Abstract
The relative contributions of interleukin-12 (IL-12) and IL-23 to viral pathogenesis have not been extensively studied. IL-12p40 mRNA rapidly increases after neurotropic coronavirus infection. Infection of mice defective in both IL-12 and IL-23 (p40−/−), in IL-12 alone (p35−/−), and in IL-23 alone (p19−/−) revealed that the symptoms of coronavirus-induced encephalitis are regulated by IL-12. IL-17-producing cells never exceeded background levels, supporting a redundant role of IL-23 in pathogenesis. Viral control, tropism, and demyelination were all similar in p35−/−, p19−/−, and wild-type mice. Reduced morbidity in infected IL-12 deficient mice was also not associated with altered recruitment or composition of inflammatory cells. However, gamma interferon (IFN-γ) levels and virus-specific IFN-γ-secreting CD4 and CD8 T cells were all reduced in the central nervous systems (CNS) of infected p35−/− mice. Transcription of the proinflammatory cytokines IL-1β and IL-6, but not tumor necrosis factor, were initially reduced in infected p35−/− mice but increased to wild-type levels during peak inflammation. Furthermore, although transforming growth factor β mRNA was not affected, IL-10 was increased in the CNS in the absence of IL-12. These data suggest that IL-12 does not contribute to antiviral function within the CNS but enhances morbidity associated with viral encephalitis by increasing the ratio of IFN-γ to protective IL-10.
Resistance to infection, as well as the extent of clinical symptoms, is directly influenced by the pattern and magnitude of cytokine induction. Specifically, gamma interferon (IFN-γ) is closely associated with control of many viruses and other intracellular pathogens (31, 45). IFN-γ secretion in turn is enhanced by interleukin-12 (IL-12), which in concert with the related cytokine IL-23, participates in regulating both innate and adaptive immune responses (15, 18, 26, 31, 49). IL-12 is a heterodimeric cytokine composed of two subunits (IL-12p35 and IL-12p40) secreted primarily by macrophages and dendritic cells. It is a potent inducer of IFN-γ secretion by CD4 T cells, even in the presence of T regulatory cells (23). It also increases cytokine secretion, as well as cytolytic potential of NK cells and CD8 T cells (49). IL-12 is rapidly induced in the periphery and the central nervous system (CNS) after a variety of viral infections (9, 31, 36, 49); however, its contribution to virus resistance varies depending upon tissue tropism and predominant effector mechanisms used to control virus replication. For example, IL-12 is critical to control murine cytomegalovirus, while it is dispensable for immune responses to lymphocytic choriomeningitis virus; IFN-γ is nevertheless required for resistance to both of these infections (31, 33, 34). Although IFN-γ is a critical component in the protective host immune response to many viruses, not all viral infections are associated with IL-12-mediated enhancement of the IFN-γ response (31, 49).
IL-23 shares both the IL-12p40 subunit and the IL-12 receptor (IL-12R) β1 subunit with IL-12 (15, 26, 49) and, similar to IL-12, IL-23 is primarily secreted by macrophages and dendritic cells. The role(s) of IL-23 in innate and adaptive immunity have only recently begun to come to light (15, 26). A primary consequence is induction of CD4 T cells secreting IL-17, which play a prominent role in neutrophil recruitment to sites of inflammation (1, 25, 26, 29). Unlike IL-12R, IL-23R is not expressed by naive T cells but is present on memory T cells (29). Differential receptor expression is consistent with IL-23-independent induction of IL-17-secreting CD4 T cells (26, 29); however, it is required for sustained expression of autoimmune T-cell effector function associated with autoimmune demyelination of the CNS (10, 29). During chronic mycobacterial and human immunodeficiency virus (HIV) infections, IL-23 functions as a regulator of the protective IFN-γ response (21, 31) but also dampens immune pathology during herpes simplex virus infection of the eye (22). However, IL-23 has a limited capacity to influence resistance in the absence of IL-12 (21, 27).
Infection of the CNS with the neurotropic JHM strain of mouse hepatitis virus (JHMV) induces an acute encephalomyelitis accompanied by myelin loss. Virus replicates in microglia, astrocytes, and oligodendroglia but only rarely in neurons and is controlled by a vigorous inflammatory response localized to the CNS (4). CD8 T cells exert crucial antiviral functions via perforin and IFN-γ-mediated mechanisms (4, 5, 37); however, recent data indicates that CD4 T cells alone can also mediate virus clearance (43, 46). The critical requirement for IFN-γ in combating JHMV infection is demonstrated by prolonged virus replication, and increased clinical disease and mortality in IFN-γ deficient mice (37). In the absence of IFN-γ, virus preferentially resides in oligodendroglia demonstrating a cell specific action of IFN-γ within the CNS (13). Furthermore, morbidity and mortality are dramatically increased in JHMV-infected immunodeficient recipients of CD4 T cells unable to secrete IFN-γ (38, 43). Although demyelination, a hallmark of JHMV infection, is an immune mediated manifestation of tissue destruction rather than a direct viral cytopathic effect (4, 28), a contribution of IFN-γ to myelin loss is controversial. Similar myelin loss in infected IFN-γ−/− mice compared to controls (37), as well as demyelination induced by IFN-γ deficient CD4 or gamma delta T cells in the context of immunodeficient hosts implicate an immune component other than IFN-γ in contributing to demyelination (5, 11). In contrast, T-cell transferred into infected immunodeficient recipients suggest that IFN-γ secretion by CD4 T cells limits demyelination (38), while IFN-γ secretion by CD8 T cells contributes to demyelination (5, 6, 39).
IFN-γ thus plays a key role in regulating clinical disease, mortality, control of virus replication, and myelin loss after JHMV infection of the CNS. To determine the contribution of IL-12 to JHMV pathogenesis, as well as a possible indirect contribution of IL-23 to myelin loss, antiviral immune responses and tissue damage were examined in infected IL-12/IL-23 (p40−/−), IL-12 (p35−/−), and IL-23 (p19−/−) mice. The data demonstrate that IL-12 enhances the magnitude of the IFN-γ response in the CNS after infection, albeit without affecting viral control. However, decreased clinical disease in IL-12-deficient mice indicates that IL-12 induced IFN-γ and a concomitant reduction in IL-10 contributes to the disability associated with JHMV-induced encephalomyelitis. In contrast, no differences in demyelination were detected in JHMV-infected mice deficient in either IL-12 or IL-23, suggesting that neither of these cytokines influences virus induced demyelination.
MATERIALS AND METHODS
Mice.
C57BL/6 mice, purchased from the National Cancer Institute (Frederick, MD), were used as wild-type (WT) controls in all experiments. Congenic IL-12/IL-23-deficient (p40−/−) and IL-12-deficient (p35−/−) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 IL-23-deficient (p19−/−) mice were previously described (10). Mice were housed and bred in an accredited animal facility at the Cleveland Clinic, Cleveland, OH. All procedures were in accordance with approved IACUC guidelines.
Virus infection and clinical disease.
Six-week-old mice were infected via intracerebral injection of 250 PFU of the J2.2v-1 neutralizing monoclonal antibody (MAb)-derived variant JHMV (12) in 30 μl of endotoxin-free Dulbecco modified phosphate-buffered saline (PBS). Animals were scored for clinical signs of disease by using the following scale: 0, healthy; 1, ruffled fur and hunched back; 2, hind-limb paralysis or inability to turn to upright position; 3, complete hind-limb paralysis and wasting; and 4, moribund or dead (12). Differences in clinical symptoms were confirmed by a blinded observer.
Cell isolation and virus titers.
Mononuclear cells were isolated from the brain, spleen, and cervical lymph nodes (CLN) as previously described (3, 5, 43, 46). Briefly, spleen and CLN tissues were dissociated, washed, and resuspended in RPMI medium. In addition, splenocytes were treated with Gey's solution to lyse red blood cells. Brain cells were isolated by tissue disruption in 4 ml of Dulbecco PBS using chilled Tenbroeck glass homogenizers. After centrifugation at 400 × g for 7 min, clarified supernatants were collected and stored at −80°C. Cell pellets were resuspended in a final concentration of 30% Percoll (Pharmacia, Uppsala, Sweden) and concentrated by centrifugation for 30 min at 800 × g at 4°C onto a 70% Percoll cushion. Mononuclear cells were collected from the 30%/70% Percoll interface and washed twice with RPMI medium containing 25 mM HEPES (pH 7.2). Viable cells were counted by using a hemacytometer and trypan blue exclusion. Virus titers were determined by plaque assay on confluent monolayers of DBT cells, a continuous murine astrocytoma cell line, as previously described (3, 5, 12, 43, 46). Plaques were counted after 48 h of incubation at 37°C.
Flow cytometry.
Cells isolated from the CNS, spleen, and CLN were incubated in 1% mouse serum and anti-mouse CD16/32 (clone 2.4G2; BD Pharmingen, San Diego, CA) for 15 min at 4°C to block nonspecific binding. Cells were stained with phycoerythrin-, fluorescein isothiocyanate-, peridinin chlorophyll protein-, or allophycocyanin-conjugated MAb specific for CD45 (30-F11), CD4 (RM4-5), CD8 (53-6.7), Ly-6G (1A8), and major histocompatibility complex (MHC) class II (IA/IE, 2G9), all from BD Pharmingen. JHMV-specific CD8 T cells were detected using a phycoerythrin-conjugated H-2Db-S510 tetramer (Beckman Coulter, Fullerton, CA). To examine the expression of MHC on infiltrating macrophages and CNS resident microglia, cells were stained with anti-CD45 and anti-F4/80 MAb (Serotec, Raleigh, NJ). Infiltrating macrophages were characterized by their CD45hi/F4/80+ phenotype, while CD45low/F4/80+ cells were distinguished as microglia.
Virus-specific IFN-γ production by the CNS-, spleen-, and CLN-derived CD8 and CD4 T cells were evaluated after peptide stimulation. Briefly, 2 × 106 splenocytes or CLN cells and 5 × 105 CNS derived cells were incubated with 3 × 105 EL-4 or CHB3 feeder cells as described previously (51). Cells were cultured in the absence or presence of either 1 μM S510 peptide encompassing the H-2Db CD8 T-cell epitope or 5 μM CD4 T-cell specific M133 peptide in a total volume of 200 μl of RPMI supplemented with 10% fetal calf serum for 5 h at 37°C with 1 μl of Golgi Stop (BD Pharmingen)/ml. After stimulation, cells were stained for surface expression of CD8, CD4, and CD45 and fixed and permeabilized by using a Cytofix/Cytoperm kit (BD Pharmingen) according to the manufacturer's instructions. Intracellular cytokine expression was detected using a fluorescein isothiocyanate-conjugated MAb specific for IFN-γ. Cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA) using FlowJo software (TreeStar, Inc., Ashland, OR).
ELISA.
IFN-γ and IL-10 concentrations in cell-free brain supernatants were determined by enzyme-linked immunosorbent assay (ELISA) (51). Briefly, plates were coated with MAb specific for either IFN-γ (1 μg/ml; R4-6A2) or IL-10 (3.5 μg/ml; JES5-2A5) (BD Pharmingen) by overnight incubation at 4°C. Plates were washed (0.5% Tween20 in PBS) and blocked with 10% fetal calf serum in PBS for 1 h at room temperature, and samples were incubated overnight at 4°C. Bound cytokine was detected with biotinylated MAb specific for IFN-γ (XMG1.2) or IL-10 (JES5-I6E3) (BD Pharmingen), avidin-horseradish peroxidase, and 3,3′,5,5′-tetramethylbenzidine (BD Pharmingen) as colorimetric substrate. The absorbance was measured at 450 nm with a microplate reader (Bio-Rad Laboratories, Hercules, CA) and analyzed with SoftMax Pro software (Molecular Devices, Sunnyvale, CA).
Histology.
Tissues were fixed in 10% formalin and embedded in paraffin. Longitudinal spinal cord sections were stained with hematoxylin and eosin or Luxol fast blue to evaluate inflammation and myelin loss, respectively, as described previously (5, 13, 36). Viral antigen was detected by immunoperoxidase staining (Vectastain-ABC kit; Vector Laboratory, Burlingame, CA) using anti-JHMV MAb J.3.3 specific for the carboxyl terminus of the viral nucleocapsid protein as the primary antibody, horse anti-mouse antibody as a secondary antibody, and 3,3′-diaminobenzidine substrate. Sections were scored in a blinded manner for inflammation, demyelination, and viral antigen. Representative sections identified by blinded scoring were chosen for photography.
Real-time PCR.
Tissues were frozen in liquid nitrogen and stored at −80°C. RNA was extracted by dissociation in TRIzol reagent (Invitrogen, Carlsbad, CA) using sterile Tenbroeck glass homogenizers according to the manufacturer's instructions. RNA was precipitated with isopropyl alcohol, washed with 75% ethanol, and resuspended in RNase-free water (Gibco/Invitrogen, Grand Island, NY). RNA was treated with a DNase treatment kit (Ambion, Austin, TX) to remove DNA contamination. RNA integrity and concentration were evaluated by electrophoresis on 1.2% formaldehyde gels. cDNA was synthesized using 2 μg of RNA, Moloney murine leukemia virus reverse transcriptase (Invitrogen), a 10 mM deoxynucleoside triphosphate mix, and 250 ng of random hexamer primers (Invitrogen). Quantitative real-time PCR was performed for expression levels of IL-12/23 (p40), IL-23 (p19), IL-12 (p35), tumor necrosis factor (TNF), IL-6, CXCL-10, CXCL-9, CCL-5, transforming growth factor β1 (TGF-β1), and TGF-β3 using SYBR green master mix (Applied Biosystems, Foster city, CA). The primers used for amplification of IL-6 and TNF-α were described previously (50). The primer sequences for p40, p19, p35, CXCL-10, CXCL-9, CCL-5, TGF-β1, and TGF-β3 were as follows (F, forward; R, reverse): p40, F, 5′-ACAGCACCAGCTTCTTCATCAG-3′, and R, 5′-TCTTCAAAGGCTTCATCTGCAA-3′; p19, F, 5′-CAGCAGCTCTCTCGGAAT-3′, and R, 5′-ACAACCATCTTCACACTGGATACG-3′; p35, F, 5′-ACAGCACCAGCTTCTTCATCAG-3′, and R, 5′-TCTTCAAAGGCTTCATCTGCAA-3′; CXCL-10, F, 5′-GACGGTCCGCTGCAACTG-3′, and R, 5′-GCTTCCCTATGGCCCTCATT-3′; CXCL-9, F, 5′-TGCACGATGCTCCTGCA-3′, and R, 5′-AGGTCTTTGAGGGATTTGTAGTGG-3′; CCL-5, F, 5′-GCAAGTGCTCCAATCTTGCA-3′, and R, 5′-CTTCTCTGGGTTGGCACACA-3′; TGF-β1, F, 5′-CCCGAAGCGGACTACTATGC-3′ and R, 5′-CGAATGTCTGACGTATTGAAGAACA-3′; and TGF-β3, F, 5′-CAATTACTGCTTCCGCAACCT-3′ and R, 5′-AGCACCGTGCTATGGGTTGT-3′. Expression was compared relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA as previously described (17). Reactions were monitored using the 7500 Fast Real Time PCR system (Applied Biosystems) under the following conditions: 95°C for 10 min, followed by 40 cycles of denaturation at 94°C for 10 s, annealing at 60°C for 30 s, and elongation at 72°C for 30 s. IL-1β expression levels were determined by using TaqMan primers and 2× universal TaqMan fast master mix (Applied Biosystems). GAPDH TaqMan probes were used as controls. TaqMan reactions were performed in 10-μl final reaction volumes containing specific master mix, 1 mM concentrations of each primer, and 4 μl of cDNA using the ABI 7500 fast PCR and 7500 software. For TaqMan probes, reactions were initiated by incubation at 95°C for 20 s, followed by 40 cycles of denaturation at 95°C for 3 s and annealing and extension at 60°C for 30 s.
RESULTS
IL-12 enhances JHMV-induced encephalitis.
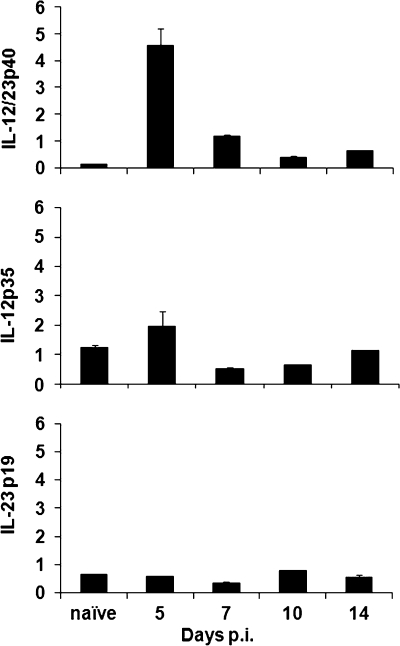
Increased transcription of IL-12p40 occurs within the CNS, rapidly following JHMV infection (36), suggesting a possible role for IL-12 and/or IL-23 in the pathogenesis of JHMV encephalitis. Recent data further suggested that IL-12p35 mRNA is rapidly induced and that IL-23p19 mRNA transcription increases subsequent to virus clearance from the CNS (14). However, the latter results were not correlated with IL-12p40 mRNA expression. Since previous reports relied upon semiquantitative measures of mRNA, we sought to confirm the activation of IL-12 and/or IL-23 during JHMV infection of the CNS by real-time PCR. Expression of p40 mRNA was below detection levels in the CNS of naive animals but was rapidly induced after infection (Fig. 1A), a finding consistent with previous data (36). Although p40 mRNA declined rapidly after day 5 postinfection (p.i.), it remained above basal levels to day 14 p.i. In contrast to the mRNA encoding the IL-12 and IL-23 common p40 chain, both the IL-12 specific p35 subunit and the IL-23 specific p19 subunit were detected at relatively low levels in the CNS of naive animals (Fig. 1A). However, expression of both p35 and p19 mRNA remained at or near basal levels after JHMV infection (Fig. 1A). Despite the inability to detect upregulation of these mRNAs, the increase of IL-12/23 p40 mRNA suggested that either IL-12 or IL-23 may play important roles in the pathogenesis of JHMV.
FIG. 1.
JHMV infection induces IL-12 p40 within the CNS. Expression of IL-12p40, IL-12p35, and IL-23p19 mRNA in the CNSs of JHMV-infected mice at various times p.i. The data reflect induction relative to GAPDH, using the following formula: (2[CT − CT{target}]) × 100, where CT is the threshold cycle. The data are the means of three mice per time point ± the standard error of the mean (SEM).
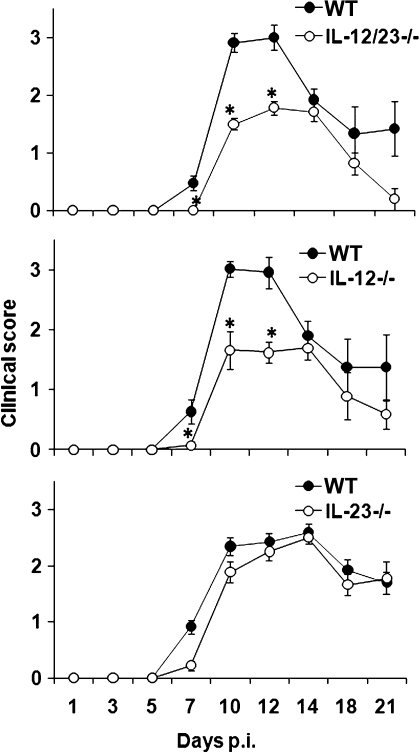
The potential role(s) of IL-12 and IL-23 on JHMV-induced encephalomyelitis were thus investigated in IL-12/IL-23-deficient (p40−/−) mice. After infection of WT mice, clinical symptoms of encephalitis were initially detected at day 7 p.i., progressed to a peak between days 10 to 12 p.i., and then began to decline (Fig. 2). In contrast, the initial onset of symptoms was delayed in p40−/− mice (Fig. 2). Furthermore, although clinical symptoms in infected p40−/− mice peaked at days 10 to 12 p.i. similar to WT mice, the severity was substantially reduced (Fig. 2). To determine a dominant contribution of either IL-12 or IL-23 to morbidity, the progression and severity of clinical disease was examined after infection of mice specifically deficient in either IL-12 (p35−/−) or IL-23 (p19−/−). Delayed onset, as well as a significant reduction in peak symptoms, was only observed after infection of IL-12-deficient mice (Fig. 2). IL-23-deficient mice exhibited no difference in onset or maximum clinical symptoms compared to WT mice (Fig. 2). Mortality was also similar (∼20%) in infected WT and p19−/− mice. In contrast, mortality in infected p35−/− and p40−/− mice was reduced (∼5%) compared to WT mice. These data indicate that IL-12, but not IL-23, plays a critical role in regulating the morbidity and mortality associated with acute viral encephalitis. Subsequent studies thus focused on analysis of p35−/− mice.
FIG. 2.
IL-12 deficiency decreases the symptoms of virus-induced encephalitis. JHMV-infected p40−/−, p35−/−, and p19−/− mice were examined for disease severity at various times postinfection. The data are representative of two separate experiments for each strain with >20 mice per experiment ± the SEM. *, P ≤ 0.05 (compared to the WT).
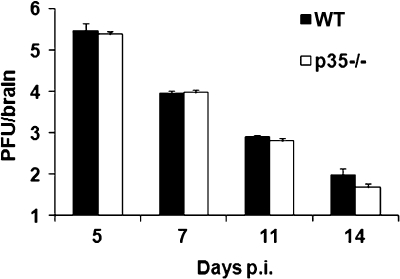
Clearance of infectious virus from the CNS of p35−/− and control mice was compared to assess whether reduced morbidity reflected enhanced control of virus replication. Infectious virus peaked in the CNS to similar levels at day 5 p.i. It was subsequently controlled with equal efficiency in both groups, and by day 14 p.i. infectious virus was at the limit of detection in both groups of mice (Fig. 3). The apparent redundancy of IL-12 in clearance of infectious virus is consistent with the absence of altered CNS virus replication in mice treated with anti-IL-12 antibody (14). Similarly, no alteration in virus replication within the CNS was detected in mice treated with anti-IL-23 antibody (14), and no differences were detected in virus replication or clearance comparing JHMV-infected p19−/− and WT mice (data not shown). These data indicate that while IL-12 plays an important role in the onset and severity of morbidity, it does not influence virus clearance.
FIG. 3.
IL-12 does not influence control of infectious virus. Virus replication in the CNS of p35−/− and WT mice after JHMV infection. The data are representative of two independent experiments and show the means of four individual mice per time point ± the SEM.
IL-12 does not alter CNS inflammation.
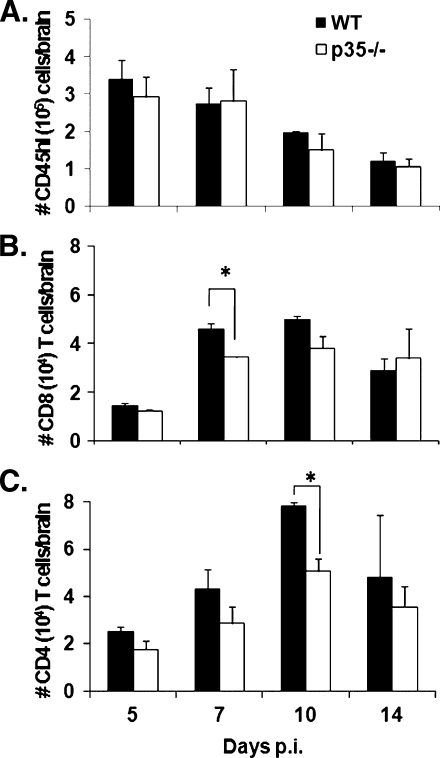
Reduced morbidity during acute infection suggested that leukocyte infiltration into the CNS might be altered in the absence of IL-12. However, analysis of CD45hi bone marrow-derived inflammatory leukocytes showed no difference in overall recruitment into the CNS of JHMV-infected p35−/− mice compared to WT mice throughout infection (Fig. 4A). Recruitment of inflammatory cells was also similar after infection of p19−/− mice (data not shown). There were also no differences in recruitment of Ly-6G+ neutrophils or F4/80+ monocytes within the CD45hi inflammatory populations at any time point postinfection in either p35−/− or p19−/− mice (data not shown). Lastly, to determine whether reduced clinical disease correlated with altered recruitment of T-cell subsets, CD8 and CD4 T cells within the infiltrating lymphocyte populations were examined. A slight decrease in total CNS infiltrating CD8 T cells was detected in infected p35−/− relative to WT mice at days 7 and 10 p.i., which only reached statistical significance at day 7 p.i. (Fig. 4B). Virus-specific tetramer+ cells specific for the dominant S510 epitope encoded within the viral spike glycoprotein comprised ∼40% of CD8 T cells at day 7 and ∼60% at day 10 p.i. in both groups (data not shown). Similar recruitment of virus-specific CD8 T cells was consistent with the ability of the p35−/− mice to control infectious virus (Fig. 3). The absence of IL-12 was also associated with a slight decrease in CD4 T-cell CNS infiltration throughout infection (Fig. 4C), reaching statistical significance at day 10 p.i. In summary, although IL-12 did not affect the recruitment of innate immune cells, total CD8 and CD4 T cells were reduced at 7 and 10 days p.i., respectively.
FIG. 4.
Decreased CD4 T-cell recruitment into the CNS of p35−/− mice. (A) Flow cytometric analysis of bone marrow-derived CD45hi infiltrating cells within the CNS after JHMV infection. Total numbers of CD8 T cells (B) and CD4 T cells (C) isolated from the brains of infected mice. The data represent the means of three independent experiments ± the SEM.
IFN-γ is compromised in the absence of IL-12.
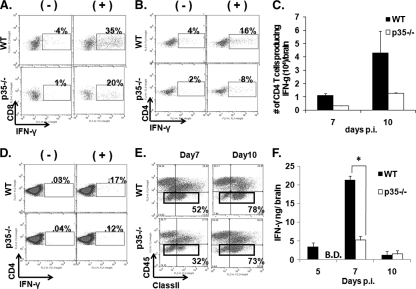
In addition to its antiviral function (4, 13, 36), IFN-γ has been suggested to regulate clinical symptoms during JHMV-induced encephalitis (38). IFN-γ is primarily secreted by activated T cells during JHMV infection, with no contribution by NK cells (4, 13, 43, 51). Although IL-12 is a powerful inducer and enhancer of IFN-γ secretion by T cells, IL-12 independent activation of IFN-γ-producing T cells has been described after a number of viral infections (31, 49). Thus, the frequency of CNS-derived virus-specific T cells producing IFN-γ was examined. In the absence of IL-12, the frequencies of virus-specific CD8 and CD4 T cells secreting IFN-γ were consistently reduced at day 7 p.i. and. Although slightly variable between independent assays, on average the frequencies of CD4 and CD8 T cells were reduced by 30 to 40% compared to T cells from the CNS of WT mice. Overall, the impact of IL-12 deficiency was greater on the CD4 T-cell population at day 7 p.i. (Fig. 5B). The enhancing affect of IL-12 on IFN-γ secretion by CD4 T cells becomes especially evident when the data reflect total numbers of virus specific T cells per brain (Fig. 5C). At day 10 p.i., the proportion of IFN-γ-secreting CD8 T cells was comparable in WT and p35−/− mice (∼36%); however, the fraction of CD4 T cells secreting IFN-γ in the CNS of the p35−/− mice remained at ∼60% of WT levels (data not shown). In contrast, no difference in the frequency of IFN-γ-producing CD4 T cells was detected in CLN (Fig. 5D) or spleen (data not shown), excluding a peripheral impairment of T-cell activation and expansion. Furthermore, reduced frequencies of virus-specific IFN-γ-producing cells were not attributable to an increase in IL-17-secreting cells, since these remained below 0.5% in both the CD4 and the CD8 T-cell compartments in WT as well as p35−/− mice (data not shown).
FIG. 5.
IL-12 enhances IFN-γ production and class II expression. The frequencies of CNS-derived IFN-γ-secreting T cells at day 7 p.i. were determined in CD8 T cells after stimulation with S510 peptide (A) and CD4 T cells with M133 peptide (B). The data are representative of two separate experiments analyzing cells combined from four to six individuals. (C) Number of CNS-derived CD4 T cells producing IFN-γ after ex vivo peptide stimulation in infected WT and p35−/− mice at days 7 and 10 p.i. The data represent the means of two independent experiments ± the SEM. (D) Frequencies of CLN derived IFN-γ-secreting CD4 T cells at day 7 p.i. after stimulation in the presence or absence of M133 peptide. The data are representative of two separate experiments analyzing cells combined from four to six individuals. (E) IFN-γ-dependent MHC class II expression on CD45lo microglia (boxed cells) at days 7 and 10 p.i. (F) Cell-free homogenates of the infected CNS were examined for IFN-γ by ELISA. The data show the means of four mice per time point ± the SEM and are representative of two separate experiments. *, P ≤ 0.05 (compared to the WT).
During JHMV infection MHC class II expression on microglia correlates with IFN-γ levels within the CNS (5). To determine whether the absence of IL-12 also compromised IFN-γ secretion in the CNS in vivo, microglia were examined for upregulation of MHC class II molecules. Microglia from infected p35−/− mice were indeed characterized by an initial delay in class II expression relative to WT mice (Fig. 5E). However, by day 10 p.i. class II expression on microglia was identical in both groups. IFN-γ within the CNS was measured by ELISA to assess to what extent the delay in class II upregulation correlates with IFN-γ protein levels. In infected WT mice, IFN-γ was detectable at day 5 p.i., increased significantly by day 7 p.i., and then declined substantially by day 10 p.i. In contrast, in the absence of IL-12 IFN-γ was undetectable at day 5 p.i., and only increased modestly by day 7 p.i. (Fig. 5F). IFN-γ levels within the CNS were similarly low in both groups by day 10 p.i. (Fig. 5F), when infectious virus was already reduced. These data suggest that even under optimal restimulation conditions the capacity of T cells derived from an IL-12-deficient CNS environment to produce IFN-γ is reduced (Fig. 5C). This deficit was more strongly manifested in vivo, where MHC/antigen density, and thus T-cell stimulation, is more limited. Significantly reduced IFN-γ levels in the CNS of p35−/− mice correlated with delayed class II expression on microglia and reduced morbidity. Nevertheless, similar and sustained class II expression at day 10 p.i. suggested that the reduced IFN-γ in the absence of IL-12 were still sufficient to drive optimal class II expression late during infection and sustain viral control.
Viral induced demyelination is IL-12 and IL-23 independent.
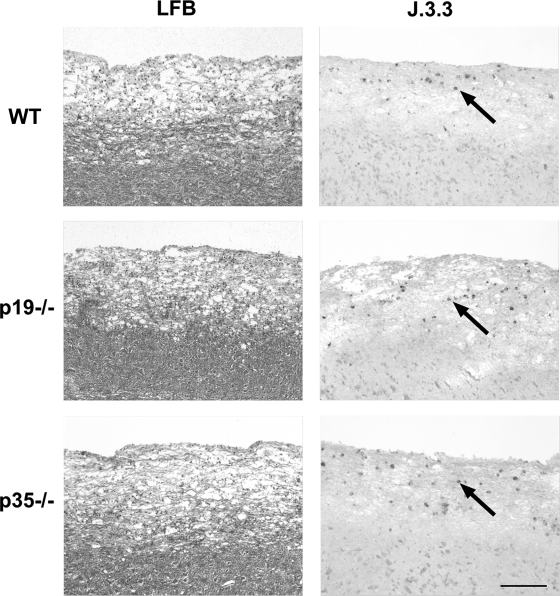
IFN-γ is critical for the control of JHMV infection in oligodendroglia (13, 37); however, demyelination in the host with an otherwise intact immune system is independent of IFN-γ (13, 37). Although demyelination associated with experimental autoimmune encephalitis is dependent upon IL-23 expression (10, 26, 29), the mechanism of myelin loss after JHMV infection is unclear (28). Therefore, potential alterations in inflammation, demyelination, and viral tropism in p35−/− and p19−/− mice were compared to those seen in WT mice. Despite the delayed onset of clinical symptoms and reduced severity in p35−/− mice (Fig. 2), no difference in inflammation or cells expressing viral antigen were detected compared to WT (Fig. 6). Although a slight increase in myelin loss was apparent in infected p35−/− mice (Fig. 6), it did not reach statistical significance due to variability between individuals. Similarly, the inability to secrete IL-23 did not alter viral induced inflammation or demyelination (Fig. 6). These data are consistent with an IFN-γ-independent mechanism of demyelination (13).
FIG. 6.
IL-12 and IL-23 do not influence viral pathogenesis. Longitudinal sections of spinal cords from JHMV-infected WT, p35−/−, and p19−/− mice at day 10 p.i. are shown. The extent of demyelination as shown by Luxol fast blue (LFB). Viral antigen (arrows) were detected by immunohistochemical analysis for viral nucleocapsid using MAb J.3.3. Bar, 200 μm.
IL-12 regulation of innate cytokine, chemokine, and anti-inflammatory cytokines.
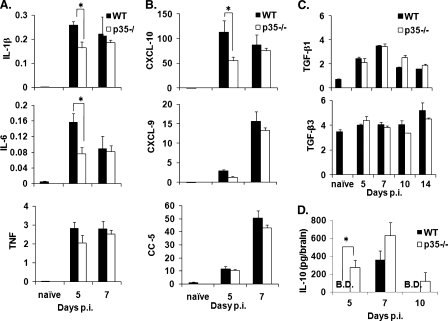
Ameliorated clinical symptoms in the absence of IL-12 suggested three possible mechanisms: (i) diminished activation of proinflammatory genes, (ii) reduced chemokine expression, or (iii) increased activation of anti-inflammatory genes. Expression of genes associated with the acute JHMV response or with induction or severity of clinical disease were compared in p35−/− and WT mice. Based on the cross talk between IL-12 and type I IFNs in regulating IFN-γ responses (7, 8, 31, 33, 49), the IFN-α4 and IFN-β mRNA levels were initially compared. Transcription of both mRNAs in the CNS was reduced by ∼40%-50% in the absence of IL-12 (data not shown). Nevertheless, the reduction was insufficient to alter the infectious virus load or increase the tropism for neurons (Fig. 6), previously shown to correlate with the inability to respond to IFN-α/β (17). IL-12 enhances the secretion of IFN-γ indirectly via enhanced IL-1β and TNF secretion, thereby initiating the production of inducible nitric oxide synthase in addition to other IFN-γ inducible genes (31). Decreased IFN-γ in the absence of IL-12 was indeed reflected in decreased CNS expression of the mRNA encoding inducible nitric oxide synthase (data not shown). Furthermore, consistent with their role in regulating clinical symptoms IL-6 and IL-1β mRNA were reduced in infected p35−/− mice at day 5 p.i. (Fig. 7A). However, no difference in the transcription of these genes was detected at day 7 p.i. (Fig. 7A), when the p35−/− group exhibited a significant decrease in morbidity (Fig. 2). Although expression of TNF mRNA was also slightly reduced in the CNS of infected IL-12−/− mice at day 5 p.i., the difference did not reach statistical significance (Fig. 7A). These data suggest that the initial decrease in proinflammatory cytokines contributes to the delayed onset of clinical symptoms.
FIG. 7.
IL-12 alters the expression of proinflammatory and anti-inflammatory cytokines. Brains from infected WT and p35−/− mice were compared for mRNA expression of the proinflammatory cytokines IL-1β, IL-6, and TNF (A); the chemokines CXCL-10, CXCL-9, and CCL-5 (B); and the anti-inflammatory cytokines TGF-β1 and TGF-β3 (C) by real-time PCR. Expression levels were normalized to GAPDH using the following formula: (2[CT{GAPDH} − CT{target}]) × 1,000, where CT is the threshold cycle. The data represent the means of three mice per time point ± the SEM. (D) IL-10 levels in brains of infected WT and p35−/− mice determined by ELISA. The data are representative of two experiments (four mice per time point) ± the SEM. B.D., below detection. *, P ≤ 0.05 (compared to the WT).
Reduced recruitment of T cells into the CNS of infected p35−/− mice suggested a possible defect in chemokine induction after infection in the absence of IL-12. However, CXCL-10 mRNA expression was only transiently reduced ∼2-fold at day 5 p.i. in the absence of IL-12. CXCL-10 mRNA increased to WT levels by day 7 p.i., and no differences were observed in the expression of CXCL-9 or CCL-5 mRNA (Fig. 7B). Although expression levels were equivalent by day 7 p.i., early differences presumably shape the responsiveness to chemokines and cytokines later during infection, thus maintaining overall decreased morbidity.
Based on their association with diminished clinical symptoms of EAE (40), expression of TGF-β and IL-10 were examined as downregulatory candidates in JHMV-infected p35−/− mice. In contrast to the proinflammatory cytokines, no significant alterations in TGF-β mRNA were detected relative to WT controls (Fig. 7C). However, IL-10 was increased in the CNS of p35−/− mice at days 5, 7, and 10 p.i. (Fig. 7D). Decreased morbidity was thus associated with decreased expression of proinflammatory cytokines, as well as increased expression of the anti-inflammatory cytokine IL-10.
DISCUSSION
IL-12 regulates both innate and adaptive immune responses and plays a major role in controlling bacterial and intracellular protozoa infections predominantly via stimulation of IFN-γ (18, 26, 49). Similarly, IL-23 contributes to host defense against bacterial infections (18, 26, 49). However, many viral infections resolve without IL-12 participation (49), and the contribution(s) of IL-23 have not been extensively explored (14, 22, 24, 31). Importantly, both cytokines also have the potential to influence the severity of pathological lesions and/or clinical disease (15, 26, 31, 49). Rapid CNS induction of IL-12p40 mRNA in a neurotropic coronavirus induced demyelination model (36) thus prompted analysis of the relative contributions of IL-12 and IL-23 to viral clearance and disease severity. Although quantitative PCR analysis confirmed upregulation of IL-12p40 mRNA (36), we were unable to detect statistically significant increases in either IL-12p35 or IL-23p19 mRNA after infection. Discrepancies with the previously reported early IL-12p35 mRNA induction and delayed IL-23p19 mRNA induction after clearance of infectious virus (14) may reside in distinct viral inoculum doses or our quantitative measurements of PCR replicons. Nevertheless, similar kinetics of virus control in p35−/− and p40−/− mice relative to WT mice are consistent with reports demonstrating no enhancing effects of IL-12 on mouse hepatitis virus clearance from the liver and CNS (14, 44). Similarly, infected p19−/− mice provided no evidence for IL-23-mediated enhancement of host defense (data not shown), a finding consistent with our inability detect upregulation of p19 transcripts in JHMV-infected WT mice. A transient increase in virus replication observed by treatment with anti-IL-23 (14) could thus not be confirmed in p19−/− mice infected with slightly lower virus doses.
A primary role of IL-12 is the enhancement of cytolytic activity and IFN-γ secretion by NK cells as well as T cells (49). Nevertheless, IL-12-independent T-cell activation and IFN-γ secretion has been noted after a number of viral infections, including systemic lymphocytic choriomeningitis virus infection (32) and influenza virus infection of the respiratory tract (30). This may be attributed to type I IFN, which can also promote IFN-γ secretion by lymphocytes (8). Type I IFN and IFN-γ are both essential to control acute JHMV infection, as shown by the inability of either virus-induced IFN-α/β or IFN-γ alone to achieve protection (17, 37). The absence of an IL-12 affect on JHMV clearance from the CNS may thus be attributed to the rapid induction of IFN-α (17), which may act as both an inhibitor of IL-12 and an enhancer of IFN-γ production (8). Nevertheless, IFN-γ levels were significantly decreased in the CNS of p35−/− mice, suggesting that type I IFN was insufficient to compensate for the absence of IL-12 in augmenting IFN-γ responses. Indeed, the modest decrease in IFN-α/β mRNA levels in p35−/− mice supports a role of IL-12 in promoting IFN-γ responses locally within the CNS. Impaired NK cell function in this context was ruled out, since NK cells do not contribute to either IFN-γ-dependent MHC class II expression on microglia or virus control in otherwise immunocompetent mice (51). Reduced IFN-γ correlated with decreased frequencies as well as total numbers of virus-specific CD8 and CD4 T cells derived from the CNS of infected p35−/− mice compared to WT mice. However, differences were not observed in the frequencies of virus-specific IFN-γ-producing CD4 T cells (Fig. 5D) or CD8 T cells (data not shown) in CLN or IFN-γ-producing CD4 or CD8 T cells in splenocytes (data not shown), which is consistent with a role of IL-12 in directly promoting local T-cell mediated IFN-γ secretion within the CNS. Although the control of JHMV in oligodendrocytes, the predominant cell type infected, is IFN-γ dependent (13, 37), no differences in viral tropism, viral antigen distribution, or viral clearance were noted in comparisons of p35−/− and WT mice. This suggested that the CD8 T-cell response in WT mice, the dominant adaptive effector response controlling viral replication (4), exceeds the minimum requirement to effectively inhibit viral replication. This is supported by similar results in IL-15- and B-cell-deficient mice, which also show reduced numbers of virus-specific CD8 T cells in the CNS after JHMV infection (42, 51).
A striking finding was the early effect of IL-12 on clinical disease, without alterations in either virus replication or demyelination. A contribution of IL-12 to clinical symptoms of virus-induced encephalitis, independent of virus load, has not been previously reported to our knowledge and provides a novel insight into viral pathogenesis of the CNS. The absence of a direct influence of IL-12 on tissue destruction is consistent with similar findings in infected mice treated with anti-IL-12 antibody (14) and other models of virus-induced demyelination. IL-12 contributes to limiting Semliki Forest virus infection of neurons and yet has no affect on subsequent demyelination (20). Furthermore, IL-12 does not influence either the acute phase or demyelination induced by Theiler's murine encephalitis virus (16). The contribution of IFN-γ to the pathogenesis of CNS disease is complex, since it can act as a prominent mediator of pathogenesis during some experimental viral infections but can also exert anti-inflammatory functions and decrease clinical symptoms (2, 22, 43), similar to its role in autoimmunity (19). For example, inhibition of IFN-γ enhances both morbidity and mortality after Semliki Forest virus, Theiler's murine encephalitis virus, and JHMV induced CNS disease (37, 41, 47).
IFN-γ plays a critical role in shaping the inflammatory cells recruited into the CNS (48). It is thus difficult to assess how increased virus replication and altered inflammation, e.g., neutrophil accumulation within the CNS, both a result of IFN-γ deficiency, contribute to the symptoms of acute encephalitis. This dilemma is evidenced by increased clinical symptoms and mortality of JHMV-infected immunodeficient recipients of immune CD4 donor T cells deficient in IFN-γ compared to WT CD4 T cells, independent of viral load (43). Both neutrophil and monocyte recruitment into the CNS and proinflammatory cytokine levels were similar in the absence of IL-12 at day 7 p.i., correlating reduced clinical disease most prominently with reduced IFN-γ levels. The observation that clinical disease in not reduced in infected IL-15−/−, despite decreased virus-specific CD8 T cells (51), implicates the paucity of total and virus-specific CD4 T cells in disease amelioration. Although CD4 T cells may contribute to viral clearance (46), they are more prominently implicated in the clinical symptoms of encephalitis (2). Deletion of the immunodominant CD4 T-cell epitope dramatically reduced both clinical symptoms and mortality after a lethal JHMV challenge (2). Similarly, mutations of the Theiler's murine encephalitis virus immunodominant CD4 T-cell epitope ameliorate disease (35). Although IL-23-dependent IL-17-secreting CD4 T cells have recently been implicated in enhancing clinical symptoms during CNS autoimmune disease (10, 26), the frequencies of IL-17-secreting T cells in the JHMV-infected CNS remained near background levels in both WT and p35−/− mice. A negligible contribution of IL-17-secreting T cells during JHMV pathogenesis is supported by a similar disease course and demyelination in both p19−/− mice and anti-IL-23 antibody-treated mice (14) compared to WT mice. In contrast to experimental autoimmune encephalitis (10, 26), IL-23 does not act as either a positive or negative regulator of virus-induced CNS inflammation. These data support the notion that the IL-12-dependent increase in virus-specific IFN-γ-secreting CD4 T cells contributes to the expression of clinical symptoms. Furthermore, although no evidence for induction of anti-inflammatory TGF-β was detected, IL-10 was modestly increased in the absence of IL-12. JHMV infection of IL-10−/− mice resulted in increased encephalitis, suggesting that IL-12-dependent limitations on IL-10 may also contribute to morbidity by increasing CD4 Th1 cell activation.
In summary, the study of JHMV-induced encephalitis in IL-12- and IL-23-deficient mice defined IL-12, but not IL-23, as a controlling element in the enhanced expression of clinical symptoms during viral encephalitis. Importantly, the absence of IL-12 decreased IFN-γ levels within the CNS; however, neither the absence of IL-12 nor the absence of IL-23 impeded viral clearance or affected demyelination. These data support the notion that IL-12 contributes to morbidity by enhancing IFN-γ-secreting CD4 T cells and reducing IL-10-mediated protection.
Acknowledgments
This study was supported by National Institutes of Health grants NS18146 and AI47249.
We thank Wen Wei and Ireland Derek for assistance.
Footnotes
Published ahead of print on 1 April 2009.
REFERENCES
- 1.Aggarwal, S., N. Ghilardi, M. H. Xie, F. J. de Sauvage, and A. L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T-cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2781910-1914. [DOI] [PubMed] [Google Scholar]
- 2.Anghelina, D., L. Pewe, and S. Perlman. 2006. Pathogenic role for virus-specific CD4 T cells in mice with coronavirus-induced acute encephalitis. Am. J. Pathol. 169209-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmann, C. C., J. D. Altman, D. Hinton, and S. A. Stohlman. 1999. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 1633379-3387. [PubMed] [Google Scholar]
- 4.Bergmann, C. C., T. E. Lane, and S. A. Stohlman. 2006. Coronavirus infection of the central nervous system: host-virus stand-off. Nat. Rev. Microbiol. 4121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergmann, C. C., B. Parra, D. R. Hinton, R. Chandran, M. Morrison, and S. A. Stohlman. 2003. Perforin-mediated effector function within the central nervous system requires IFN-gamma-mediated MHC up-regulation. J. Immunol. 1703204-3213. [DOI] [PubMed] [Google Scholar]
- 6.Bergmann, C. C., B. Parra, D. R. Hinton, C. Ramakrishna, K. C. Dowdell, and S. A. Stohlman. 2004. Perforin and gamma interferon-mediated control of coronavirus central nervous system infection by CD8 T cells in the absence of CD4 T cells. J. Virol. 781739-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cousens, L. P., J. S. Orange, H. C. Su, and C. A. Biron. 1997. Interferon-alpha/beta inhibition of interleukin 12 and interferon-gamma production in vitro and endogenously during viral infection. Proc. Natl. Acad. Sci. USA 94634-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cousens, L. P., R. Peterson, S. Hsu, A. Dorner, J. D. Altman, R. Ahmed, and C. A. Biron. 1999. Two roads diverged: interferon alpha/beta- and interleukin 12-mediated pathways in promoting T-cell interferon gamma responses during viral infection. J. Exp. Med. 1891315-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coutelier, J. P., J. Van Broeck, and S. F. Wolf. 1995. Interleukin-12 gene expression after viral infection in the mouse. J. Virol. 691955-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cua, D. J., J. Sherlock, Y. Chen, C. A. Murphy, B. Joyce, B. Seymour, L. Lucian, W. To, S. Kwan, T. Churakova, S. Zurawski, M. Wiekowski, S. A. Lira, D. Gorman, R. A. Kastelein, and J. D. Sedgwick. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421744-748. [DOI] [PubMed] [Google Scholar]
- 11.Dandekar, A. A., and S. Perlman. 2002. Virus-induced demyelination in nude mice is mediated by gamma delta T cells. Am. J. Pathol. 1611255-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming, J. O., M. D. Trousdale, F. A. el-Zaatari, S. A. Stohlman, and L. P. Weiner. 1986. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J. Virol. 58869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez, J. M., C. C. Bergmann, C. Ramakrishna, D. R. Hinton, R. Atkinson, J. Hoskin, W. B. Macklin, and S. A. Stohlman. 2006. Inhibition of interferon-gamma signaling in oligodendroglia delays coronavirus clearance without altering demyelination. Am. J. Pathol. 168796-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Held, K. S., W. G. Glass, Y. I. Orlovsky, K. A. Shamberger, T. D. Petley, P. J. Branigan, J. M. Carton, H. S. Beck, M. R. Cunningham, J. M. Benson, and T. E. Lane. 2008. Generation of a protective T-cell response following coronavirus infection of the central nervous system is not dependent on IL-12/23 signaling. Viral. Immunol. 21173-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter, C. A. 2005. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 5521-531. [DOI] [PubMed] [Google Scholar]
- 16.Inoue, A., C. S. Koh, M. Yamazaki, H. Yahikozawa, M. Ichikawa, H. Yagita, and B. S. Kim. 1998. Suppressive effect on Theiler's murine encephalomyelitis virus-induced demyelinating disease by the administration of anti-IL-12 antibody. J. Immunol. 1615586-5593. [PubMed] [Google Scholar]
- 17.Ireland, D. D., S. A. Stohlman, D. R. Hinton, R. Atkinson, and C. C. Bergmann. 2008. Type I interferons are essential in controlling neurotropic coronavirus infection irrespective of functional CD8 T cells. J. Virol. 82300-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jouanguy, E., R. Doffinger, S. Dupuis, A. Pallier, F. Altare, and J. L. Casanova. 1999. IL-12 and IFN-gamma in host defense against mycobacteria and salmonella in mice and men. Curr. Opin. Immunol. 11346-351. [DOI] [PubMed] [Google Scholar]
- 19.Kelchtermans, H., A. Billiau, and P. Matthys. 2008. How interferon-gamma keeps autoimmune diseases in check. Trends Immunol. 29479-486. [DOI] [PubMed] [Google Scholar]
- 20.Keogh, B., G. J. Atkins, K. H. Mills, and B. J. Sheahan. 2002. Avirulent Semliki Forest virus replication and pathology in the central nervous system is enhanced in IL-12-defective and reduced in IL-4-defective mice: a role for Th1 cells in the protective immunity. J. Neuroimmunol. 12515-22. [DOI] [PubMed] [Google Scholar]
- 21.Khader, S. A., J. E. Pearl, K. Sakamoto, L. Gilmartin, G. K. Bell, D. M. Jelley-Gibbs, N. Ghilardi, F. deSauvage, and A. M. Cooper. 2005. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J. Immunol. 175788-795. [DOI] [PubMed] [Google Scholar]
- 22.Kim, B., P. P. Sarangi, A. K. Azkur, S. D. Kaistha, and B. T. Rouse. 2008. Enhanced viral immunoinflammatory lesions in mice lacking IL-23 responses. Microbes Infect. 10302-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King, I. L., and B. M. Segal. 2005. Cutting edge: IL-12 induces CD4+ CD25− T-cell activation in the presence of T regulatory cells. J. Immunol. 175641-645. [DOI] [PubMed] [Google Scholar]
- 24.Kohyama, S., S. Ohno, A. Isoda, O. Moriya, M. L. Belladonna, H. Hayashi, Y. Iwakura, T. Yoshimoto, T. Akatsuka, and M. Matsui. 2007. IL-23 enhances host defense against vaccinia virus infection via a mechanism partly involving IL-17. J. Immunol. 1793917-3925. [DOI] [PubMed] [Google Scholar]
- 25.Kolls, J. K., and A. Linden. 2004. Interleukin-17 family members and inflammation. Immunity 21467-476. [DOI] [PubMed] [Google Scholar]
- 26.Langrish, C. L., B. S. McKenzie, N. J. Wilson, R. de Waal Malefyt, R. A. Kastelein, and D. J. Cua. 2004. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol. Rev. 20296-105. [DOI] [PubMed] [Google Scholar]
- 27.Lieberman, L. A., F. Cardillo, A. M. Owyang, D. M. Rennick, D. J. Cua, R. A. Kastelein, and C. A. Hunter. 2004. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J. Immunol. 1731887-1893. [DOI] [PubMed] [Google Scholar]
- 28.Matthews, A. E., S. R. Weiss, and Y. Paterson. 2002. Murine hepatitis virus: a model for virus-induced CNS demyelination. J. Neurovirol. 876-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGeachy, M. J., and D. J. Cua. 2008. Th17 cell differentiation: the long and winding road. Immunity 28445-453. [DOI] [PubMed] [Google Scholar]
- 30.Monteiro, J. M., C. Harvey, and G. Trinchieri. 1998. Role of interleukin-12 in primary influenza virus infection. J. Virol. 724825-4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novelli, F., and J. L. Casanova. 2004. The role of IL-12, IL-23 and IFN-gamma in immunity to viruses. Cytokine Growth Factor Rev. 15367-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orange, J. S., and C. A. Biron. 1996. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense: studies of natural killer and T-cell responses in contrasting viral infections. J. Immunol. 1561138-1142. [PubMed] [Google Scholar]
- 33.Orange, J. S., and C. A. Biron. 1996. Characterization of early IL-12, IFN-α/β, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J. Immunol. 1564746-4756. [PubMed] [Google Scholar]
- 34.Oxenius, A., U. Karrer, R. M. Zinkernagel, and H. Hengartner. 1999. IL-12 is not required for induction of type 1 cytokine responses in viral infections. J. Immunol. 162965-973. [PubMed] [Google Scholar]
- 35.Palma, J. P., R. L. Yauch, H. K. Kang, H. G. Lee, and B. S. Kim. 2002. Preferential induction of IL-10 in APC correlates with a switch from Th1 to Th2 response following infection with a low pathogenic variant of Theiler's virus. J. Immunol. 1684221-4230. [DOI] [PubMed] [Google Scholar]
- 36.Parra, B., D. R. Hinton, M. T. Lin, D. J. Cua, and S. A. Stohlman. 1997. Kinetics of cytokine mRNA expression in the central nervous system following lethal and nonlethal coronavirus-induced acute encephalomyelitis. Virology 233260-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parra, B., D. R. Hinton, N. W. Marten, C. C. Bergmann, M. T. Lin, C. S. Yang, and S. A. Stohlman. 1999. IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J. Immunol. 1621641-1647. [PubMed] [Google Scholar]
- 38.Pewe, L., J. Haring, and S. Perlman. 2002. CD4 T-cell-mediated demyelination is increased in the absence of gamma interferon in mice infected with mouse hepatitis virus. J. Virol. 767329-7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pewe, L., and S. Perlman. 2002. Cutting edge: CD8 T cell-mediated demyelination is IFN-gamma dependent in mice infected with a neurotropic coronavirus. J. Immunol. 1681547-1551. [DOI] [PubMed] [Google Scholar]
- 40.Prud'homme, G. J. 2000. Gene therapy of autoimmune diseases with vectors encoding regulatory cytokines or inflammatory cytokine inhibitors. J. Gene Med. 2222-232. [DOI] [PubMed] [Google Scholar]
- 41.Pullen, L. C., S. D. Miller, M. C. Dal Canto, P. H. Van der Meide, and B. S. Kim. 1994. Alteration in the level of interferon-gamma results in acceleration of Theiler's virus-induced demyelinating disease. J. Neuroimmunol. 55143-152. [DOI] [PubMed] [Google Scholar]
- 42.Ramakrishna, C., S. A. Stohlman, R. D. Atkinson, M. J. Shlomchik, and C. C. Bergmann. 2002. Mechanisms of central nervous system viral persistence: the critical role of antibody and B cells. J. Immunol. 1681204-1211. [DOI] [PubMed] [Google Scholar]
- 43.Savarin, C., C. C. Bergmann, D. R. Hinton, R. M. Ransohoff, and S. A. Stohlman. 2008. Memory CD4+ T-cell-mediated protection from lethal coronavirus encephalomyelitis. J. Virol. 8212432-12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schijns, V. E., B. L. Haagmans, C. M. Wierda, B. Kruithof, I. A. Heijnen, G. Alber, and M. C. Horzinek. 1998. Mice lacking IL-12 develop polarized Th1 cells during viral infection. J. Immunol. 1603958-3964. [PubMed] [Google Scholar]
- 45.Scott, P., and S. H. Kaufmann. 1991. The role of T-cell subsets and cytokines in the regulation of infection. Immunol. Today 12346-348. [DOI] [PubMed] [Google Scholar]
- 46.Stohlman, S. A., D. R. Hinton, B. Parra, R. Atkinson, and C. C. Bergmann. 2008. CD4 T cells contribute to virus control and pathology following central nervous system infection with neurotropic mouse hepatitis virus. J. Virol. 822130-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomkins, P. T., G. A. Ward, and A. G. Morris. 1988. Role of interferon-gamma in T-cell responses to Semliki Forest virus-infected murine brain cells. Immunology 63355-362. [PMC free article] [PubMed] [Google Scholar]
- 48.Tran, E. H., E. N. Prince, and T. Owens. 2000. IFN-gamma shapes immune invasion of the central nervous system via regulation of chemokines. J. Immunol. 1642759-2768. [DOI] [PubMed] [Google Scholar]
- 49.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3133-146. [DOI] [PubMed] [Google Scholar]
- 50.Zhou, J., N. W. Marten, C. C. Bergmann, W. B. Macklin, D. R. Hinton, and S. A. Stohlman. 2005. Expression of matrix metalloproteinases and their tissue inhibitor during viral encephalitis. J. Virol. 794764-4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuo, J., S. A. Stohlman, J. B. Hoskin, D. R. Hinton, R. Atkinson, and C. C. Bergmann. 2006. Mouse hepatitis virus pathogenesis in the central nervous system is independent of IL-15 and natural killer cells. Virology 350206-215. [DOI] [PMC free article] [PubMed] [Google Scholar]