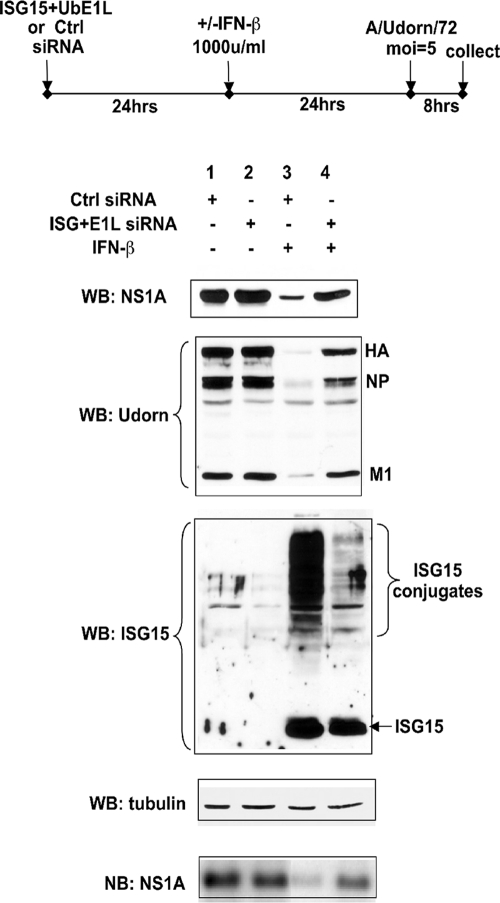
The basic experimental design of our experiments with human cells is diagrammed at the top of Fig. 1. Human cells were treated with a high level of IFN-β (1,000 units/ml) for 24 h to achieve efficient ISG15 conjugation. As a control, a different set of cells was not treated with IFN. Twenty-four hours prior to IFN treatment, the cells were transfected with siRNA(s) directed against an enzyme in the ISG15 conjugation pathway to selectively suppress IFN-induced ISG15 conjugation. As a control, another set of cells was treated with a control siRNA. After the successive siRNA and IFN treatments, the cells were infected with Ud virus at a high MOI, 5 PFU/cell, for 8 h, and the synthesis of viral proteins and RNAs was measured by immunoblotting and Northern blotting as described below.
FIG. 1.
siRNA knockdown of UbE1L in A549 cells inhibits ISG15 conjugation and causes a 5- to 20-fold increase in the synthesis of several viral proteins during influenza A virus infection. The experimental design is shown at the top. Extracts collected 8 h after Ud virus infection of cells subjected to the indicated treatments were analyzed by immunoblotting (WB) using antibodies against the indicated proteins. Quantitation of the immunoblots was carried out as described in Materials and Methods. In addition, the RNA extracted from these extracts was analyzed by a Northern blotting (NB) for the amount of NS1A mRNA as described in Materials and Methods.
We first targeted UbE1L, the E1 enzyme in ISG15 conjugation (27). However, siRNA knockdown of UbE1L in human epithelial lung A549 cells alone did not achieve efficient inhibition of ISG15 conjugation, and it was necessary to add a siRNA directed against ISG15 itself. As analyzed from an immunoblot, the combination of these two siRNAs effectively inhibited most (>90%) IFN-induced ISG15 conjugation (Fig. 1, compare lanes 3 and 4 of the ISG15 Western blot). In contrast, the reduction in free ISG15 was much less (∼20%). Consequently, it was not clear why it was necessary to add the siRNA directed against ISG15 to achieve efficient inhibition of ISG15 conjugation. As shown later, efficient inhibition of IFN-induced free ISG15 was obtained in a different human cell line, Calu3 cells (see Fig. 3). By inhibiting ISG15 conjugation in A549 cells with the UbE1L and ISG15 siRNAs, the synthesis of the viral NS1A, HA, NP, and M1 proteins was partially rescued (Fig. 1, NS1A and Udorn Western blots). The amounts of the NS1A, NP, and M1 proteins in the cells transfected with these two siRNAs (lane 4) were approximately five- to sevenfold higher than those in the cells transfected with the control siRNA (lane 3). The difference was consistently higher for HA, approximately 20-fold (compare lanes 3 and 4), because HA synthesis was more strongly inhibited by IFN treatment than that of the other three viral proteins (compare lane 3 to lanes 1 and 2). The amount of the restored viral proteins, including HA, was approximately 50% of the amount produced in infected cells not treated with IFN (compare lane 4 to lanes 1 and 2), indicating that ISG15 conjugation plays a substantial role in the antiviral state induced by IFN in human cells. A similar restoration (∼40%) of the synthesis of NS1A mRNA was observed in the cells treated with the two specific siRNAs, as assayed by Northern analysis. Consequently, we concluded that ISG15 conjugation inhibits the synthesis of several influenza A viral proteins and at least one viral mRNA.
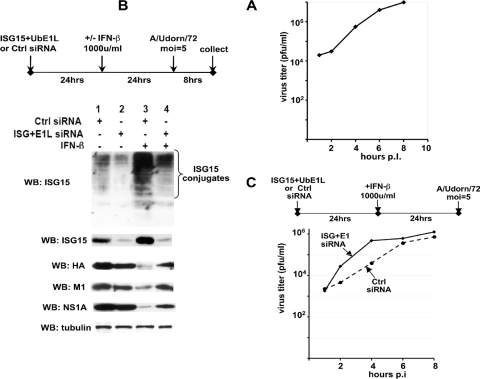
FIG. 3.
Inhibition of IFN-induced ISG15 conjugation in Calu3 cells by siRNA knockdown of UbE1L leads to a significant increase in the rate of influenza A virus replication at early times after infection. (A) Single-cycle growth curve for Ud virus in Calu3 cells. (B) Extracts of cells collected 8 h after Ud virus infection of Calu3 cells subjected to the indicated treatments were analyzed by immunoblotting (WB) using antibodies against the indicated proteins. Quantitation of the immunoblots was carried out as described in Materials and Methods. (C) Calu3 cells were transfected with either ISG15 and UbE1L siRNAs or a control siRNA and 24 h later were treated with 1,000 units/ml IFN-β. After an additional 24 h at 37°C, the two sets of cells were infected with 5 PFU/cell of the Ud virus, and the amounts of virus produced at 2, 4, 6, and 8 h postinfection were determined by plaque assays of MDCK cells.
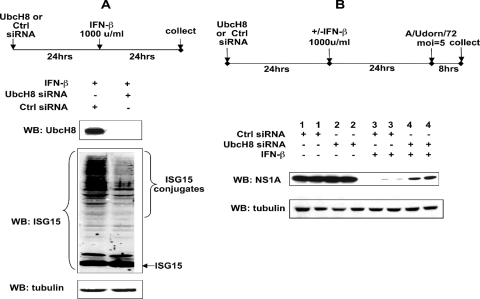
To establish that ISG15 conjugation rather than free ISG15 mediated the inhibition of viral protein synthesis, we used an siRNA that targeted UbcH8, the E2 enzyme in ISG15 conjugation (5, 10, 29). The UbcH8 siRNA effectively inhibited UbcH8 protein production and ISG15 conjugation (greater than 95% and 85%, respectively), whereas no effect on the accumulation of free ISG15 was detected (Fig. 2A). We determined the effect of the UbcH8 siRNA on the synthesis of the NS1A protein. The amount of the NS1A protein in the cells transfected with the UbcH8 siRNA (lane 4) was approximately 12-fold higher than that in the cells transfected with the control siRNA (lane 3), and the amount of rescued NS1A protein was approximately 40% of the amount of the NS1A protein in infected cells not treated with IFN (compare lane 4 to lanes 1 and 2). Similar results were obtained for other viral proteins (data not shown). These results verify that IFN-induced ISG15 conjugation rather than IFN-induced free ISG15 is primarily responsible for the inhibition of the synthesis of influenza A virus proteins in human cells.
FIG. 2.
siRNA knockdown of UbcH8 in A549 cells inhibits ISG15 conjugation and causes a 12-fold reduction in the IFN-induced antiviral activity against the synthesis of the NS1A protein during influenza A virus infection. (A) Extracts of cells transfected with a siRNA against UbcH8 or a control siRNA, followed by IFN-β treatment for 24 h, were analyzed by immunoblotting (WB) using antibody against UbcH8, ISG15, or tubulin. (B) Extracts collected 8 h after Ud virus infection of cells subjected to the indicated treatments were analyzed by quantitative immunoblotting using antibody against the viral NS1A protein or against tubulin.
The inhibition of influenza A virus gene expression by ISG15 conjugation in human cells is sufficient to result in inhibition of virus replication at early times of infection.
To determine whether ISG15 conjugation inhibits the replication of influenza A virus in human cells, we screened several human cell lines to identify the cell line that afforded the highest rate of influenza A virus replication during single-cycle growth. Calu3 cells (24) were chosen. Remarkably, progeny virus was detected as early at 2 h after infection of Calu3 cells with 5 PFU/cell of the Ud virus, and a 40-fold increase in virus titer had already occurred by 4 h postinfection (Fig. 3A). The linear phase of virus replication continued until at least 8 h postinfection, at which time 107 PFU/ml were produced. Virus yield at 8 h varied been 107 and 7 × 107 PFU/ml.
We determined whether the UbE1L and ISG15 siRNAs also effectively inhibited ISG15 conjugation in Calu3 cells. Unexpectedly, transfection of Calu3 cells with the control siRNA in the absence of IFN treatment induced the production of a substantial amount of free ISG15 as well as a small amount of ISG15 conjugation (Fig. 3B, lane 1), which was not observed in A549 cells (Fig. 1, lane 1). The UbE1L and ISG15 siRNAs in Calu3 cells strongly inhibited the production of free ISG15 as well as the ISG15 conjugation that occurred in the absence of IFN (Fig. 3B, compare lanes 1 and 2). The amount of free ISG15 in cells transfected with these two siRNAs (lane 2) was only 10% of that in cells transfected with the control siRNA (lane 1). IFN treatment coupled with transfection of the control siRNA led to a substantial (10-fold) increase in both the production of free ISG15 and ISG15 conjugation compared to transfection of the control siRNA alone (compare lanes 3 and 1). The UbE1L and ISG15 siRNAs reduced the IFN-induced production of free ISG15 and ISG15 conjugation 12- and 8-fold, respectively, and the amounts of the HA, M1, and NS1A proteins synthesized in cells transfected by these two siRNAs were 6- to 8-fold higher than those in cells transfected with the control siRNA (compare lanes 3 and 4). The amounts of these three viral proteins that were rescued by the UbE1L and ISG15 siRNAs were approximately 50% of the amounts produced in the absence of IFN treatment (compare lane 4 to lanes 1 and 2). Consequently, the overall effects of these two siRNAs on the rescue of viral protein synthesis after IFN treatment were similar to the effects in A549 cells.
Based on these results, we measured the rate of virus replication in IFN-treated Calu3 cells that had been transfected with either the control siRNA or the UbE1L and ISG15 siRNAs (Fig. 3C). A clear difference between the effects of these two transfections was seen at early times after infection: the rate of virus replication during the first 4 h of infection in cells transfected with the UbE1L and ISG15 siRNAs was approximately 10- to 20-fold greater than that in cells transfected with the control siRNA. At 6 h postinfection, the rate of virus replication in cells transfected with either the control siRNA or the UbE1L and ISG15 siRNAs leveled off, presumably reflecting the inhibition of virus replication by IFN-induced antiviral proteins other than ISG15 conjugates. We were able to detect this inhibition of influenza A virus replication by IFN-induced ISG15 conjugation at early times of infection because of the remarkably early rapid rate of virus replication in Calu3 cells.
IFN-induced ISG15 conjugation in MEFs does not contribute to IFN-induced antiviral activity against influenza A virus gene expression.
Although replication of influenza A/WSN/33 (WSN) virus was shown to be enhanced in ISG15−/− mice compared to that in ISG15+/+ mice, it was not enhanced in ISG15−/− MEFs in tissue culture (16). In these experiments ISG15−/− and ISG15+/+ MEFs, which were not pretreated with IFN, were infected with an intermediate MOI of 0.1 PFU/cell. Similar, but minimal levels of replication of the WSN virus in ISG15−/− and ISG15+/+ MEFs were observed. However, it was not determined whether significant ISG15 conjugation occurred in the virus-infected ISG15+/+ MEFs in the absence of IFN pretreatment. Further, these investigators measured only the production of infectious virus and hence could have missed effects on virus gene expression that did not result in significant effects on virus yield.
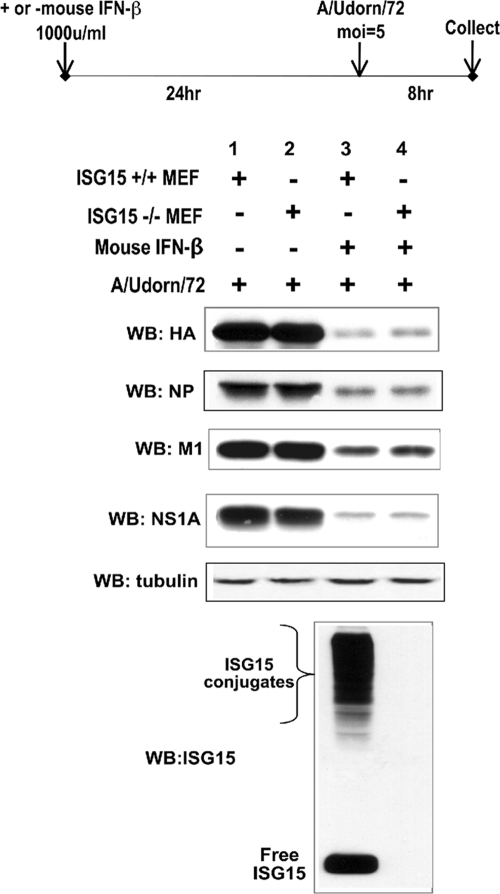
To address these issues, we treated ISG15−/− and ISG15+/+ MEFs with a high level of mouse IFN-β for 24 h, followed by influenza A (Ud) virus infection at high MOI (5 PFU/cell) and determined whether influenza A virus gene expression was enhanced in ISG15−/− MEFs compared to ISG15+/+ MEFs (Fig. 4). Viral gene expression was determined by monitoring the synthesis of the HA, NP, M1, and NS1A proteins. Whereas substantial amounts of these viral proteins were synthesized in both ISG15−/− and ISG15+/+ MEFs in the absence of IFN treatment (lanes 1 and 2), only small amounts of the these proteins were produced in both sets of MEFs that had been pretreated with IFN (lanes 3 and 4). Essentially the same small amounts of these viral proteins were synthesized in the two sets of MEFs that were pretreated with IFN. Similar results were obtained using the mouse-adapted WSN virus (data not shown). An immunoblot using antibody against mouse ISG15 (21) showed that large amounts of ISG15 conjugates were produced in IFN-treated ISG15+/+ MEFs (Fig. 3, lane 3). These results show that IFN-induced conjugation of the large number of proteins in ISG15+/+ MEFs does not contribute to the IFN-induced antiviral activity against influenza A virus. Consequently, IFN-induced antiviral proteins other than ISG15 conjugates must be responsible for the strong inhibition of influenza A virus gene expression in both ISG15−/− and ISG15+/+ MEFs.
FIG. 4.
ISG15 conjugation in MEFs does not contribute to the IFN-induced antiviral activity against influenza A virus gene expression. ISG15+/+ and ISG15−/− MEFs were treated with 1,000 units/ml of mouse IFN-β (Sigma) or were untreated. After 24 h of incubation, the MEFs were infected with Ud virus, and cells were collected 8 h later. Extracts of the cells were analyzed by immunoblotting (WB) using the indicated antibodies. Quantitation of the immunoblots was carried out as described in Materials and Methods.
DISCUSSION
IFN-α/-β treatment of human cells induces the synthesis of ISG15 and three of its conjugating enzymes, resulting in the conjugation of a large number of proteins (4-6, 9, 10, 12, 18, 22, 26, 27, 29, 30). In the present study we demonstrated that IFN-induced ISG15 conjugation inhibits influenza A virus gene expression in human cells in tissue culture. By siRNA-mediated depletion of either of two enzymes (UbE1L and UbcH8) that catalyze ISG15 conjugation, we demonstrated that inhibition of ISG15 conjugation in human cells causes a 5- to 20-fold reduction in IFN-induced antiviral activity against influenza A virus protein synthesis. The amounts of the viral proteins that were restored by this siRNA treatment were substantial, specifically, 40 to 50% of the amounts produced in cells that were not pretreated with IFN. The results obtained with the siRNA against UbE1L did not rule out the possibility that the effects were due to free ISG15 as well as ISG15 conjugation because it was necessary to include a siRNA against ISG15 to obtain efficient knockdown of ISG15 conjugation. In contrast, the results obtained with a siRNA against UbcH8 alone established that IFN-induced ISG15 conjugation rather than IFN-induced free ISG15 is primarily responsible for the inhibition of the synthesis of influenza A virus proteins in human cells. Nonetheless, these results do not rule out the possibility that free ISG15 may have an additional antiviral activity against influenza A virus in human cells.
Further, we showed that inhibition of influenza A virus gene expression by ISG15 conjugation in human cells is sufficient to result in inhibition of virus replication at early times of infection. We used Calu3 cells for these experiments because these cells afforded a remarkably early rapid rate of influenza A virus replication. The rate of virus replication during the first 4 h of infection in Calu3 cells transfected with the UbE1L and ISG15 siRNAs was approximately 10- to 20-fold greater than that in cells transfected with the control siRNA. This difference disappeared at subsequent times after infection because virus replication essentially ceased at 6 h postinfection whether the cells were transfected with the UbE1L and ISG15 siRNAs or with the control siRNA. We interpret these results as indicating that ISG15 conjugation is the predominant IFN-induced antiviral activity acting at early times of influenza A virus infection, whereas other IFN-induced antiviral proteins act predominately at later times to cause the cessation of virus replication. Based on this interpretation, it is reasonable to propose that ISG15 conjugation targets early events in influenza A virus replication.
In contrast, we show that in one type of mouse cell, MEFs, IFN-induced ISG15 conjugation does not contribute to the IFN-induced antiviral activity against influenza A virus gene expression. It was shown previously that similar, albeit minimal, levels of replication of influenza A virus occurred in ISG15−/− and ISG15+/+ MEFs (16). In the present study, we treated both of these sets of MEFs with mouse IFN to induce the production of large amounts of ISG15 conjugates in the ISG15+/+ MEFs, but not in the ISG15−/− MEFs, and determined whether viral protein synthesis during a subsequent influenza A virus infection was inhibited to a greater degree in the ISG15+/+ MEFs. Essentially the same small amounts of viral proteins were synthesized in the two sets of MEFs that were pretreated with IFN. We conclude that IFN-induced antiviral proteins other than ISG15 conjugates are responsible for the strong inhibition of influenza A virus gene expression in both ISG15−/− and ISG15+/+ MEFs. These results demonstrate a fundamental difference between MEFs and human cells in tissue culture with respect to the role of ISG15 conjugation in the IFN-induced antiviral activity against influenza A virus. In mice the anti-influenza virus activity caused by ISG15 conjugation apparently occurs primarily in radioresistant stromal cells (14). It will be of interest to determine whether influenza A virus infection of such mouse cells in tissue culture exhibits the same sensitivity to IFN-induced ISG15 conjugation as that exhibited in human tissue culture cells.
Recent studies have shown that ISG15 has antiviral activities against other viruses. In the case of human immunodeficiency virus type 1 and Ebola virus, the antiviral function is most likely mediated by free ISG15 alone, which suppresses the ubiquitination of viral proteins that is required for efficient virus release (17, 19, 20). In contrast, ISG15 conjugation is responsible for the antiviral activity against Sindbis virus (7, 15), which is predominately the case for influenza A virus, as shown in the present study.
ISG15 conjugation could exert its antiviral activity against influenza A virus by two mechanisms that may not be mutually exclusive. In one mechanism, ISG15 conjugation of cellular target proteins, e.g., the previously identified antiviral MxA and p56 protein targets (30), is required for, or at least strongly enhances, their antiviral activities. In a second mechanism, ISG15 conjugation of a viral protein(s) inhibits one or more of its essential functions. Based on the present study, human cells in tissue culture should provide tractable systems to delineate the mechanisms by which ISG15 conjugation inhibits influenza A virus replication.
Acknowledgments
This work was supported by Public Health Service grant AI-11772 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 8 April 2009.
REFERENCES
- 1.Biron, C. A., and G. C. Sen. 2001. Interferons and other cytokines, p. 321-351. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 2.Chen, B. J., G. P. Leser, E. Morita, and R. A. Lamb. 2007. Influenza virus hemagglutinin and neuraminidase, but not the matrix protein, are required for assembly and budding of plasmid-derived virus-like particles. J. Virol. 817111-7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chin, L. S., J. P. Vavalle, and L. Li. 2002. Staring, a novel E3 ubiquitin-protein ligase that targets syntaxin I for degradation. J. Biol. Chem. 27735071-35079. [DOI] [PubMed] [Google Scholar]
- 4.Dastur, A., S. Beaudenon, M. Kelley, R. M. Krug, and J. M. Huibregtse. 2006. Herc5, an interferon-induced HECT E3 enzyme, is required for conjugation of ISG15 in human cells. J. Biol. Chem. 2814334-4338. [DOI] [PubMed] [Google Scholar]
- 5.Durfee, L. A., M. L. Kelley, and J. M. Huibregtse. 2008. The basis for selective E1-E2 interactions in the ISG15 conjugation system. J. Biol. Chem. 28323895-23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrell, P. J., R. J. Broeze, and P. Lengyel. 1979. Accumulation of an mRNA and protein in interferon-treated Ehrlich ascites tumor cells. Nature 279523-525. [DOI] [PubMed] [Google Scholar]
- 7.Giannakopoulos, N. V., E. Arutyunova, C. Lai, D. J. Lenschow, A. L. Haas, and H. W. Virgin, I. V. 2009. ISG15 Arg151 and the ISG15-conjugating enzyme UbE1L are important for innate immune control of Sindbis virus. J. Virol. 831602-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giannakopoulos, N. V., J. K. Luo, V. Papov, W. Zou, D. J. Lenschow, B. S. Jacobs, E. C. Borden, J. Li, H. W. Virgin, and D.-E. Zhang. 2005. Proteomic identification of proteins conjugated to ISG15 in mouse and human cells. Biochem. Biophys. Res. Commun. 336496-506. [DOI] [PubMed] [Google Scholar]
- 9.Haas, A. L., P. Ahrens, P. M. Bright, and H. Ankel. 1987. Interferon induces a 15-kilodalton protein exhibiting marked homology to ubiquitin. J. Biol. Chem. 26211315-11323. [PubMed] [Google Scholar]
- 10.Kim, K. I., N. V. Giannakopoulos, H. W. Virgin, and D.-E. Zhang. 2004. Interferon-inducible ubiquitin E2, Ubc8, is a conjugating enzyme for protein ISGylation. Mol. Cell. Biol. 249592-9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, M. J., A. G. Latham, and R. M. Krug. 2002. Human influenza viruses activate an interferon-independent transcription of cellular antiviral genes: outcome with influenza A virus is unique. Proc. Natl. Acad. Sci. USA 9910096-10101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korant, B. D., D. C. Blomstrom, G. J. Jonak, and E. Knight. 1984. Interferon-induced proteins. Purification and characterization of a 15,000-dalton protein from human and bovine cells induced by interferon. J. Biol. Chem. 25914835-14839. [PubMed] [Google Scholar]
- 13.Kumar, S., W. H. Kao, and P. M. Howley. 1997. Physical interaction between specific E2 and Hect E3 enzymes determines functional cooperativity. J. Biol. Chem. 27213548-13554. [DOI] [PubMed] [Google Scholar]
- 14.Lai, C., J. J. Struckhoff, J. Schneider, L. Martinez-Sobrido, T. Wolff, A. Garcia-Sastre, D.-E. Zhang, and D. J. Lenschow. 2009. Mice lacking the ISG15 E1 enzyme UbE1L demonstrate increased susceptibility to both mouse-adapted and non-mouse-adapted influenza B virus infection. J. Virol. 831147-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenschow, D. J., N. V. Giannakopoulos, L. J. Gunn, C. Johnston, A. K. O'Guin, R. E. Schmidt, B. Levine, and H. W. Virgin. 2005. Identification of interferon-stimulated gene 15 as an antiviral molecule during Sindbis virus infection in vivo. J. Virol. 7913974-13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenschow, D. J., C. Lai, N. Frias-Staheli, N. V. Giannakopoulos, A. Lutz, T. Wolff, A. Osiak, B. Levine, R. E. Schmidt, A. Garcia-Sastre, D. A. Leib, A. Pekosz, K.-P. Knobeloch, I. Horak, and H. W. Virgin. 2007. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc. Natl. Acad. Sci. USA 1041371-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malakhova, O. A., and D. E. Zhang. 2008. ISG15 inhibits Nedd4 ubiquitin E3 activity and enhances the innate antiviral response. J. Biol. Chem. 2838783-8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narasimhan, J., J. L. Potter, and A. L. Haas. 1996. Conjugation of the 15-kDa interferon-induced ubiquitin homolog is distinct from that of ubiquitin. J. Biol. Chem. 271324-330. [DOI] [PubMed] [Google Scholar]
- 19.Okumura, A., G. Lu, I. Pitha-Rowe, and P. M. Pitha. 2006. Innate antiviral response targets HIV-1 release by the induction of ubiquitin-like protein ISG15. Proc. Natl. Acad. Sci. USA 1031440-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okumura, A., P. M. Pitha, and R. N. Harty. 2008. ISG15 inhibits Ebola VP40 VLP budding in an L-domain-dependent manner by blocking Nedd4 ligase activity. Proc. Natl. Acad. Sci. USA 1053974-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osiak, A., O. Utermohlen, S. Niendorf, I. Horak, and K.-P. Knobeloch. 2005. ISG15, an interferon-stimulated ubiquitin-like protein, is not essential for STAT1 signaling and responses against vesicular stomatitis and lymphocytic choriomeningitis virus. Mol. Cell. Biol. 256338-6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pattyn, E., A. Verhee, I. Uyttendaele, J. Piessevaux, E. Timmerman, K. Gevaert, J. Vandekerckhove, F. Peelman, and J. Tavernier. 2008. HyperISGylation of Old World monkey ISG15 in human cells. PLoS ONE 3e2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng, C. T., J. Tseng, L. Perrone, M. Worthy, V. Popov, and C. J. Peters. 2005. Apical entry and release of severe acute respiratory syndrome-associated coronavirus in polarized Calu-3 lung epithelial cells. J. Virol. 799470-9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tumpey, T. M., C. F. Basler, P. V. Aguilar, H. Zeng, A. Solorzano, D. E. Swayne, N. J. Cox, J. M. Katz, J. K. Taubenberger, P. Palese, and A. Garcia-Sastre. 2005. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 31077-80. [DOI] [PubMed] [Google Scholar]
- 25.Urano, T., T. Saito, T. Tsukui, M. Fujita, T. Hosoi, M. Muramatsu, Y. Ouchi, and S. Inoue. 2002. Efp targets 14-3-3 sigma for proteolysis and promotes breast tumour growth. Nature 417871-875. [DOI] [PubMed] [Google Scholar]
- 26.Wong, J. J., Y. F. Pung, N. S.-K. Sze, and K.-C. Chin. 2006. HERC5 is an IFN-induced HECT-type E3 protein ligase that mediates type I IFN-induced ISGylation of protein targets. Proc. Natl. Acad. Sci. USA 10310735-10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan, W., and R. M. Krug. 2001. Influenza B virus NS1 protein inhibits conjugation of the interferon (IFN)-induced ubiquitin-like ISG15 protein. EMBO J. 20362-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang, Y., J. Gao, K. K. K. Chung, H. Huang, V. L. Dawson, and T. M. Dawson. 2000. Parkin functions as an E2-dependent ubiquitin-protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc. Natl. Acad. Sci. USA 9713354-13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao, C., S. L. Beaudenon, M. L. Kelley, M. B. Waddell, W. Yuan, B. A. Schulman, J. M. Huibregtse, and R. M. Krug. 2004. The UbcH8 ubiquitin enzyme is also the E2 enzyme for ISG15, an IFN-α/β-induced ubiquitin-like protein. Proc. Natl. Acad. Sci. USA 1017578-7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao, C., C. Denison, J. M. Huibregtse, S. Gygi, and R. M. Krug. 2005. Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways. Proc. Natl. Acad. Sci. USA 10210200-10205. [DOI] [PMC free article] [PubMed] [Google Scholar]