Abstract
Standardized green tea extract was evaluated for exposure and toxicity in Beagle dogs following oral dosing by capsules. The main component (–)-epigallocatechin gallate (EGCG) accounted for 56-72% of the material. A 9-month chronic study (0, 200, 500, and 1000 mg/kg/day) was done in fasted dogs to take advantage of the reported improved catechin bioavailability with fasting. Extensive morbidity, mortality, and pathology of many major organs led to its early termination at 6.5 months and prevented identification of the toxicity mechanisms. A follow-up 13-week study examined the exposure to and toxicity of the extract. In general, toxicities were less severe than in the chronic study during the same interval. Dosing in a fed state resulted in considerably lower and less variable exposure than found under fasted conditions. Toxicity was less frequent and of lesser severity with lower exposure but limited sample size and large variability prevented reaching that definitive conclusion. Differences in mortality and morbidity between the preliminary terminated chronic and follow-up subchronic studies with the same dose of the same drug lot and similar exposure were not fully resolved as there may be other as yet unclear confounding factors.
Keywords: green tea, toxicity, polyphenols, (–)-epigallocatechin gallate (EGCG), dogs, fasted
1. Introduction
Tea is one of the most commonly consumed beverages worldwide, especially in Asian countries where it has been believed for centuries to posses a number of health promoting and curative properties. Green tea, like black and Oolong teas, is manufactured from dried leaves of Camelia sinensis, a species of Theaceae family. It differs from the other tea types by the processing method. Green tea is obtained by dry heat treatment of freshly harvested leaves to inactivate oxidative enzymes and by omitting fermentation, thereby preserving polyphenolic catechins with their high antioxidant activities. Four polyphenolic compounds, (–)-epigallocatechin gallate (EGCG), (–)-epigallocatechin (EGC), (–)-epicatechin gallate, (ECG), and (–)-epicatechin (EC), constitute the main polyphenolic components of green tea (Fig. 1). Green tea products are commonly available, marketed and used as dietary supplements (nutraceuticals) in the United States, mainly for purported weight loss and antioxidant properties. A number of different health benefits have been attributed to green tea, including prevention and/or control of atherosclerosis, hypertension, coronary heart disease, diabetes, metabolic syndrome, obesity, and cancer as well as having antibacterial, antiviral, antifungal, and neuroprotective activities (Cabrera et al. 2006; Cheng 2006; Cooper et al. 2005a, b; Crespy and Williamson 2004; Friedman 2007; Fujiki 2005; Khan and Mukhtar 2007; Pham-Huy et al. 2008; Shankar et al. 2007; Shukla 2007; Zaveri 2006). Due to potential public health benefits, green tea products have been and continue to be a subject of considerable attention from the general public and the scientific community.
Fig. 1.
Chemical structures of EGCG, EGC, ECG, and EC
Green tea polyphenolic catechins have shown promising cancer chemopreventive activities in a variety of animal tumor models, including lung, urinary bladder, mammary gland, prostate and skin (Lubet et al. 2007; Shukla 2007; Yan et al. 2006; Yang et al. 2007). A highly purified and standardized green tea extract (Polyphenon E®; PPE) is in drug development, presently Phase 2 testing, as a candidate cancer chemopreventive agent in the Division of Cancer Prevention at the National Cancer Institute. PPE contains 85-95% total catechins and the main component is EGCG. EGCG accounts for 56-72% of the material and the other major ingredients EGC, ECG, and EC are present at approximately 3, 8, and 8%, respectively. PPE may also contain small amounts of caffeine (<1%), theobromine (<1%), and gallic acid (<0.5%). PPE as a topical antiviral cream was the first botanical drug approved (http://www.fda.gov/cder/foi/label/2006/021902lbl.pdf) under the FDA’s new Botanical Drug Products guidance (http://www.fda.gov/cder/guidance/4592fnl.htm).
Chronic 9-month and the follow-up 13-week subchronic dog studies of PPE were carried out at different laboratories. Doses for the 9-month chronic study (0, 200, 500 and 1000 mg/kg/day) were selected on the basis of an earlier 13-week subchronic oral toxicity study in dogs in which the No-Observed-Adverse-Effect-Level (NOAEL) was > 600 mg/kg body weight (highest dose tested) in fed dogs (Johnson et al. 1999). However, in view of the recognized low oral bioavailability of green tea catechins (Cai et al. 2002; Walle 2007) and increased oral bioavailability of free catechins following administration of Polyphenon E® capsules to human volunteers on an empty stomach (Chow et al. 2005), dosing under fasted conditions was selected for the chronic study. Unexpected high morbidity and mortality in the chronic study led to its premature termination at 6.5 months. Due to high morbidity and mortality in the chronic study, it was not possible to ascertain causes for the unexpected severe toxicity that was observed. Therefore, the 13-week follow-up study was initiated to determine if the results could be replicated in another laboratory and to attempt to identify and characterize toxicities of Polyphenon E® following oral capsule dosing in dogs and examine confounding factors such as dosing under fed or fasted conditions.
2. Materials and methods
Prior to initiation of in vivo experimentation, study protocols were reviewed and approved by the institutional Animal Care and Use Committee. All aspects of animal care, use, and welfare were performed in compliance with United States Department of Agriculture regulations and the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). All studies were conducted in full compliance with the Good Laboratory Practice Regulations of the United States Food and Drug Administration (21 CFR Part 58).
2.1. Animals
16 male and 16 female Beagle dogs, 4 animals per gender per group (Marshall Farms USA, Inc., North Rose, NY), approximately 5.7-7.2 kg and 5-6 months at the start of the study, were used in the chronic study (scheduled 9-month study) performed in a different laboratory than the earlier 13-week study (Johnson et al. 1999). They were housed individually in stainless steel cages and 300-400 g/day of Certified Diet # 5007 (PMI Feeds, Inc., St. Louis, MO) was available for 2 hours starting 2 hours after dosing.
Fifteen male Beagle dogs, 3 animals per group (Covance Laboratories, Inc., Kalamazoo, MI), approximately 6.4-8.5 kg and 5-6 months at the start of the study, were used in a follow-up subchronic 13-week study performed in a third laboratory. They were singly housed in runs and approximately 350 g of Harlan Teklad Certified Diet No. 2025C (Harlan Teklad, Madison, WI) was provided daily from arrival until termination.
In all studies, the animals were housed in an AALAC Intl.-accredited facility in a temperature (64-84°F) and humidity (50 ± 20%) controlled room with a 12 hour light/12 hour dark cycle. Local tap water from an automatic watering system was provided ad libitum from arrival until termination. Animals were quarantined for approximately 21 days prior to randomization into experimental groups and start of the study. During the quarantine period, animals underwent a complete physical examination, including body temperature, a fecal examination for parasites, clinical pathology, and body weights. They were also observed for general health and acceptability for use in these studies.
2.2. Test article
PPE (lot A, PE-041014 and lot B, PE-050808) was obtained from Mitsui Norin Co., LTD., Shizuoka, Japan and its clinical formulation (lot C, 21110905) from ThermoFisher, Rockville, MD. Lot A was used in both the chronic 9-month study and the follow-up 13-week study. In addition to lot A, two additional lots (newer lot B and a clinical lot C) were used in the follow-up 13-week study for comparison. Lot C was a formulated clinical lot and contained microcrystalline cellulose, croscarmellose sodium, colloidal silicon dioxide, and magnesium stearate excipients in size 0 gelatin capsules. Different lots contained approximately 63.3-64.8% (–)-epigallocatechin gallate (EGCG; CAS 989-51-5), 3.0-3.7 % (–)-epigallocatechin (EGC; CAS 970-74-1), 6.0-8.0 % (–)-epicatechin gallate (ECG; CAS 1257-08-5), and 7.6-12.3% (–)-epicatechin (EC; CAS 490-46-0) (Fig. 1). PPE was stored at approximately 2-8°C at ambient humidity and protected from light. All test article lots were shown to be stable under the storage conditions. Except for lot C, PPE was administered as neat, unformulated substance in gelatin capsules. Lot C was formulated and already in gelatin capsules and was administered as such. Control group animals received the same number of the same size empty gelatin capsules each day as test animals.
It was re-confirmed that the levels of heavy metals, pesticides, microbial contamination, decomposition products, caffeine and methylxanthines in the PPE lots used in these studies were within acceptable ranges and comparable to other lots.
2.3. Treatment
In the chronic study, animals were dosed with 0, 200, 500, and 1000/800 mg/kg/day (approximately 0, 4000, 10,000, and 20,000/16,000 mg/m2/day) of neat (unformulated) PPE in gelatin capsules (1-2 capsules/dog/day) on empty stomach. Food was available 2 hours after dosing for 2 hours.
In the 13-week follow-up study, animals were grouped according to test article lot. PPE (200 mg/kg/day ~ 4000 mg/m2/day) was orally administered neat (unformulated) in gelatin capsules, with the exception of lot C. This lot was administered as 6 clinical capsules each containing approximately 300 mg of PPE (176 mg/kg/day ~ 3416 mg/m2/day). PPE was administered on an empty stomach in 3 groups and to fed animals (lot A) in one group. Starting from day 1, the “fed” group had food available approximately two hours prior to dosing. All other groups had food available for 2 hours starting 2 hours after dosing.
2.4. Toxicological evaluation
Throughout the study, animals were observed a minimum of twice daily at least 4 hours apart to monitor their general health status and for signs of mortality/morbidity. Morning observation was within an hour following dosing to look for the onset and duration of any clinical signs of toxicity.
Body weight measurements were performed weekly and food consumption was quantified daily. Each dog was removed from its cage once each week for a physical exam that involved close examination for detailed signs of toxicity. This detailed physical examination including the eyes and all orifices. All dogs underwent ophthalmic examination by indirect ophthalmoscopy at least once during the quarantine period and during the study. The eyelids, conjunctiva, cornea, sclera, iris, lens, fundus, anterior chamber lens, anterior vitreous and posterior chambers were examined. A tear production test (Schirmer Test) was also performed as deemed warranted.
All dogs also underwent electrocardiographic examinations during the quarantine period and during the study. Electrocardiograms were evaluated for heart rate and rhythm, amplitude of the P wave and QRS complex, and duration of the P wave, PR, QRS, and QT intervals.
Blood samples for clinical chemistry, hematology and coagulation evaluations were collected from fasted dogs at pre-test, at the terminal necropsy and when significant toxicity was observed. Clinical pathology assays were performed using standard automated instruments. Hematology parameters were measured using an Advia 120 Hematology Analyzer by using standardized methods. Tests included Hemoglobin (cyanomethemoglobin method); Hematocrit, Erythrocyte Count, Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), Mean Corpuscular Hemoglobin Concentration (MCHC), Leukocyte Count, Platelet Count, Reticulocyte Count, Leukocyte Differential Count, Nucleated RBCs, and RBC Morphology. In addition to this routine battery of tests, spherocytes were hand counted (10 fields at 100x magnification). The following coagulation parameters were measured on an MLA 900 coagulation machine: Activated Partial Thromboplastin Time (APTT); Prothrombin Time (PT); Fibrinogen. The following clinical chemistry parameters were measured using a Hitachi 912 Clinical Chemistry Analyzer using standardized methods: Glucose, Urea Nitrogen (BUN), Inorganic Phosphorus, Creatinine, Total Protein, Albumin, Calcium, Aspartate Aminotransferase (AST/GOT), Alanine Aminotransferase (ALT/GPT), Alkaline Phosphatase (AKP), Total Bilirubin, Creatine Kinase (CK), sodium, Potassium, and Chloride. The following chemistry parameters were measured using specific assay kits: Folic Acid (Dualcount Reagent Kit, DPC); Insulin (Diagnostics Products Coat-A Insulin Kit); Histamine (Histamine RIA kit, Beckman Coulter); IgE (Dog IgE ELISA Quantitation Kit, Bethyl Laboratories); IgG, IgA, IgM (Radial Immune Diffusion Reagent Kits, Bethyl Laboratories); Glucagon (Glucagon RIA kit, Linco Research); Lipase (Randox method of Colorimetric Hitachi 717, Randox); Pancreatic Amylase (Pancreatic α-Amylase test, Randox); Glutathione peroxidase (Glutathione Peroxidase Assay Kit, Cayman Chemical Company); Lipid Peroxidation (Lipid Peroxidation Assay Kit, Cayman Chemical Company); Total antioxidant activity (Antioxidant Activity Kit, Cayman Chemical Company); Total Glutathione (Measured by fluorescence HPLC); Reduced Glutathione (Measured by fluorescence HPLC); Oxidized Glutathione (calculated from difference between total and reduced glutathione measurements).
Urine samples were collected from fasted dogs at pre-test and during the final week of dosing, and were analyzed for color, appearance, specific gravity, urinalysis parameters by dipstick and sediment microscopically.
At necropsy, all gross lesions and approximately 45 tissues were collected from each animal and fixed in 10% neutral buffered formalin. All tissues collected from all study dogs were processed by routine histological methods and evaluated histopathologically.
2.5. Plasma analysis
The concentration of EGCG, EGC, ECG and EC in plasma (EDTA-tubes) was determined using high performance liquid chromatography and tandem mass spectrometry (LC/MS/MS). Chromatographic separation was achieved using Waters Symmetry Shield™ RP8 (50 × 2.1mm, 3.5 μm) (Waters, Milford, MA) analytical and Thermo Hypersil Gold (10 × 2.1 mm, 3 μm) (Thermo Fisher Scientific, Inc., Waltham, MA) guard columns at ambient temperature and a flow rate of 400 μl/min. Linear gradient elution was from 95% A (5 mM ammonium acetate with 0.4% formic acid): 5% B (50:50 methanol:acetonitrile with 0.4% formic acid) to 10% A:90% B over 6.5 min followed by a 1.5 min re-equilibration. Detection was performed using multiple reaction monitoring mode with negative polarity on Micromass Quattro Micro™ API (Waters, Milford, MA) unit. The following parent-daughter transitions were monitored 457.10 to 169.2, 305.20 to 125.20, 441.20 to 169.30, 289.20 to 125.35, and 301.24 to 151.24 for EGCG, EGC, ECG, EC and quercetin (internal standard), respectively. Quantification was based on peak areas. Free catechins (unconjugated) and total (unconjugated plus conjugated) were determined without and with hydrolysis by β-glucuronidase/arylsulfatase (Sigma-Aldrich, St. Louis, MO) for 2 hr at 37°C. After combining plasma samples (250 μl) with 25 μl of 10% ascorbic acid, 500 μl of acetonitrile and 50 μl of the internal standard were added to each tube. Tubes were vortexed, centrifuged and supernatants were transferred to clean tubes and evaporated to dryness under nitrogen. The dried extracts were reconstituted in 100 μl of mobile phase (95% A: 5% B), centrifuged, and supernatants were transferred to autosampler vials for analysis.
2.6. Pharmacokinetic analysis
Due to low levels of some catechins in a number of samples, data were not adequate for reliable determination of the elimination half-lives so full pharmacokinetic modeling could not be performed. Area-under-plasma concentration-time curve was determined using the trapezoidal rule from time equals zero to the last time point, 24 hr. Maximal plasma concentration (Cmax) was based on the highest measured plasma test article concentration.
2.7. Ancillary tests
Fasting serum levels of amylase, lipase, insulin, and glucagon were measured once in the quarantine period (day -11) and in weeks 4 (day 23), 8 (day 51) and 13 (day 86) as indices of pancreatic function. Immunoglobulins (IgE, IgG, IgM and IgA) and folic acid levels in serum were tested once from serum collected during quarantine period and from serum collected on the day of necropsy from fasted animals. Histamine levels were tested from EDTA-plasma collected on the day of necropsy from fasted animals. Tests for oxidative stress (levels of reduced and oxidized glutathione, glutathione peroxidase, lipid peroxidation and total antioxidant activity) were performed by AniLytics (Gaithersburg, MD) in plasma processed from the heparinized blood samples collected from all animals at necropsy. Additional liver samples (approximately 1.0 g) were obtained from all scheduled sacrifice animals at necropsy, rinsed three times in separate aliquots of ice-cold phosphate buffered saline until free of blood and stored frozen at approximately -80°C until shipped on dry ice to AniLytics, Inc. (Gaithersburg, MD), for the five tests for oxidative stress (reduced and oxidized glutathione, glutathione peroxidase, lipid peroxidation and total antioxidant activity).
2.8. Statistical analyses
Where appropriate, group comparisons were made by analysis of variance. If a significant F ratio (P < 0.05) was obtained, Dunnett’s Test was used for comparisons with a control. In all cases, the lower limit for statistical significance was defined as p < 0.05.
3. Results
3.1 Chronic study
Unexpected morbidity and mortality were encountered in the chronic 9-month dog toxicology study with PPE. Doses selected for this study (200, 500 and 1000 mg/kg/day) were based on an earlier 13-week study where NOAEL was > 600 mg/kg/day, the highest dose tested (Johnson et al. 1999). Unlike in that earlier study, PPE was administered on an empty stomach in the chronic study and food was provided for two hours starting two hours after dosing. Toxicity was evident by day 9 when clinical signs, including one death, significant body weight and food consumption decreases were seen in the 1000 mg/kg/day group. Dosing of that group was then discontinued and resumed one week later with 800 mg/kg/day after some body weight recovery. By the early termination of the study at 6.5 months, there were 16 deaths out of 24 PPE-treated animals. Incidence of mortality was 0 out of 4 in both male and female control groups, 2 out of 4 and 1 out of 4 in male and female 200 mg/kg groups, respectively, 3 out of 4 and 2 out of 4 in male and female 200 mg/kg groups, respectively, and 4 out of 4 in both male and female high dose groups. The majority of unscheduled deaths occurred during the first 13-weeks. It was not possible to correlate time of dosing and time of death. Control animals did not exhibit toxicity or death, suggesting that toxicity and mortality were related to the test article. In general, the incidence of mortality was dose- related and the time-to-death was inversely related to the dose of PPE. Male animals appeared more susceptible than their female counterparts. With the high rate of mortality and morbidity, and manifestation of significant toxicities in multiple organs and systems, it was not possible to identify major cause(s) of toxicity or determine if incidences were dose-related.
The most prevalent adverse events observed for PPE-treated dogs in that study were diarrhea, emesis, and excessive salivation. Ophthalmoscopic and electrocardiographic examinations were unremarkable. However, a few dogs exhibited keratoconjuctivitis during the study that resolved with sterile saline flush and/or application of ocular ointment (Paralube Vet Ointment, Pharmaderm, Melville, NY).
The dogs that survived to the end of the early terminated study gained weight throughout. The average weight gain from Day 1 to the terminal necropsy for these survivors (number of survivors in parentheses) was 37.4% (4), 43.4% (2), and 30.5% (1) for male dogs and 28.3% (4), 35.9% (3), and 13.1% (2) for female dogs in the 0, 200, and 500 mg/kg/day dose groups, respectively.
Food consumption data were complicated by a number of factors including normal week-to-week variability of food consumption for a given dog, supplementation of the normal dry diet with prescription diet to encourage dogs to consume their food, and presence of wet feed in the food cup, presumably resulting from the dog depositing unknown amounts of drinking water in its food cup. Generally, prescription diet, which was soft and moist, was not offered to an affected dog until it had not consumed any normal diet for several consecutive days.
Hematological effects in PPE-treated dogs included mild to marked increases in white blood cells, neutrophil, monocyte, platelet, and platelet crit (percentage volume of platelets) values; mild to marked decreases (≤85% of vehicle control) in red blood cells (RBC), hemoglobin (HGB), and hematocrit (HCT) values and a trend for higher reticulocyte counts. In addition, increases were seen in group mean and/or individual Red blood cell Distribution Width (RDW) values and more variable increases in Mean Cell Volume (MCV), Platelet Distribution Width (PDW), Mean Platelet Volume (MPV) values. The increases in group mean and/or individual WBC, neutrophil, and/or monocyte values noted for dogs in the PPE dose groups, both at scheduled blood collection intervals and prior to moribund sacrifice, were considered test article-related findings; the lowest incidence of changes was observed for dogs in the 200 mg/kg/day dose group. Increases in WBC and neutrophil counts (≥1.7-fold over vehicle control), and/or monocyte counts (≥2.7-fold over vehicle control) were consistent with test article-related inflammation and necrosis, and consistent with concurrent clinical chemistry and coagulation findings of increases in globulin and fibrinogen values, bone marrow observations of myeloid hyperplasia, and histopathology findings of tissue necrosis and inflammation. Increases in group mean and/or individual eosinophil counts (≥3-fold over vehicle control) were considered part of the test article-associated granulocytic hyperplastic response or may suggest an allergic/immune response to the test article. Increases in MPV and PDW were less consistently observed but were consistent with a trend for a platelet regenerative response. The increases (≥140% of vehicle control) in platelet counts and platelet crit values were considered test article-related, potentially associated with generalized marrow hyperplastic responses. The observed anemia varied from macrocytic poorly regenerative to macrocytic nonregenerative. Decreased presence of polychromatophilic cells in bone marrow of a number of PPE-treated dogs was consistent with poorly regenerative to nonregenerative erythropoiesis. Bone marrow cytology of the high-dose animals showed low cellularity, low megakaryocyte counts, relative myeloid/monocytoid hyperplasia and erythroid/lymphocytic hypoplasia as well as increased nuclear debris and brown-pigment macrophages. A few animals in the lower dose groups also exhibited similar abnormalities. There were also rare findings of spherocytes in the two high-dose groups. In general, the incidence and/or severity of the hematological findings were less in the 200 mg/kg/day dose group than in dogs in the two higher dose groups. Histopathological and cytological observation of increased myeloid percents (shift towards early precursors) and a lack of complete erythroid maturation indicated effects on erythropoiesis. Due to the varied onset and severity of the anemia and reticulocyte responses as well as concurrent findings of inflammatory leukograms, spherocytes, high bilirubin values and/or observations of brown urine, both decreased erythropoiesis and erythrocyte hemolysis were considered potential contributing factors to the observed anemia.
Altered clinical chemistry parameters in PPE-treated dogs included decreases in group mean and/or individual albumin, albumin/globulin, sodium, potassium, chloride and calcium values and increases in group mean and/or individual globulin values and findings of individual dogs with increased AST, ALT, ALP, total bilirubin, and triglyceride levels. Increases in amylase, insulin, glucagon, BUN, creatinine, phosphorus, and lipase (low incidence) values in individual dogs prior to early sacrifice were considered potentially test article-related, but may also have been secondary to test article-related dehydration, associated with vomiting and diarrhea, and to decreased renal and pancreatic perfusion and tissue injury associated with the moribund condition of some dogs.
Increases in APTT and fibrinogen values, and to a lesser extent, increases in PT values were observed in individual dogs in all dose groups, prior to early sacrifice. Increases in fibrinogen values observed in individual dogs prior to moribund sacrifice were consistent with histopathological findings of tissue inflammation and necrosis. Increases in APTT and/or PT values were consistent with thromboembolic events secondary to inflammation and necrosis and observations of leukocytosis, monocytosis, neutrophilia, and increased globulin.
Amber- or brown-colored urine and low pH values were noted in individual dogs in the 200 mg/kg dose group and also in the two high-dose groups which also had high urine bilirubin levels. Brown color and high bilirubin levels in urine are consistent with hemolysis.
Organ weights were only recorded for dogs euthanized at scheduled necropsy and thus data were limited. In view of that, possible dose-related increases in weights of the adrenals, liver, testes, ovary, spleen, and thyroid/parathyroid and possible decreases in thymus weights were observed in PPE-treated dogs in comparison to the same-gender control group animals. Decreases in thymic weights are common in stressed animals, especially when they have signs of clinical toxicities.
Macroscopic and microscopic pathological examinations showed test article-related lesions in many tissues. Gross examination of the gastro-intestinal tract primarily showed diffuse lesions in the stomach (grey plaques, mottled and granular, and thick and mottled) and small and large intestine (thick and mottled, thick and dark, granular and red, and thick and red). Histopathologically, there was minimal to marked (ulceration) necrosis of the mucosal epithelium with hemorrhage, infiltration of neutrophils, brown pigment and cellular debris as well as epithelial hyperplasia, probably a regenerative response to necrosis. In addition to stomach and small intestine, epithelial necrosis of esophagus was noted microscopically in 3 of 4 high dose males. Centrolobular necrosis and chronic-active inflammation with infiltration of neutrophils and mononuclear cells were evident in the liver along with brown intracytoplasmic pigment in Kupffer cells. Tubular epithelial necrosis (involving proximal tubules of cortex and medulla), tubular dilation with granular or hyaline protein casts, epithelial regeneration, acute inflammation and transitional epithelium hyperplasia were evident in the kidneys. However, it wasn’t clear if these were a direct result of the drug or secondary to decreased renal blood flow and glomerular filtration resulting from vomiting- and diarrhea-caused dehydration. Lymphoid tissue showed enlargement, dark discoloration or a gelatinous appearance. Histologically there was atrophy or necrosis in spleen, lymph nodes and tonsils as well as lymphoid aggregates of the small and large intestines, salivary gland, gall bladder and urinary bladder. The predominant necrotic lesion observed in the lymphoid tissue was necrosis, apoptotic (Elmore 2006). There were few tissues in individual animals which had mixed apoptotic and oncotic necrosis or just oncotic necrosis. It wasn’t clear if these were a direct result of the drug or indirect effects due to debilitation and stress caused by increased endogenous corticoids. Testicular germinal epithelial atrophy and aspermia, atrophy of prostate and ovarian atrophy were also noted on histological examination. Grossly, lungs showed discoloration (dark, mottled, focus) or increased thickness while heart showed mass, nodule and focal lesions and discoloration. Gross lesion of mass or nodule corresponded to the microscopic lesion of thrombus. Thromboses of heart and lung were noted histologically and considered a significant factor in the deaths of several male dogs. However, it also wasn’t clear if these were direct effects of the drug or secondary to vascular damage caused by dehydration (with hemodynamic shock) from vomiting and diarrhea. Bacteria were not observed in the necrotic lesions of the digestive tracts and only normal bacterial flora was observed on the mucosal surface. Therefore, there was no evidence of endotoxin presence but it could not be definitely discounted. Thromboses, in addition to inflammation and necrosis in multiple tissues, were considered to be factors in the deteriorating condition of these animals.
With the high rate of mortality and morbidity, it was not possible to determine if incidences and severities of macro- and microscopic lesions were dose-related.
3.2. 13-week follow-up study
This study was a follow-up to the above chronic study and was intended to examine possible causes of unexpected morbidity and mortality in the chronic study. A single dose of 200 mg/kg/day was administered to male dogs for 13 weeks. This dose was selected for comparison with the chronic study where it was the lowest dose used and produced significant morbidity and mortality. Male animals were used because they seemed more sensitive to toxicity in the chronic study than their female counterparts. It was felt that limiting gender and group size (3 per group) would be sufficient based on the observations in the chronic study and would minimize the use of animals. Since the majority of morbidity and mortality in the chronic study occurred within the first 13 weeks, it was expected that this shorter evaluation period would also be adequate.
Treatment-related effects are summarized in Tables 1-3.
Table 1.
Summary of treatment-related effects in the 13-week follow-up study
| LOT | A | A | B | C |
|---|---|---|---|---|
| DOSE mg/kg/day mg/m2/day |
200 3846 |
200 3846 |
200 3862 |
176 3416 |
| FOOD | No | Yes | No | No |
| FREE EGCG AUC (hr × ng/ml) | 29,191 ± 21,591 | 14,841 ± 4,375 | 45,160 ± 33,145 | 41,189 ± 33,145 |
| TOTAL EGCG AUC (hr × ng/ml) | 123,590 ± 123,330 | 32,185 ± 4,938 | 118,450 ± 91,381 | 123,760 ± 70,649 |
| BODY WEIGHT GAIN | NE | ↓↓ | NE | NE |
| FOOD CONSUMPTION | NE | ↓ | NE | NE |
| TEAR PRODUCTION | NE | ↓ | ↓ | ↓ |
| CLINICAL SIGNS | VR, DI, RM | BL, DE, PE | VR, DI, RM, BL, DE, PE, DA, DH, LB, ES | VR, DI, DE |
| CLINICAL CHEMISTRY | NE | ↑ ALT, ↑ HIST, ↑IgE | ↑ ALT, AST, ↑ HIST, ↑ IgG, ↑ IgA, ↑↑ GLOB, ↑↑ TBIL, ↓↓ A/G | ↑ ALT, ↑ HIST, ↑ IgG, ↑↑ GLOB, ↓↓ A/G |
| HEMATOLOGY | NE | NE | ↓ RBC, ↓ HGB, ↓ HCT, ↑ RETIC, ↑ WBC, ↑ NEUT, ↑ MONO, ↑ BASO, ↑ SPHER, marginal eosinophilia, slight to moderate large platelets, marked hypochromia | ↓ RBC, ↓ HGB, ↓ HCT, ↑ RETIC, ↑ MAC, ↑ SPHER, marginal eosinophilia, moderate large platelets, slight hypochromia |
| ORGAN WEIGHTS | ↑ Prostate ↑ Spleen | ↑ Prostate | ↑ Prostate ↑ Spleen | ↑ Prostate ↑ Spleen |
| PATHOLOGY | NE | NE | Liver: hematopoiesis, pigmented macrophages Bone marrow (sternal): hyperplasia Spleen: erythropoiesis | Liver: hematopoiesis, pigmented macrophages Bone marrow (sternal): hyperplasia |
NE = No Effect ↑↑ = Statistically Significant Increase ↑ = Biologically Significant Increase ↓↓ = Statistically Significant Decrease ↓ = Biologically Significant Decrease
BL = Blepharospasm, DA = Decreased Activity, DE = Discharge from Eyes, DH = Dehydrated, DI = Diarrhea/Loose Stool, ES = Excessive Salivation, LB = Labored Breathing, PE = Pink Eyes, RM = Red Material in Run, VR = Vomit in Run
A/G = albumin/globulin ratio, ALT = Alanine aminotransferase, AST = Aspartate aminotransferase, GLOB = Globulin, HIST = Histamine, IgA = Immunoglobulin A, IgE = Immunoglobulin E, IgG= Immunoglobulin G, TBILI = Total bilirubin
HCT = Hematocrit concentration, HGB = Hemoglobin Concentration, BASO = Basophil Count, MAC = Macrocyte Count, MONO = Monocyte Count, NEUT = Neutrophil Count, RBC = Erythrocyte Count, RETICS = % Reticulocytes, SPHER = Spherocyte Count, WBC = White Blood Cell Count
Table 3.
Selected hematology parameters (week 13) in male dogs dosed with PPE in the 13-week follow up study.
| RBC (106/μL) | HGB (g/dL) | HCT (%) | Reticulocytes (%) | Spherocytes (Count/10 fields) | Eosinophils (103/μL) | |
|---|---|---|---|---|---|---|
| Control-Fasted Group | 5.84 ± 0.63 | 13.3± 0.63 | 38.6 ± 4.19 | 0.4 ± 0.10 | 18 ± 3.5 | 0.18 ±0.098 |
| Lot A Fasted - 200 mg/kg/day | 6.30 ± 0.106 | 14.3 ± 0.20 | 41.4 ± 0.75 | 0.3 ± 0.06 | 27 ± 11.9 | 0.33 ± 0.257 |
| Lot A Fed - 200 mg/kg/day | 6.83 ± 0.193 | 15.2 ± 0.57 | 44.6 ± 1.90 | 0.3 ± 0.17 | 19 ± 18.2 | 0.46 ± 0.131 |
| Lot B Fasted - 200 mg/kg/day | 6.01 ± 0.535 | 13.5 ± 1.67 | 41.3 ± 1.68 | 2.0 ± 1.89 | 40 ± 17.2 | 0.39 ± 0.120 |
| Lot C Fasted - 176 mg/kg/day | 6.06 ± 0.670 | 13.4 ± 1.35 | 40.7 ± 3.24 | 1.3 ± 1.08 | 36 ± 18.0 | 0.73 ± 0.438 |
Although no statistically significant differences were seen for most parameters, biologically significant changes were seen in one or two animals per dose group (especially evident in groups administered with Lots B and C, see Table 2) and also evident from the high standard deviations for mean group numbers in the Tables 8 and 9.
No mortalities occurred in any group. However, two dogs (lot B dog 1146 and lot C dog 1140) exhibited serious clinical signs of toxicity in weeks 8-9. The more severely affected dog (lot B) showed marked signs of clinical toxicity including inactivity, respiratory distress, dangerously low oxygen saturation of hemoglobin (~60%), and cyanotic mucous membrane on one occasion in week 8. This animal was found unresponsive to touch and oxygen was administered to save the dog. Both dogs recovered and remained clinically normal until termination.
All three groups of animals being administered PPE on an empty stomach exhibited gastro-intestinal irritation as evidenced by vomiting, mild diarrhea/loose stool and/or red material in the run occasionally. These symptoms were totally absent when PPE was not administered on an empty stomach.
Comparison of treatment-related toxicities in the 4 groups in this study (Table 1) showed that administration of lot A on a full or empty stomach resulted in the least toxicity. This is surprising since this lot is one of the lots used in the prior chronic study which showed marked morbidity and mortality at the same dose. The AUC of both free and total EGCG for lot A in fed animals was lower than the corresponding AUCs for lots A, B and C in unfed animals (Table 1). However, while the mean AUC of free EGCG for lot A in unfed animals was lower than AUCs for lots B and C in unfed animals, there were no statistical differences between the means due to variability in this parameter in all groups of unfed animals.
Clinical pathology parameters measured (Table 2 and 3) showed increases in serum globulin levels in the animals dosed with lot B. Eosinophils were slightly increased in all PPE dosed groups, which corresponds with an increase in histamine levels in these groups. Special clinical chemistry tests for pancreatic function (lipase, amylase, insulin and glucagon), oxidative stress tests and urinalysis parameters did not reveal consistent or significant drug-treatment-related changes. Statistically non-significant increases in alanine aminotransferase (ALT) were observed in the lot C dose group compared to the control group. Statistically non-significant increases were seen in aspartate aminotransferase (AST) levels in the lot B dose group. These increased levels were due to the high levels in one of three animals in both ALT and AST parameters. There was a significant increase in serum total bilirubin levels in the lot B dose group. Animals in the lot B and lot C dose groups had increased reticulocyte percentages; however, these were not statistically significant due to high variability. Spherocytes were non-significantly increased in all PPE dose groups in comparison with the control group.
Table 2.
Selected blood chemistry parameters in male dogs dosed with PPE in the 13-week follow up study.
| ALT (IU/L) | AST (IU/L) | Total Bilirubin (mg/dL) | Globulin (g/dL) | IgG (mg/dL) | IgA (mg/dL) | Histamine (ng/mL) | |
|---|---|---|---|---|---|---|---|
| Control –Fasted Group | 37 ± 5.1 | 39 ± 6.1 | 0.06 ± 0.012 | 1.4 ± 0.17 | 927 ± 323.9 | 46 ± 13.5 | 0.12 ± 0.035 |
| Lot A Fasted - 200 mg/kg/day | 29 ± 3.6 | 34 ± 1.5 | 0.08 ± 0.012 | 1.5 ± 0.06 | 963 ± 126.6 | 46 ± 10.8 | 0.29 ± 3.6 |
| Lot A Fed - 200 mg/kg/day | 52 ± 34.2 | 33 ± 2.6 | 0.07 ± 0.006 | 1.7 ± 0.12 | 1075 ± 373.3 | 39 ± 6.4 | 1.15 ± 1.193 |
| Lot B Fasted- 200 mg/kg/day | 53 ± 10.8 | 90 ± 84.3 | 0.11 ± 0.006a | 2.5 ± 1.24 | 1950 ± 1602.3 | 191 ± 241.6 | 0.96 ± 1.124 |
| Lot C Fasted - 176 mg/kg/day | 123 ± 146.4 | 39 ± 3.6 | 0.09 ± 0.026 | 1.9 ± 0.31 | 1250 ± 458.3 | 42 ± 1.2 | 0.58 ± 0.433 |
p < 0.05 versus control group
Histopathology revealed some limited signs of toxicity. In liver, hematopoiesis, characterized by the presence of immature granulocytes and erythrocyte precursors in perivascular regions, was seen in the lot B and lot C dose groups. Pigmented macrophages, occurring as 2-5 cell foci of pigmented Kupffer cells in and adjacent to foci of hematopoietic cells, were also seen in the lot B and lot C dose groups. In sternal bone marrow, hyperplasia, characterized by a lack of lipid cells in the bone marrow due to the presence of both granulocytic and erythrocytic precursor cells, was observed in the lot B and lot C dose groups. In spleen, erythropoiesis, characterized by the presence of foci of amphoteric nucleated erythrocyte precursor cells in the red pulp, was seen in one male from lot B dose group. These findings were interpreted as consistent with a history of recent hemolytic blood loss. There were no qualitative differences in bone marrow cells and no clear treatment-related quantitative differences in group mean results for marrow differential counts in any Polyphenon E-treated group when compared to the control group.
3.3. Exposure to PPE
AUC and Cmax values were used as indices of exposure to the four major PPE components. AUC values for free and total EGCG, EGC, ECG and EC following a 13-week treatment with 200 mg/kg/day of PPE are shown in Table 4 and 5 for the chronic and the follow-up study, respectively. In general, the mean AUC values were similar between the two studies and different lots and not statistically different due to large standard deviations. Administration of the clinically formulated lot (C) resulted in most consistent plasma levels of free and total catechins (Table 5). On the other hand, the older lot (A) yielded most variable plasma levels of free and total catechins. Administration of PPE to non-fed animals resulted in approximately 2- to 4-fold increase in AUC for free and total PPE components relative to fed animals being administered the same dose and lot (Table 6). Markedly larger exposure in a fasted as opposed to a fed state was consistent with that observed following administration of PPE in human volunteers (Chow et al. 2005) and EGCG in dogs (Isbrucker et al. 2006). It is also worthwhile to note that variability in AUC values of all four catechins, free and total, was much less under the fed as opposed to fasted conditions (Table 6).
Table 4.
AUC values for four major components of PPE at 13 weeks in the chronic dog study
| AUC (hr × ng/ml) | ||
|---|---|---|
| FREE | TOTAL | |
| EGCG | 33,651 ± 33,652 | 147,976 ± 169,423 |
| EGC | 2,301 ± 2,022 | 3,437 ± 2904 |
| ECG | 1,524 ± 1794 | 8,046 ± 9666 |
| EC | 867 ± 799 | 4,151 ± 4,079 |
200 mg/kg/day (3602 mg/m2/day) PPE for 13 weeks to fasted dogs.
Values represent mean ± SD for n = 3.
AUC (0-last) where last = 24 hr.
Table 5.
AUC values for four major components of PPE in fasted dogs at 13 weeks in the 13-week follow-up dog study
| AUC (hr × ng/ml) | |||
|---|---|---|---|
| FREE | TOTAL | ||
| EGCG | Lot A | 29,191 ± 21,591 | 123,590 ± 123,330 |
| Lot B | 45,160 ± 33,145 | 118,450 ± 91,381 | |
| Lot C | 41,189 ± 27582 | 123,760 ± 70,649 | |
| EGC | Lot A | 2,144 ± 2,800 | 2,950 ± 3,434 |
| Lot B | 1,765 ± 1,034 | 2,614 ± 995 | |
| Lot C | 1,887 ± 364 | 3,383 ± 1,213 | |
| ECG | Lot A | 3,392 ± 4,321 | 9,071 ± 11,022 |
| Lot B | 2,912 ± 2,190 | 6,663 ± 5,831 | |
| Lot C | 2,587 ± 1,449 | 7,354 ± 4,804 | |
| EC | Lot A | 2820 ± 3843 | 5593 ± 6226 |
| Lot B | 1,898 ± 768 | 4,517 ± 1,036 | |
| Lot C | 1,533 ± 209 | 5,877 ± 1,986 | |
Lot A: 200 mg/kg/day (3846 mg/m2/day) PPE for 13 weeks to fasted dogs.
Lot B: 200 mg/kg/day (3862 mg/m2/day) PPE for 13 weeks to fasted dogs.
Lot C: 176 mg/kg/day (3416 mg/m2/day) PPE for 13 weeks to fasted dogs.
Values represent mean ± SD for n = 3.
AUC (0-last) where last = 24 hr.
Table 6.
AUC values for four major components of PPE at 13 weeks in the 13-week follow-up study in fasted and fed dogs administered lot A
| AUC (hr × ng/ml) | |||
|---|---|---|---|
| FREE | TOTAL | ||
| EGCG | FASTED | 29,191 ± 21,591 | 123,590 ± 123,330 |
| FED | 14,841 ± 4,375 | 32,185 ± 4,938 | |
| EGC | FASTED | 2,144 ± 2,800 | 2,950 ± 3,434 |
| FED | 570 ± 71 | 1,309 ± 235 | |
| EGC | FASTED | 3,392 ± 4,321 | 9,071 ± 11,022 |
| FED | 1,193 ± 312 | 2,271 ± 341 | |
| EC | FASTED | 2820 ± 3843 | 5593 ± 6226 |
| FED | 806 ± 238 | 3905 ± 494 | |
Lot A: 200 mg/kg/day (3846 mg/m2/day) PPE for 13 weeks to fasted dogs.
Values represent mean ± SD for n = 3.
AUC (0-last) where last = 24 hr.
4. Discussion
Green tea extracts have been implicated in control, mitigation or prevention of a number of different diseases or conditions (Cabrera et al. 2006; Cheng 2006; Cooper et al. 2005a, b; Crespy and Williamson 2004; Friedman 2007; Fujiki 2005; Khan and Mukhtar 2007; Pham-Huy et al. 2008; Shankar et al. 2007; Shukla 2007; Zaveri 2006), including in the area of cancer prevention (Lubet et al. 2007; Shukla 2007; Yan et al. 2006; Yang et al. 2007). However, epidemiological studies on cancer preventive activities of green tea extracts have been inconsistent (Higdon and Frei 2003; Yang et al. 2007). Purified and standardized green tea extract, PPE, is undergoing evaluation as a candidate cancer preventive agent http://www.clinicaltrials.gov/ct2/results?term=Polyphenon+E). The Division of Cancer Prevention of the National Cancer Institute is evaluating this substance as a part of public health initiative.
Initial subchronic preclinical toxicology studies with this agent have been done in fed animals and the agent was well tolerated in both rats and dogs (Johnson et al. 1999). In a 13-week oral toxicology study in Beagle dogs administered PPE two hours after feeding, the NOAEL was greater than 600 mg/kg/day of PPE, the highest dose tested. Subsequently, a marked improvement in bioavailability of PPE was demonstrated in fasted human volunteers (Table 7) and it was proposed that dosing should be done under fasting conditions in order to optimize the biological effects of this agent (Chow et al. 2005). Based on this information, chronic preclinical oral toxicology studies have been undertaken in fasted animals. PPE doses of 200, 500 and 1000 mg/kg/day were administered to fasted male and female Beagle dogs. The high dose had to be discontinued on day 9 due to toxicity including mortality in one animal, and dosing was restarted 8 days later at 800 mg/kg/day. In contrast to lack of toxicity in the prior 13-week study in fed Beagle dogs, marked morbidity and mortality were encountered in all PPE-treated groups in fasted dogs. Unscheduled mortality occurred in 16 out of 24 PPE-treated animals. A multitude of PPE-caused toxicities were observed including hematology (decreases in red blood cells, hemoglobin, and hematocrit, increases in white blood cells, neutrophils, monocytes, platelet count), gross pathology (lesions in gastrointestinal tract, lymph nodes, liver, kidney, lung, heart and tonsils), histopathology (epithelial necrosis in gastrointestinal tract, inflammation of the liver including centrilobular necrosis and congestion, renal tubular necrosis, atrophy of reproductive organs, and atrophy and necrosis of hematopoietic tissues) as well as abnormalities in clinical chemistry, coagulation and urinalysis parameters. Due to excessive morbidity and mortality, the study was terminated prematurely at 6.5 months. It was not possible to ascertain mechanisms of the toxicities because of large number of animals in moribund condition and dying at different times in the study, extensive organ and system involvement, and severity of toxicity.
Table 7.
Pharmacokinetic variables of free and total EGCG after oral administration of a single dose of PPE to human volunteers in fasted and fed states *
| AUC (hr × ng/ml) | Cmax (ng/ml) | |||
|---|---|---|---|---|
| FREE | TOTAL | FREE | TOTAL | |
| FASTED | 11,433 ± 6567 a | NA | 3,372 ± 1,651 a | 3,988 ± 2,068 a |
| FED | 4,990 ± 4767 | NA | 924 ± 755 | 872 ± 625 |
Adapted from (Chow et al. 2005)
25.5 mg/kg (998 mg/m2) PPE.
Values represent mean ± SD for n = 10.
Significantly different from the fed condition, p < 0.05.
NA – data not available.
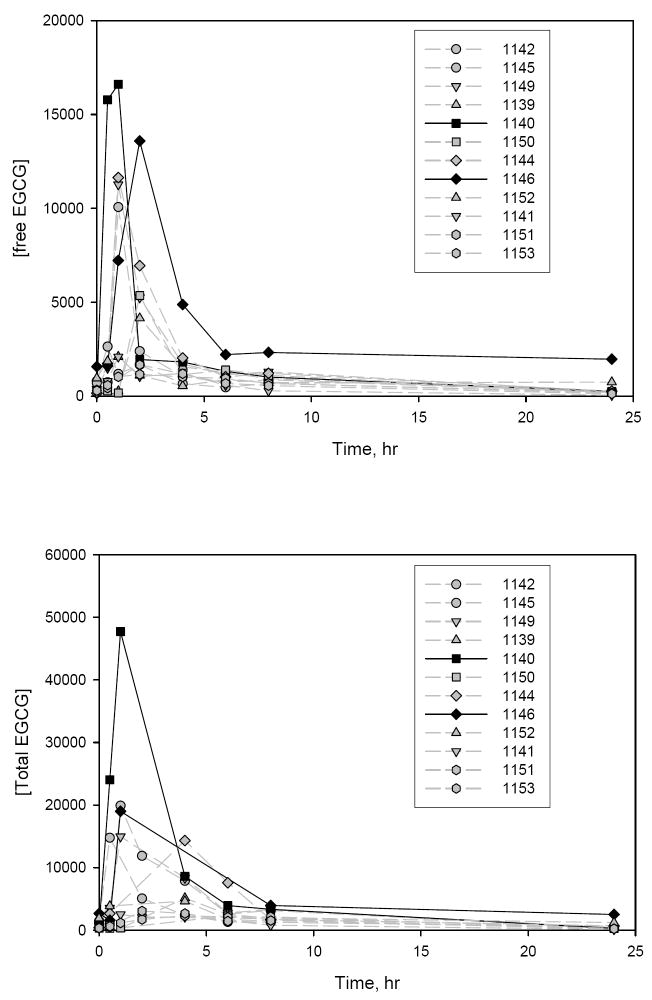
In order to ascertain factors behind these unexpected and severe toxicities encountered with PPE in the chronic dog study, a 13-week follow-up study was initiated. It compared effects of different lots and feeding status on toxicity and exposure in a limited number of male animals treated with the lowest dose, 200 mg/kg, from the chronic study. The same lot used in the chronic study was compared in fed and fasted animals to examine inter-laboratory response and effect of food. Also a newer lot and clinically formulated lot were compared with the older lot to examine lot-to-lot variability and effects of excipients. While there was no mortality in this study, two animals exhibited serious clinical signs of toxicity, one of which was in respiratory distress and unresponsive to touch and had to be revived by oxygen administration. Afterwards until the termination of the study, both animals were clinically unremarkable. It is noteworthy to point out that these two animals had the highest Cmax and AUC values for free and total EGCG, ECG, and EC of all 12 PPE-treated animals in week 4 as shown for EGCG in Fig. 2. Overall, the toxicity was lower with the same dose and during the same time interval in this follow-up study than in the previous chronic study. Many of the same toxicities were seen in at least some animals in the two studies, but to a different extent. Evidence of digestive system disturbances, mild liver damage, moderate to progressive anemia (in individual animals that were not statistically significant between groups), mild disturbances in hematopoiesis (in liver, spleen, bone marrow), and transient ocular symptoms were seen in 1 to 3 animals in one or more PPE-treated groups and were more prevalent and pronounced with lots B and C in the 13-week study. Limited animal group size and high variability in the tested parameters made it difficult to reach conclusions based on statistical significance.
Fig. 2.
Plasma concentration-time profiles for free EGCG (top panel) and total EGCG (bottom panel) in individual dogs at 4 weeks in a 13-week follow-up study (lot A: 200 mg/kg/day (3846 mg/m2/day PPE). Animals 1140 and 1146 exhibited signs of serious clinical toxicity in weeks 8-9.
Administration of PPE in a fed state avoided signs of gastrointestinal irritation and resulted in the lowest exposure of all four catechins; in terms of their free and total plasma AUC levels (Table 1 and 6). It also resulted in a more consistent exposure as evidenced by considerably lesser variability in AUC values for free and conjugated catechins. Lot A yielded lower levels of free EGCG under fasting or fed conditions than lots B and C and this could account for its lower overall toxicity observed in this 13-week study. However, limited sample size and high variability precludes reaching definite conclusions relating toxicity to the exposure.
AUC values for free and total EGCG measured once in week 13 following 200 mg/kg dose in a fasted state were similar between the chronic study and the 13-week follow-up. Differences in toxicities between groups and studies remain unclear although they could be related to differences in exposure at different, unmonitored time points in the studies and sensitivities of different animals. In general, administration of PPE in a fed state results in lower and more consistent exposure with lower manifestation of gastrointestinal irritation.
Exposure, in terms of AUC or Cmax values of free EGCG in fed state, in current clinical trials under the FDA dosing recommendation (see below), is about four fold lower than in the present dog study (Table 8). It is interesting to note that the dose ratio based on the body surface area differs by a similar four-fold factor between these human and dog studies (Table 8).
Table 8.
Pharmacokinetic variables of free EGCG after oral administration of PPE for 4 weeks to dogs and human volunteers in fed states *
| FREE EGCG | ||
|---|---|---|
| STUDY | AUC (hr × ng/ml) | Cmax (ng/ml) |
| DOG | 11,555 ± 2059 | 1,637 ± 466 |
| HUMAN | 2,640 ± 1,497 | 288 ± 124 |
Adapted from (Chow et al. 2003)
Dog (fed): 13-week follow-up study, lot A: 200 mg/kg/day (3846 mg/m2/day) PPE for 4 weeks to dogs, n = 3.
Man (fed): 16.3 mg/kg (651 mg/m2/day) PPE for 4 weeks, n = 8.
Values represent mean ± SD for n = 3.
AUC (0-last) where last = 24 hr.
Mechanisms behind the observed dog toxicities still remain unclear. Regenerative anemia (low levels of RBC, HGB, HCT, reticulocytosis), hematopoiesis and pigmented macrophages in liver, splenic erythropoiesis, bone marrow hyperplasia, increase in average spherocyte counts are possibly suggestive of immune-mediated hemolysis in individual dogs. However, definitive diagnosis would be possible with the use of Coomb’s test which requires fresh red blood cells and they were not available at the time. Statistically significant marginal increases in eosinophil counts in lot B and C groups and increases in IgG and IgA and histamine suggests hypersensitivity. Green tea has been reported to induce histamine release and asthma in tea factory workers (Shirai et al. 2003; Shirai et al. 1997). EGCG has been reported to have an in vitro antifolate activity (Navarro-Peran et al. 2005) which could lead to anemia. However, the present data did not demonstrate a test article- related effect on folate levels. Clinical chemistry tests for pancreatic function (lipase, amylase, insulin, and glucagon) did not reveal consistent or significant drug-related changes. PPE did not affect levels of the markers of oxidative stress (levels of reduced and oxidized glutathione, glutathione peroxidase, lipid peroxidation and total antioxidant activity) in the 13-week study. Therefore, oxidative stress changes seen in some individual dogs in the aborted chronic toxicity study may be attributed to the moribund condition rather than the toxicity of the test article.
Various other toxicities of catechins under various conditions have been reported in literature. Catechins are known to be unstable at neutral and basic pH and undergo oxidation leading to production of reactive oxygen species. EGCG has been reported to have both antioxidant and prooxidative activities, be involved in redox cycling and quinone formation (Sang et al. 2005a) and may induce oxidative stress in vivo (Lambert et al. 2007; Sang et al. 2005b). Cysteine conjugates, indicative of formation of reactive species, have been detected after 200 and 400 mg/kg i.p. EGCG (Sang et al. 2005b). EGCG was also reported to be capable of inducing liver, kidney and gastrointestinal toxicity which seemed to be correlated with bioavailability of EGCG (Isbrucker et al. 2006; Lambert et al. 2007). In addition, EGCG has also been reported to be a low-affinity inhibitor of hERG potassium channels (Kelemen et al. 2007). However, it is unclear if any of these have any relationship to the toxicities noted in the present studies.
In summary, results of these studies seem to allow exclusion of individual content of each of four catechins in different test article lots as a being responsible for dramatic differences in toxicity seen in the preliminary aborted chronic and 13-week studies. Systemic exposure of each of the major four components of PPE was not markedly different when compared under fasting conditions in 13-week and chronic toxicity studies. However, plasma drug levels were only measured on two occasions in each study and it may be possible that plasma profiles may have varied at different times during the studies. The discrepancies in mortality and morbidity between the chronic study and a follow-up 13-week study after dosing with the same dose and lot of drug and with similar systemic exposure levels were not completely resolved in these studies. Other potential confounding factors may be the source of dogs (variability in individual sensitivity), different food, geographical locations of the testing sites, and seasons when the studies were performed.
Recently, there have been a number of published adverse events, including hepatic failure, associated with human use of green tea extracts in Europe for their putative antioxidant and weight loss activities (Bjornsson and Olsson 2007; Bonkovsky 2006; Gloro et al. 2005; Javaid and Bonkovsky 2006; Jimenez-Saenz and Martinez-Sanchez 2007; Jimenez-Saenz and Martinez-Sanchez Mdel 2006; Molinari et al. 2006). These were products other than PPE and tended to be alcoholic as opposed to aqueous extracts and there may have been other extenuating circumstances in the affected individuals. These reports of human toxicity led to restrictions on marketing of these products in Spain and France where these cases took place.
In general, use of standardized green extracts substances has been well tolerated in human clinical trials. Eight-hundred milligrams of EGCG (~ 660 mg PPE/m2) or PPE (equivalent to 8-16 cups of green tea) once a day or in divided doses twice a day administered to healthy non-fasted volunteers was well tolerated over a 4-week treatment period (Chow et al. 2003). Ten day repeated dosing with 800 mg of 94% pure EGCG (~ 660 mg PPE/m2) to healthy volunteers was also well tolerated (Ullmann et al. 2004). In another study, a single oral dose of 1200 mg PPE (~ 1000 mg/m2) resulted in 70% and 20% of subjects showing nausea when dosed in fasting and fed states, respectively (Chow et al. 2005).
In view of human toxicities observed with some green tea extract products overseas and the observed dog toxicities in fasted states, the Division of Drug Oncology Products at FDA has recommended that PPE be taken with food (within one hour of substantial meal) and that the clinical study subjects have liver function tests performed at baseline and repeated every four weeks while on treatment. Following any elevation in alanine aminotransferase (ALT), PPE should be withheld (grade 1 toxicity) or discontinued (grade ≥ 2 toxicity), and liver function monitored until recovery to normal.
Acknowledgments
The work was conducted under contract N01-CN-43306.
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BUN
blood urea nitrogen
- APTT
activated partial thromboplastin time
- AUC
area under the (plasma concentration-time) curve
- Cmax
maximal concentration
- EC
(–)-epicatechin
- ECG
(–)-epicatechin gallate
- EDTA
ethylene diamine tetraacetic acid
- EGC
(–)-epigallocatechin
- EGCG
epigallocatechin gallate
- hERG
human Ether-a-go-go Related Gene
- HGB
hemoglobin
- HCT
hematocrit
- Ig
immunoglobulin
- LC/MS/MS
liquid chromatography with tandem mass spectrometry detection
- MCV
mean cell volume
- MPV
mean platelet volume
- PDW
platelet distribution width
- PPE
Polyphenon E®
- NOAEL
No-Observed-Adverse-Effect-Level
- PT
prothrombin time
- RBC
red blood cells
- RDW
red blood cell distribution width
- WBC
white blood cells
Footnotes
The authors have no personal or financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bjornsson E, Olsson R. Serious adverse liver reactions associated with herbal weight-loss supplements. J Hepat. 2007;47:295–297. doi: 10.1016/j.jhep.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Bonkovsky HL. Hepatotoxicity associated with supplements containing Chinese green tea (Camellia sinensis) Ann of Internal Med. 2006;144:68–71. doi: 10.7326/0003-4819-144-1-200601030-00020. [DOI] [PubMed] [Google Scholar]
- Cabrera C, Artacho R, Gimenez R. Beneficial effects of green tea--a review. J Am Coll Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- Cai Y, Anavy ND, Chow HH. Contribution of presystemic hepatic extraction to the low oral bioavailability of green tea catechins in rats. Drug Metab Dispos. 2002;30:1246–1249. doi: 10.1124/dmd.30.11.1246. [DOI] [PubMed] [Google Scholar]
- Cheng TO. All teas are not created equal: the Chinese green tea and cardiovascular health. Int J Cardiology. 2006;108:301–308. doi: 10.1016/j.ijcard.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, Dorr RT, Hara Y, Alberts DS. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–3319. [PubMed] [Google Scholar]
- Chow HH, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, Celaya CA, Rodney SR, Hara Y, Alberts DS. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin Cancer Res. 2005;11:4627–4633. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- Cooper R, Morre DJ, Morre DM. Medicinal benefits of green tea: Part I. Review of noncancer health benefits. Journal Alternative Complementary Med. 2005a;11:521–528. doi: 10.1089/acm.2005.11.521. [DOI] [PubMed] [Google Scholar]
- Cooper R, Morre DJ, Morre DM. Medicinal benefits of green tea: part II. review of anticancer properties. J Alternative Complementary Med. 2005b;11:639–652. doi: 10.1089/acm.2005.11.639. [DOI] [PubMed] [Google Scholar]
- Crespy V, Williamson G. A review of the health effects of green tea catechins in in vivo animal models. J Nutr. 2004;134:3431S–3440S. doi: 10.1093/jn/134.12.3431S. [DOI] [PubMed] [Google Scholar]
- Elmore SA. Enhanced histopathology of the thymus. Toxicol Pathol. 2006;34:656–665. doi: 10.1080/01926230600865556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol Nutr Food Res. 2007;51:116–134. doi: 10.1002/mnfr.200600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki H. Green tea: Health benefits as cancer preventive for humans. Chem Rec. 2005;5:119–132. doi: 10.1002/tcr.20039. [DOI] [PubMed] [Google Scholar]
- Gloro R, Hourmand-Ollivier I, Mosquet B, Mosquet L, Rousselot P, Salame E, Piquet MA, Dao T. Fulminant hepatitis during self-medication with hydroalcoholic extract of green tea. Eur J Gastroenterol Hepatol. 2005;17:1135–1137. doi: 10.1097/00042737-200510000-00021. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Critical Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- Isbrucker RA, Edwards JA, Wolz E, Davidovich A, Bausch J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: dermal, acute and short-term toxicity studies. Food Chem Toxicol. 2006;44:636–650. doi: 10.1016/j.fct.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Javaid A, Bonkovsky HL. Hepatotoxicity due to extracts of Chinese green tea (Camellia sinensis): a growing concern. J Hepatol. 2006;45:334–335. doi: 10.1016/j.jhep.2006.05.005. author reply 335-336. [DOI] [PubMed] [Google Scholar]
- Jimenez-Saenz M, Martinez-Sanchez Mdel C. Acute hepatitis associated with the use of green tea infusions. J Hepatol. 2006;44:616–617. doi: 10.1016/j.jhep.2005.11.041. [DOI] [PubMed] [Google Scholar]
- Jimenez-Saenz M, Martinez-Sanchez MDC. Camellia sinensis liver toxicity. Journal of Hepatology. 2007;47:297–298. [Google Scholar]
- Johnson WD, Morrissey RL, Crowell JA, McCormick DL. Subchronic oral toxicity of green tea polyphenols in rat and dogs. Toxicol Sci. 1999;48(1 Suppl):57–58. abst. No. 271. [Google Scholar]
- Kelemen K, Kiesecker C, Zitron E, Bauer A, Scholz E, Bloehs R, Thomas D, Greten J, Remppis A, Schoels W, Katus HA, Karle CA. Green tea flavonoid epigallocatechin-3-gallate (EGCG) inhibits cardiac hERG potassium channels. Biochem Biophys Res Commun. 2007;364:429–435. doi: 10.1016/j.bbrc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007;81:519–533. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JD, Sang S, Yang CS. Possible Controversy over Dietary Polyphenols: Benefits vs Risks. Chem Res Toxicol. 2007;20:583–585. doi: 10.1021/tx7000515. [DOI] [PubMed] [Google Scholar]
- Lubet RA, Yang CS, Lee MJ, Hara Y, Kapetanovic IM, Crowell JA, Steele VE, Juliana MM, Grubbs CJ. Preventive effects of polyphenon E on urinary bladder and mammary cancers in rats and correlations with serum and urine levels of tea polyphenols. Mol Cancer Ther. 2007;6:2022–2028. doi: 10.1158/1535-7163.MCT-07-0058. [DOI] [PubMed] [Google Scholar]
- Molinari M, Watt KD, Kruszyna T, Nelson R, Walsh M, Huang WY, Nashan B, Peltekian K. Acute liver failure induced by green tea extracts: case report and review of the literature. Liver Transpl. 2006;12:1892–1895. doi: 10.1002/lt.21021. [DOI] [PubMed] [Google Scholar]
- Navarro-Peran E, Cabezas-Herrera J, Garcia-Canovas F, Durrant MC, Thorneley RN, Rodriguez-Lopez JN. The antifolate activity of tea catechins. Cancer Res. 2005;65:2059–2064. doi: 10.1158/0008-5472.CAN-04-3469. [DOI] [PubMed] [Google Scholar]
- Pham-Huy L, He H, Pham-Huy C. Green tea and health: An overview. J Food Agr Env. 2008;6:6–13. [Google Scholar]
- Sang S, Hou Z, Lambert JD, Yang CS. Redox Properties of Tea Polyphenols and Related Biological Activities. Antioxidants & Redox Signaling. 2005a;7:1704–1714. doi: 10.1089/ars.2005.7.1704. [DOI] [PubMed] [Google Scholar]
- Sang S, Lambert JD, Hong J, Tian S, Lee MJ, Stark RE, Ho CT, Yang CS. Synthesis and Structure Identification of Thiol Conjugates of (-)-Epigallocatechin Gallate and Their Urinary Levels in Mice. Chem Res Toxicol. 2005b;18:1762–1769. doi: 10.1021/tx050151l. [DOI] [PubMed] [Google Scholar]
- Shankar S, Ganapathy S, Srivastava RK. Green tea polyphenols: biology and therapeutic implications in cancer. Front Biosci. 2007;12:4881–4899. doi: 10.2741/2435. [DOI] [PubMed] [Google Scholar]
- Shirai T, Reshad K, Yoshitomi A, Chida K, Nakamura H, Taniguchi M. Green tea-induced asthma: relationship between immunological reactivity, specific and non-specific bronchial responsiveness. Clin Exp Allergy. 2003;33:1252–1255. doi: 10.1046/j.1365-2222.2003.01744.x. [DOI] [PubMed] [Google Scholar]
- Shirai T, Sato A, Chida K, Hayakawa H, Akiyama J, Iwata M, Taniguchi M, Reshad K, Hara Y. Epigallocatechin gallate-induced histamine release in patients with green tea-induced asthma. Ann Allergy Asthma Immunol. 1997;79:65–69. doi: 10.1016/S1081-1206(10)63087-6. [DOI] [PubMed] [Google Scholar]
- Shukla Y. Tea and cancer chemoprevention: A comprehensive review. Asian Pacific J Cancer Prev. 2007;8:155–166. [PubMed] [Google Scholar]
- Ullmann U, Haller J, Decourt JD, Girault J, Spitzer V, Weber P. Plasma-kinetic characteristics of purified and isolated green tea catechin epigallocatechin gallate (EGCG) after 10 days repeated dosing in healthy volunteers. Int J Vitam Nutr Res. 2004;74:269–278. doi: 10.1024/0300-9831.74.4.269. [DOI] [PubMed] [Google Scholar]
- Walle T. Methylation of Dietary Flavones Greatly Improves Their Hepatic Metabolic Stability and Intestinal Absorption. Mol Pharmaceutics. 2007;4:826–832. doi: 10.1021/mp700071d. [DOI] [PubMed] [Google Scholar]
- Yan Y, Wang Y, Tan Q, Hara Y, Yun TK, Lubet RA, You M. Efficacy of polyphenon E, red ginseng, and rapamycin on benzo(a)pyrene-induced lung tumorigenesis in A/J mice. Neoplasia. 2006;8:52–58. doi: 10.1593/neo.05652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: Molecular mechanisms and human relevance. Toxicol App Pharmacol. 2007;224:265–273. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006;78:2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]