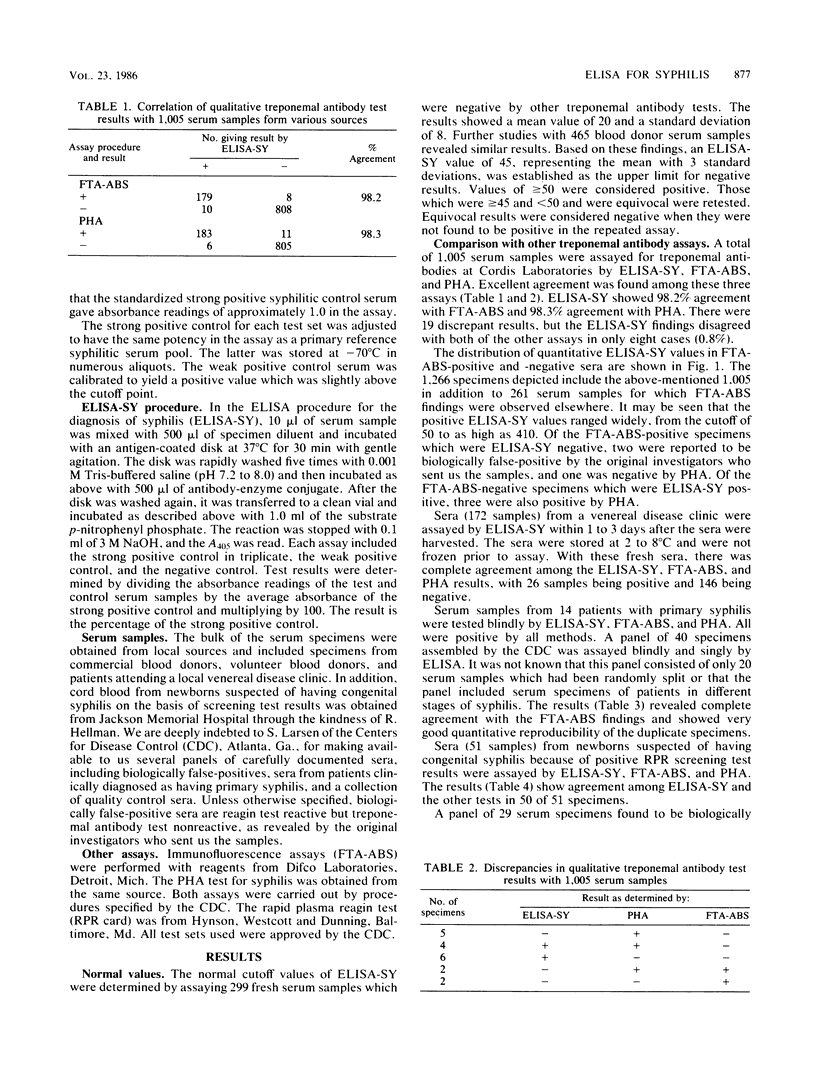
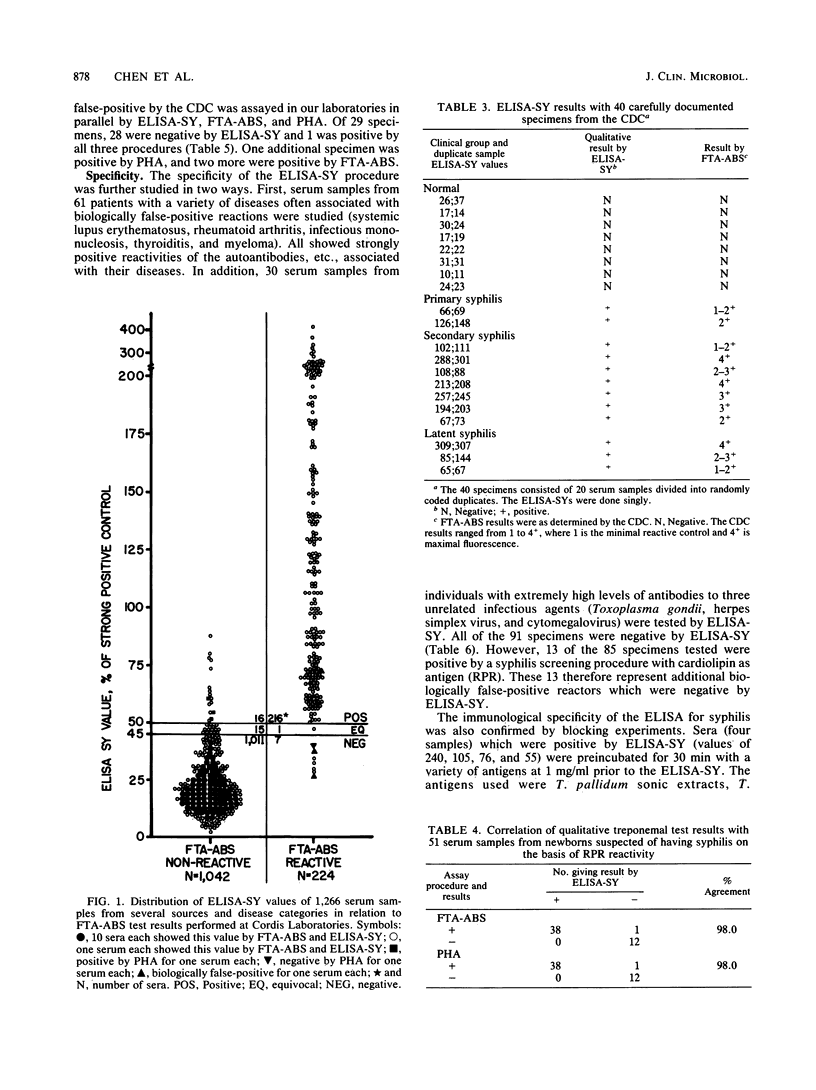
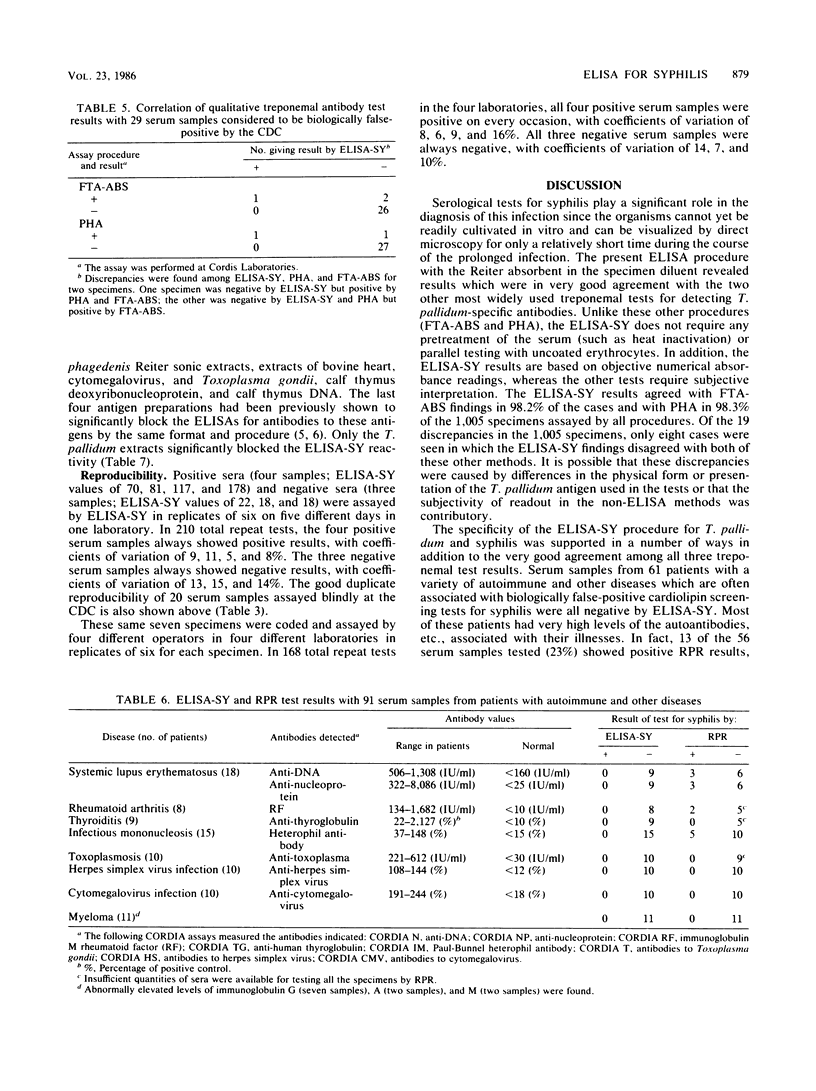
Abstract
An enzyme immunoassay for the diagnosis of syphilis (ELISA-SY) was developed with solid-phase extracts of Treponema pallidum, specimen diluent containing Reiter treponeme absorbent, and three 30-min incubations. The ELISA-SY results were determined in comparison with a standardized positive control and reported as a percentage of strong positive control. In tests with 1,005 serum samples from a venereal disease clinic and other sources, 98.2% agreement was found with fluorescent treponemal antibody-absorption (FTA-ABS) results, and 98.3% agreement was found with T. pallidum passive hemagglutination (PHA) findings. Only 1 of 29 sera originally considered to be biologically false-positive by ELISA-SY; the latter specimen was also positive by PHA and FTA-ABS tests performed in our laboratories. Serum samples from clinically diagnosed syphilitics (16 primary-stage isolates, 7 secondary-stage isolates, and 3-latent-stage isolates) were all positive by ELISA-SY, FTA-ABS, and PHA. Serum samples from 51 newborns suspected of having syphilis on the basis of positive cardiolipin flocculation tests showed 98% agreement of ELISA-SY results with FTA-ABS and PHA findings. Sera from all 61 patients with a variety of autoimmune and other diseases known to be associated with biologically false-positive reactions for syphilis were negative by this ELISA-SY. The specificity of the ELISA procedure for T. pallidum antibody was also confirmed immunologically by blocking experiments.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwood W. G., Miller J. L. Fluorescent treponemal antibodies in fractionated syphilitic sera. The immunoglobulin class. Arch Dermatol. 1969 Dec;100(6):763–769. [PubMed] [Google Scholar]
- Catt K., Niall H. D., Tregear G. W. A solid phase disc radioimmunoassay for human growth hormone. J Lab Clin Med. 1967 Nov;70(5):820–830. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Farshy C. E., Hunter E. F., Larsen S. A., Cerny E. H. Double-conjugate enzyme-linked immunosorbent assay for immunoglobulins G and M against Treponema pallidum. J Clin Microbiol. 1984 Dec;20(6):1109–1113. doi: 10.1128/jcm.20.6.1109-1113.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert S. P., Anken M. Detection of hepatitis B surface antigen (HBS Ag) with use of alkaline phosphatase-labeled antibody to HBS Ag. J Infect Dis. 1977 Oct;136 (Suppl):S318–S323. doi: 10.1093/infdis/136.supplement_2.s318. [DOI] [PubMed] [Google Scholar]
- Hunter E. F., Farshy C. E., Liska S. L., Cruce D. D., Crawford J. A., Feeley J. C. Sodium desoxycholate-extracted treponemal antigen in an enzyme-linked immunosorbent assay for syphilis. J Clin Microbiol. 1982 Sep;16(3):483–486. doi: 10.1128/jcm.16.3.483-486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. M., Chin-See M. W., Halbert S. P. The stability of prostatic acid phosphatase, as measured by a capture immunoenzyme assay. Clin Chim Acta. 1984 Mar 27;138(1):73–86. doi: 10.1016/0009-8981(84)90355-3. [DOI] [PubMed] [Google Scholar]
- Müller F., Lindenschmidt E. G. Demonstration of specific 19S(IgM) antibodies in untreated and treated syphilis. Comparative studies of the 19S(IgM)-FTA test, the 19S(IgM)-TPHA test, and the solid phase haemadsorption assay. Br J Vener Dis. 1982 Feb;58(1):12–17. doi: 10.1136/sti.58.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill P., Nicol C. S. IgM class antitreponemal antibody in treated and untreated syphilis. Br J Vener Dis. 1972 Dec;48(6):460–463. doi: 10.1136/sti.48.6.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope V., Hunter E. F., Feeley J. C. Evaluation of the microenzyme-linked immunosorbent assay with Treponema pallidum antigen. J Clin Microbiol. 1982 Apr;15(4):630–634. doi: 10.1128/jcm.15.4.630-634.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout G. W., Kellogg D. S., Jr, Falcone V. H., McGrew B. E., Lewis J. S. Preparation and standardization of the sorbent used in the fluorescent treponemal antibody-absorption (FTA-ABS) test. Health Lab Sci. 1967 Jan;4(1):5–8. [PubMed] [Google Scholar]
- Van Weemen B. K., Schuurs A. H.W.M. Immunoassay using antigen-enzyme conjugates. FEBS Lett. 1971 Jun 24;15(3):232–236. doi: 10.1016/0014-5793(71)80319-8. [DOI] [PubMed] [Google Scholar]
- Veldkamp J., Visser A. M. Application of the enzyme-linked immunosorbent assay (ELISA) in the serodiagnosis of syphilis. Br J Vener Dis. 1975 Aug;51(4):227–231. doi: 10.1136/sti.51.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53(1):55–65. [PMC free article] [PubMed] [Google Scholar]


