Summary
Tendons and ligaments mediate the attachment of muscle to bone and of bone to bone to provide connectivity and structural integrity in the musculoskeletal system. We show that TGFβ signaling plays a major role in the formation of these tissues. TGFβ signaling is a potent inducer of the tendon progenitor (TNP) marker scleraxis both in organ culture and in cultured cells, and disruption of TGFβ signaling in Tgfb2-/-;Tgfb3-/- double mutant embryos or through inactivation of the type II TGFβ receptor (TGFBR2; also known as TβRII) results in the loss of most tendons and ligaments in the limbs, trunk, tail and head. The induction of scleraxis-expressing TNPs is not affected in mutant embryos and the tendon phenotype is first manifested at E12.5, a developmental stage in which TNPs are positioned between the differentiating muscles and cartilage, and in which Tgfb2 or Tgfb3 is expressed both in TNPs and in the differentiating muscles and cartilage. TGFβ signaling is thus essential for maintenance of TNPs, and we propose that it also mediates the recruitment of new tendon cells by differentiating muscles and cartilage to establish the connections between tendon primordia and their respective musculoskeletal counterparts, leading to the formation of an interconnected and functionally integrated musculoskeletal system.
Keywords: TGFβ, Connective tissue, Ligaments, Tendons, Mouse
INTRODUCTION
Tendons transmit the force generated by muscle contraction to the skeleton, thereby determining the specific connections between muscles and their skeletal insertions (Benjamin et al., 2008). Recent studies identified scleraxis (Scx), a bHLH transcription factor gene, as a unique marker for the tendon cell fate (Cserjesi et al., 1995; Schweitzer et al., 2001), and used Scx expression to describe tendon induction and differentiation during embryogenesis (Brent et al., 2003; Schweitzer et al., 2001) (reviewed by Tozer and Duprez, 2005). In somites, tendon progenitors (TNPs) are found in the syndetome, a dorsolateral stripe of the sclerotome at the junction between adjacent myotomes (Brent et al., 2003). In limb buds, TNPs are induced in mesenchyme directly under the ectoderm, in locations that follow the proximal-to-distal outgrowth of the limb bud (Schweitzer et al., 2001); by E12.5, the TNPs align as loosely organized progenitors between the differentiating muscles and corresponding cartilage condensations. The TNPs later condense and differentiate to give rise to overtly distinct tendons by E13.5 (Murchison et al., 2007).
A molecular framework for tendon induction and differentiation is also beginning to emerge. FGF signaling plays an important role in the induction of TNPs (reviewed by Tozer and Duprez, 2005). In somites, FGFs emanate from the myotome to induce adjacent sclerotomal cells to become TNPs (Brent et al., 2003; Brent and Tabin, 2004). In limb buds the source and identity of FGFs that direct the induction of TNPs has not been established to date, but expression of FGF4 has been reported in limb muscles (Edom-Vovard et al., 2002). The subsequent condensation and differentiation of TNPs is dependent on the transcriptional activities of Scx (Murchison et al., 2007).
TGFβs comprise a small subfamily within the TGFβ superfamily (Massague et al., 2000; Shi and Massague, 2003). The regulation and function of TGFβ signaling has been the subject of numerous studies, leading to the targeting in mice of all the unique participants in TGFβ signaling, notably the ligands Tgfb1-3 (Kulkarni et al., 1993; Proetzel et al., 1995; Sanford et al., 1997) and their receptors (Dudas et al., 2006; Oshima et al., 1996). Significantly, TGFβs use a single type II receptor, TGFBR2 (also known as TβRII), which implies that targeted recombination of a conditional allele, Tgfbr2flox, is sufficient for the disruption of all TGFβ signaling, circumventing complications due to redundancy of ligands or receptors (Chytil et al., 2002). The analysis of such mutants has established a role for TGFβ signaling in numerous developmental processes (Dunker and Krieglstein, 2000; Serra and Chang, 2003), including crucial roles in skeletogenesis, the disruption of which can manifest in reduced chondrocyte proliferation, cleft palate, disrupted skeletal boundaries, fused joints and the failure of sternum development (Baffi et al., 2006; Proetzel et al., 1995; Sanford et al., 1997; Seo and Serra, 2007; Spagnoli et al., 2007). TGFβ signaling has also been associated with the connective tissues because of its capacity to induce extracellular matrix (ECM) proteins and an involvement in the development of fibrosis (reviewed by Mauviel, 2005). More recently it was demonstrated that disruption of TGFβ signaling resulted in the loss of Scx expression in cranial tissues, suggesting a role for TGFβ signaling in tendon development (Oka et al., 2008).
We show that disruption of TGFβ signaling results in the loss of most tendons and ligaments - the first demonstration of a molecular activity with an essential role in formation of these tissues. The induction of TNPs was not affected in mutant embryos and tendon loss was apparent only at E12.5, concurrent with the organization of tendon primordia that align between the differentiating muscles and the prechondrogenic mesenchymal condensations. Moreover, we have found that TGFβ signaling is a potent inducer of Scx both in organ culture and in cultured cells, suggesting a role for TGFβ signaling in tendon induction. TGFβ signaling is thus essential for maintenance of the early TNPs, and we propose that it also mediates recruitment of additional tendon cells by the adjacent muscles and cartilage condensations to establish the connections of tendon primordia with these tissues, an essential event for the subsequent differentiation and growth of mature tendons.
MATERIALS AND METHODS
Mice and histology
Existing mouse lines were previously described: Tgfb2 (Sanford et al., 1997), Tgfb3 (Proetzel et al., 1995), Tgfbr2flox (Chytil et al., 2002), Prx1Cre (Logan et al., 2002) and ScxGFP (Pryce et al., 2007). Tgfbr1-/+ mice were generated using the stochastic germline activity in Prx1Cre females (Logan et al., 2002), and the colony was expanded to verify recombination in the germline.
Tgfb2-/-;Tgfb3-/- embryos were retrieved at the expected ratio in harvests performed up to E12.5 (10/164 embryos), but the frequency decreased sharply in later stages (3/297 embryos at E14.5 and older).
In situ hybridization, antibody staining, BrdU and TUNEL assays were performed as previously described (Murchison et al., 2007).
Organ culture
Organ culture was performed as previously described (Zuniga et al., 1999). Embryos were harvested in DMEM and limb buds or trunks were dissected and placed on metal grids in six-well plates containing Nutriated Medium (Zuniga et al., 1999). Affigel beads (BioRad) were soaked in 20 μg/ml TGFβ2 or TGFβ3, or 25 μg/ml hFGF4 recombinant proteins (R&D Systems) for 1 hour on ice and grafted, and the plates were incubated at 37°C, 5% CO2. We found a progressive loss of endogenous mRNAs for E12.5 limbs (but not E10.5 trunks) incubated for 2 hours and longer and therefore limited the duration of these experiments.
Tissue culture
C3H10T1/2 cells (ATCC) were seeded in six-well plates (2.5×106 cells/well) in DMEM-10% FBS; after 24 hours the medium was supplemented with 20 ng/ml TGFβ2 protein (R&D Systems). Activation medium was maintained till harvest or replaced by DMEM-10% FBS after 1 hour. Cells were trypsinized in duplicate, RNA was prepped using RNeasy mini (Qiagen) and 1 μg RNA was used for cDNA synthesis (Invitrogen Superscript III).
Qualitative PCR primers were:
5′ScxExon1a, GAGACGGCGGCGAGAACACCC;
3′ScxExon2a, GCGTGCTCTTGGGGACCTGCG;
TNCExon12-5′, GAACACCGATGCTCTCTACTGACG; and
TNCExon13-3′, ATGTGGGCAGTCCGTTCAGCA.
Quantitative RT-PCR was performed using ABI 7900HT with SYBR green. Results were normalized to GAPDH and four samples were used for each time point. Primers were: Scx-Q5′-1 AGAGACGGCGGCGAGAACAC and Scx-Q3′-2 GTGGGGCTCTCCGTGACTCTTC.
RESULTS
TGFβ signaling is essential for the formation of limb tendons
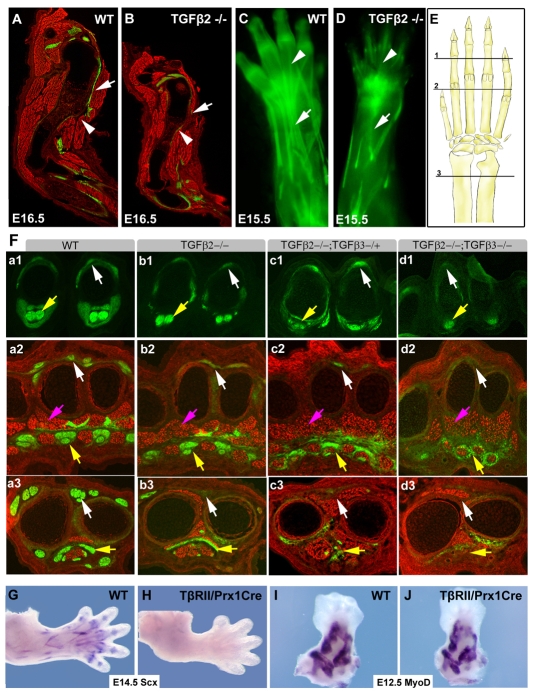
A report of TGFβ gene expression in tendons of chick embryos (Merino et al., 1998) prompted us to check whether the same was true in mouse, and we detected expression of both Tgfb2 and Tgfb3 in limb tendons at E14.5 (Fig. 5A,B). A functional link between TGFβ signaling and limb tendons was recently proposed in an analysis of the role TGFβ signaling plays in the development of the deltoid tuberosity, a lateral outgrowth of the humerus (Seo and Serra, 2007). The deltoid tuberosity was not affected when Tgfbr2flox was targeted in chondrocytes (Baffi et al., 2004), but it did not develop in Tgfb2-/- embryos (Sanford et al., 1997) or when TGFβ signaling was disrupted throughout the limb mesenchyme (Seo and Serra, 2007; Spagnoli et al., 2007). As growth of the deltoid tuberosity is dependent on biomechanical activation (Dysart et al., 1989), it was suggested that the phenotype might be attributed to a loss of muscles or tendons in these mutants (Seo and Serra, 2007). To address this possibility, Tgfb2-/+ mice were bred with the tendon reporter ScxGFP (Pryce et al., 2007). The deltoid tuberosity and deltoid tendon were apparent in sagittal sections through the humerus of a wild-type embryo at E16.5, but missing in sections from a Tgfb2-/- littermate (Fig. 1A,B, white arrowheads and arrows, respectively). Moreover, antibody staining for myosin heavy chain (MHC) revealed that the muscles around the humerus were also significantly reduced in the Tgfb2-/- embryo, suggesting a defect in tendons or muscles as being the underlying cause for the loss of biomechanical activity in Tgfb2-/- embryos.
Fig. 5.
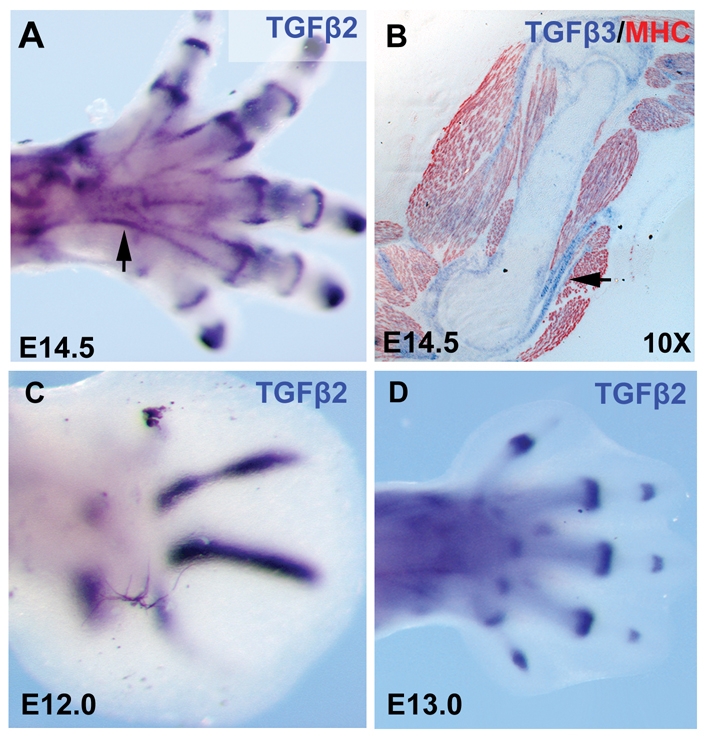
Expression of Tgfb2 and Tgfb3. (A) Whole-mount ISH for Tgfb2 on an E14.5 hindlimb. (B) Section ISH for Tgfb3 followed by immunostaining with antibodies to MHC (red) on a sagittal section through the humerus of an E14.5 embryo. (C,D) Whole-mount ISH for Tgfb2 on forelimbs from embryos at E12.0 (C) and E13.0 (D).
Fig. 1.
Loss of ScxGFP tendon signal in an allelic series of Tgfb2- and Tgfb3-null alleles. Tendons were visualized using the ScxGFP transgenic reporter and sections were counterstained with an MHC antibody. (A,B) Sagittal sections through the forelimbs of an E16.5 wild-type mouse embryo and a Tgfb2-/- littermate. White arrowheads indicate the normal and missing Deltoid tuberosity in A and B, respectively; white arrows indicate the Deltoid tendon and missing tendon in A and B, respectively. (C,D) The extensor tendons of an E15.5 wild-type embryo and a Tgfb2-/- littermate visualized using the ScxGFP tendon reporter. White arrows indicate the extensor digitorium tendon in C and missing extensors in D; white arrowheads indicate extensor tendons in the digits. (E) Schematic drawing of the forelimb. The positions of sections shown in F are marked, 1, digits; 2, metacarpals; 3, proximal to the wrist. (F) Transverse sections through the forelimb of E14.5 embryos from an allelic series of Tgfb2 and Tgfb3 mutant mice. The panels are sub-indexed with a letter to denote the genotype: a, wild type; b, Tgfb2-/-; c, Tgfb2-/-;Tgfb3-/+; d, Tgfb2-/-;Tgfb3-/-. The numeral in each panel denotes the corresponding plane of section in E. White arrows indicate extensor tendons; yellow arrows, flexor tendons; pink arrows, muscles. (G-J) Whole-mount ISH on forelimbs from wild-type and Tgfbr2Prx1Cre embryos with a Scx probe at E14.5 (G,H) and a Myod probe at E12.5 (I,J).
Detection of ScxGFP in whole limbs revealed a more severe tendon phenotype, most of the extensor tendons were missing in the forelimb of E15.5 Tgfb2-/- embryos, but segments of the extensor tendons persisted in the digits (Fig. 1C,D, white arrows and arrowheads, respectively). To study the phenotype in greater detail, we analyzed cross sections through the forelimbs of E14.5 embryos. In digit sections, the extensor tendons appeared similar in wild-type and Tgfb2-/- embryos, but in more proximal positions the extensor tendons were missing or rudimentary in mutant embryos in agreement with the tendon loss we saw in whole limbs (Fig. 1F, a1-b3, white arrows). Surprisingly, the flexor tendons appeared normal in the mutant embryos (Fig. 1F, a1-b3, yellow arrows).
We found expression of Tgfb3 in tendons as well, but tendons were not disrupted in Tgfb3-/- embryos (not shown). However, an allelic series, combining mutations in both TGFβ genes resulted in a dramatic phenotypic series. In Tgfb2-/-;Tgfb3-/+ embryos, the loss of extensor tendons was enhanced relative to the loss in Tgfb2-/- embryos and flexor tendons were severely reduced as well (Fig. 1F, c1-c3, white and yellow arrows, respectively). Remarkably, in double mutant Tgfb2-/-;Tgfb3-/- embryos, no tendons were detected at all limb levels (Fig. 1F, d1-d3), except for a remnant of the flexor profundus tendon in the digit, whose signal is disproportionately enhanced in Fig. 1F, d1. Some ScxGFP-expressing cells were also found at the level of the metacarpals, encircling the muscles in a pattern that was not related to tendons in this position (Fig. 1F, d2).
Double mutant Tgfb2-/-;Tgfb3-/- embryos were retrieved at a very low frequency (see Materials and methods), prompting us to study some aspects of the phenotype using the conditionally targeted Tgfbr2flox allele (Chytil et al., 2002) in conjunction with Prx1Cre (Logan et al., 2002), a limb-specific Cre deletor that shows early activity through the limb mesenchyme, resulting in the targeting of all tendon cells (see Fig. S1 in the supplementary material). This combination leads to a complete disruption of TGFβ signaling in limb mesenchyme and will be denoted Tgfbr2Prx1Cre (Seo and Serra, 2007; Spagnoli et al., 2007). Similar to Tgfb2-/-;Tgfb3-/- embryos, ScxGFP could not be detected in limbs from Tgfbr2Prx1Cre embryos at E14.5 (not shown) and in situ hybridization (ISH) analysis showed a complete loss of Scx expression in these limbs as well (Fig. 1G,H).
Tgfb2-/-;Tgfb3-/- and Tgfbr2Prx1Cre embryos represent a broad disruption of TGFβ signaling, hence we cannot rule out the possibility that the effects on Scx expression were secondary to effects on other tissues. It is, however, important to note that cartilage condensation appeared normal in these mutants (Seo and Serra, 2007), and ISH with a Myod probe showed that early muscle differentiation was not affected either (Fig. 1I,J). Subsequent aspects of skeletal differentiation were, however, affected when TGFβ signaling was disrupted (Seo and Serra, 2007; Spagnoli et al., 2007). Although muscle pattern was not drastically altered, the positions of some muscles were changed and the muscles appeared less compact, with increased spaces between myotubes (Fig. 1F, a2,b2,c2,d2, pink arrows). We did not determine at this time if these effects represent a requirement for TGFβ signaling in muscles or a secondary consequence of the tendon phenotype.
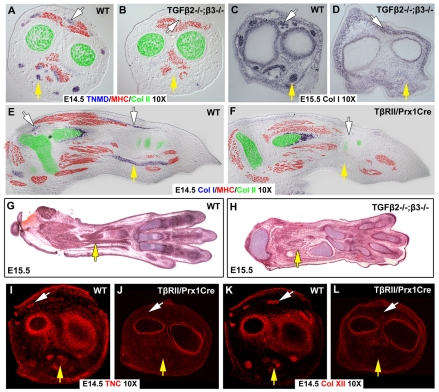
The complete loss of Scx expression in TGFβ signaling mutants prompted us to evaluate the expression of other tendon markers. Expression of tenomodulin (Tnmd), encoding a tendon-specific transmembrane protein (Brandau et al., 2001), could not be detected in Tgfb2-/-;Tgfb3-/- embryos (Fig. 2A,B), nor did we detect expression of Col1a1, the most robust ISH probe in tendon cells, which encodes the most abundant protein in tendons, collagen 1 (Murchison et al., 2007). In transverse sections of forelimbs from a Tgfb2-/-;Tgfb3-/- embryo (Fig. 2C,D), or sagittal sections from a Tgfbr2Prx1Cre forelimb (Fig. 2E,F, white and yellow arrows), the ISH signal for Col1a1 did not highlight tendon cells, even when the ISH reactions were over-developed and other cells, notably osteoblasts, showed a robust Col1a1 signal. Moreover, even in Hematoxylin and Eosin stained sections, in which tendons have a distinct condensed morphology, tendons could not be detected in mutant embryos (Fig. 2G,H, yellow arrow). Finally, in studies of the tendon phenotype in Scx-/- embryos, we found that some tendon rudiments that lost expression of Scx, Tnmd and Col1a1, retained the capacity to attach tissues and expressed tendon matrix proteins such as collagen XII and tenascin C (Murchison et al., 2007). However, in limbs from Tgfbr2Prx1Cre embryos, we could not detect any tendon-related expression of tenascin C or collagen XII (Fig. 2I-L). Taken together, these results imply that tendon cells do not exist in the limbs of embryos in which TGFβ signaling is disrupted, the first mutant combination in which a complete loss of limb tendons is identified.
Fig. 2.
All limb tendons are lost in Tgfb2-/-;Tgfb3-/- and Tgfbr2Prx1Cre mutant embryos. Comparison of tendon markers in sections from the limbs of wild-type and TGFβ signaling mutants. In all panels white arrows indicate extensor tendons, yellow arrows indicate flexor tendons. (A-D) ISH with a Tnmd probe (A,B) and a collagen I probe (C,D) on transverse sections through the zeugopod of wild-type embryos and Tgfb2-/-;Tgfb3-/- littermates at E14.5 and E15.5. Sections in A and B were subsequently stained with antibodies for MHC (red) and collagen II (green). (E,F) ISH with a collagen I probe on sagittal sections through the limbs of an E14.5 wild-type embryo and a Tgfbr2Prx1Cre littermate was followed by antibody staining for MHC (red) and collagen II (green). (G,H) Hematoxylin and Eosin staining of planar sections through limbs of an E15.5 wild-type embryo and a Tgfb2-/-;Tgfb3-/- littermate. (I-L) Antibody staining for tenascin C (I,J) and collagen XII (K,L) on transverse sections through the zeugopod of a wild-type embryo (I,K) and a Tgfbr2Prx1Cre littermate (J,L).
Tendons and ligaments throughout the body are missing in mutants that disrupt TGFβ signaling
The essential role of TGFβ signaling in the development of limb tendons prompted us to examine whether other tendons were also affected when TGFβ signaling was disrupted. Although limb tendons are derived from the lateral plate mesoderm, the axial tendons originate in a distinct somitic domain, the syndetome (Brent et al., 2003), and cranial tendons originate from cranial neural crest cells (Chai et al., 2000; Kontges and Lumsden, 1996). The different embryonic origins of axial, cranial and appendicular tendons suggest that there may also be divergent aspects to the regulation of their differentiation and development (reviewed by Tozer and Duprez, 2005).
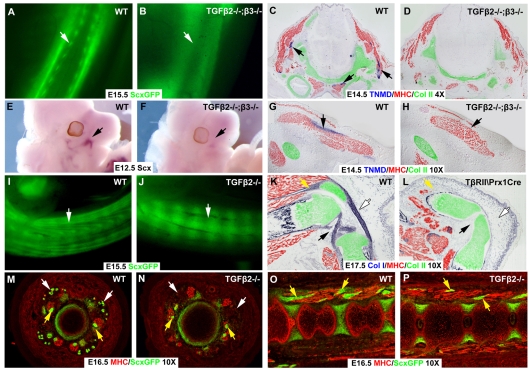
The distinctive structures of trunk tendons seen in wild-type skinned embryos was missing in Tgfb2-/-;Tgfb3-/- littermates (Fig. 3A,B, white arrows). Moreover, in transverse sections through the head of embryos at E14.5, in which staining for collagen II and MHC was used to highlight the neck muscles and cartilage, Tnmd-expressing tendons cells could not be detected in sections from a Tgfb2-/-;Tgfb3-/- embryo (Fig. 3C,D). The same transverse sections extended also through the jaws and the masseter, the major cranial muscle (Fig. 3G,H). Tnmd-expressing tendons anchor the masseter to the jaws, but Tnmd could not be detected next to any of the cranial muscles in the Tgfb2-/-;Tgfb3-/- embryos (Fig. 3G,H, arrows). Interestingly, Scx-expressing progenitors of the masseter tendons could not be detected in heads of Tgfb2-/-;Tgfb3-/- mutant embryos already at E12.5 (Fig. 3E,F, arrows).
Fig. 3.
Tendons and ligaments throughout the body are lost in TGFβ signaling mutants. (A,B) The ScxGFP tendon reporter in trunks of skinned E15.5 wild-type embryos and Tgfb2-/-;Tgfb3-/- littermates. Arrows indicate tendons. (C,D,G,H) Transverse sections through the heads of a wild-type embryo (C,G) and a Tgfb2-/-;Tgfb3-/- littermate (D,H) at E14.5 were processed for ISH for Tnmd, followed by immunostaining with antibodies to MHC (red) and collagen II (green). (C,D) Neck muscles. (G,H) The masseter muscle of the jaw. Arrows indicate actual and missing Tnmd signal. (E,F) Whole-mount ISH for Scx on heads of an E12.5 wild-type embryo and a Tgfb2-/-;Tgfb3-/- littermate. Arrows indicate Scx in the masseter. (I,J) ScxGFP in tails from an E15.5 wild-type embryo and a Tgfb2-/-;Tgfb3-/- littermate. Arrows indicate tendons. (K,L) Sagittal sections through the knees of E17.5 wild-type and Tgfbr2Prx1Cre embryos processed for Col1a1 ISH followed by immunostaining with antibodies to MHC (red) and collagen II (green). Yellow arrows, patellar tendons; white arrows, patellar ligament; black arrows, cruciate ligaments. (M-P) ScxGFP tendon reporter and antibodies to MHC (red) in transverse sections (M,N) and sagittal sections (O,P) through the tails of E16.5 wild-type (M,O) and Tgfb2-/- (N,P) embryos. White arrows, extrinsic tail tendons; yellow arrows, intrinsic muscles and tendons.
The most robust axial tendons in the mouse are the tail tendons that originate in sacral muscles and extend through the tail to insert at the dorsal and ventral sides of tail vertebrae (Fig. 3I, white arrow) (see also Murchison et al., 2007). In cross section, these long tendons appear as peripheral dots in each quadrant of the tail (Fig. 3M, white arrows). Minor aspects of tail movement are regulated by intrinsic muscles that extend across a single vertebra and anchor via short tendons (Fig. 3M,O, yellow arrows) (see also Shinohara, 1999). The long tendons of the tail were entirely missing in Tgfb2-/- mutant embryos (Fig. 3I,J,M,N, white arrows), but the intrinsic muscles of the tail and their tendons were intact (Fig. 3M-P, yellow arrows); these tendons persisted even in double mutant Tgfb2-/-;Tgfb3-/- embryos (not shown).
Ligaments, connecting bones across joints to provide structural integrity during joint movement, are similar to tendons in ultrastructure and cellular composition, suggesting they may also be affected by a disruption of TGFβ signaling. It was recently shown that in Tgfbr2Prx1Cre embryos the joints in the autopod do not undergo cavitation and remain fused (Seo and Serra, 2007; Spagnoli et al., 2007). Ligaments do not form in these joints (not shown), but that may be a secondary consequence to the failure of joint formation. We therefore focused on the knee, in which cavitation does occur in these embryos. ISH with a Col1a1 probe (Fig. 3K,L) on a saggital section through the hindlimb, highlighted the unique features of the knee, the cruciate ligaments that connect the femur and the tibia, and the patella, connected to the tibia with the patellar ligament and connected to the quadricep muscles with a short tendon (Fig. 3L). Limited cavitation could be seen in the knees of Tgfbr2Prx1Cre embryos, but no ligaments or tendons were found in these knees (Fig. 3M). The muscles were also drastically atrophied in the mutant knee, but at E17.5 that was most likely to be a secondary consequence of the loss of tendons. TGFβ signaling is thus the first molecular activity shown to be essential for the formation of almost all tendons and ligaments.
TGFβ signaling is required for TNP expansion and reorganization at E12.5
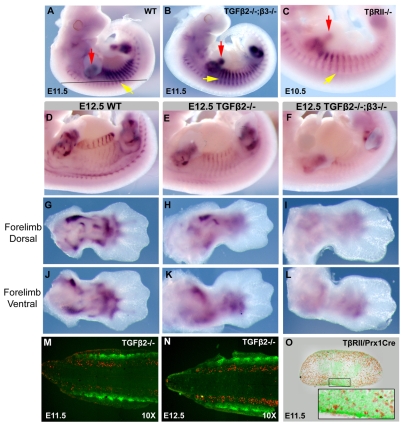
Having established an essential role for TGFβ signaling in tendon genesis, we next wanted to identify the onset of the tendon phenotype in TGFβ signaling mutants. Contrary to the dramatic tendon phenotype described above, at E11.5 Scx expression appeared normal in Tgfbr2Prx1Cre embryos (not shown) and Tgfb2-/-;Tgfb3-/- embryos (Fig. 4A,B). As it is possible that residual TGFβ signaling exists in these mutants, we generated a null allele of the type II receptor, Tgfbr2-/+, by recombining the Tgfbr2flox allele in the germline (see Materials and methods). Tgfbr2-/- embryos die at E10.5 (Oshima et al., 1996), but we found that at E10.5 Scx expression was not affected, even in Tgfbr2-/- embryos (Fig. 4C), demonstrating that the initial induction of TNPs was not dependent on TGFβ signaling.
Fig. 4.
TNPs are lost in TGFβ signaling mutants at E12.5. (A-C) Whole-mount ISH for Scx on E11.5 wild-type (A), E11.5 Tgfb2-/-;Tgfb3-/- (B) and E10.5 Tgfbr2-/- (C) embryos. Yellow arrows, Scx expression in somites; red arrows, Scx expression in limb buds. The black line in A indicates the level of a frontal section through the trunk. (D-L) Whole-mount ISH for Scx on E12.5 whole embryos and dorsal and ventral forelimbs from wild-type (D,G,J), Tgfb2-/- (E,H,K) and Tgfb2-/-;Tgfb3-/- (F,I,L) embryos. (M,N) TUNEL staining (red) superimposed on ScxGFP signal (green) on frontal sections through the back (illustrated in A) of Tgfb2-/- embryos at E11.5 (M) and E12.5 (N). (O) BrdU staining superimposed on a ScxGFP signal in a transverse section through the limb bud of a E11.5 Tgfbr2Prx1Cre embryo. The inset is an enlargement of the boxed ventral Scx-positive domain.
The first indication of tendon loss was seen in mutant embryos at E12.5, Scx expression in the somites of Tgfb2-/- embryos was markedly reduced and was almost completely lost in Tgfb2-/-;Tgfb3-/- embryos (Fig. 4D-F). Interestingly, in limbs of Tgfb2-/- embryos, Scx expression was dramatically reduced on the dorsal side but only partially reduced on the ventral side, corresponding to the loss of extensor but not flexor tendons in Tgfb2-/- embryos at later stages (Fig. 4G,H,J,K). Moreover, Scx expression could hardly be detected in Tgfbr2Prx1Cre limbs (not shown) and Tgfb2-/-;Tgfb3-/- limbs at E12.5 (Fig. 4I,L). The full scope of the tendon phenotype was thus reflected in the loss of TNPs already at E12.5, a stage in which the TNPs undergo expansion and reorganization to form loosely organized tendon primordia between the differentiating muscles and the cartilage condensations.
The dramatic loss of Scx expression between E11.5 and E12.5 could be the result of apoptosis of the TNPs in the absence of TGFβ signaling. However, TUNEL staining on frontal sections from trunks of Tgfb2-/- mutants at E11.5, E12.5 and E13.5, showed no cell death in the ScxGFP-positive TNPs, but robust TUNEL activity in the sclerotome (Fig. 4M,N; data not shown). Ectopic TUNEL labeling was also not found in sections from limb buds of Tgfbr2Prx1Cre embryos at E11.5 and E12.5 (not shown). The loss of Scx-expressing cells could also be caused by a failure of TNP proliferation, but BrdU labeling in ScxGFP-expressing cells appeared normal in limb buds of Tgfbr2Prx1Cre embryos at E11.5 (Fig. 4O). The loss of TNPs in TGFβ mutants is thus not caused by cell loss and is likely to represent a failure in maintenance of the tendon cell fate when TGFβ signaling was disrupted.
TGFβ2 and TGFβ3 are expressed in TNPs, and in the differentiating muscles and cartilage, in early embryonic stages
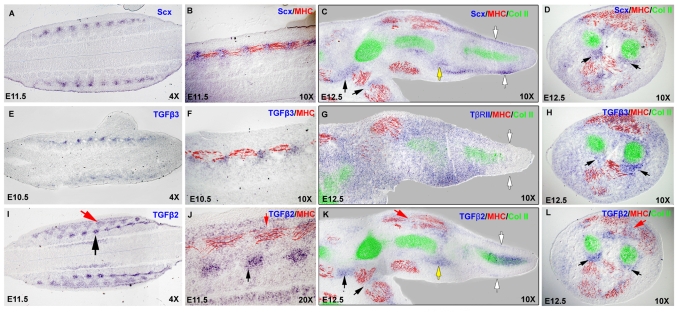
The loss of TNPs in TGFβ signaling mutants prompted us to examine the expression of TGFβ2 and TGFβ3 in the relevant embryonic stages. In somites, Scx is expressed in the syndetome, a dorsolateral stripe at the junction between adjacent myotomes (Brent et al., 2003), which can be conveniently visualized in frontal sections (illustrated in Fig. 4A), wherein the myotomes can be highlighted by antibody staining for MHC expression (see Fig. 6A,B). Expression of Tgfb3 in somites was very faint in embryos between E10.5 and E12.5, but low levels of Tgfb3 were detected in the syndetome (Fig. 6E,F). Interestingly, Tgfb2, which plays a more central role in axial tendon development, was not expressed in the syndetome, although expression was detected in condensed medial domains, the initial sites of cartilage condensation (Fig. 6I,J, black arrows), and in a triangular domain overlapping with MHC expression at the center of the differentiating myotome (Fig. 6I,J, red arrows).
Fig. 6.
Expression of Tgfb2 and Tgfb3 in and around the TNPs. (A-L) Section ISH for Scx (A-D), Tgfb3 (E,F,H), Tgfbr2 (G) and Tgfb2 (I-L) followed by immunostaining with antibodies to MHC (red) and collagen II (green). (A,B,E,F,I,J) Frontal sections through the back (illustrated in Fig. 4A) from embryos at E11.5 (A,B,I,J) and E10.5 (E,F). B, F and J are higher magnification images of A, E and I, respectively. Black arrows, early cartilage condensations; red arrows, myotome. (C,G,K) Sagittal sections thorough the forelimbs of an E12.5 embryo. White arrows, TNPs in the digits; red arrows, expression in muscle; black arrow, TNPs; yellow arrow, the wrist. (D,H,L) Transverse sections through the zeugopod of an E12.5 embryo at the level of the pronator quadratus muscle. Black arrows, TNPs; red arrow, expression in muscle.
In limb buds, Tgfb2 was again expressed in prechondrogenic mesenchymal condensations as they emerge in a proximal to distal progression, resulting in transient expression in digit condensations at E12.0 and a later restriction to the presumptive joints by E13.0 (Fig. 5C,D). Expression of Tgfb2 was also seen in differentiating muscles in the limb buds, but the expression was less distinct than that seen in somites (Fig. 6K,L, red arrows). Finally, a comparison of Tgfb2 and Scx expression in alternating sections at E12.5 showed that Tgfb2 was expressed in some TNPs as well; for example, in the TNPs that connect the pronator quadratus muscle to the radius and ulna (Fig. 5D,L, black arrows), and in other TNPs demarcated more clearly in sagittal sections through limbs at this stage (Fig. 5C,K, black arrows). However, not all TNPs expressed Tgfb2; for instance, the TNPs in the digits, the last TNPs induced at this stage, were positive for Scx but did not express Tgfb2 (Fig. 5C,K, white arrows). Tgfb2 and Scx expression were also overlapping in prospective joints, although in this case Tgfb2 expression was much broader than that of Scx, as seen in the presumptive wrist joint (Fig. 6C,K, yellow arrows). Expression of Tgfb3 in limb buds at these stages was again very faint, but similar to the expression in somites, Tgfb3 transcripts were detected in TNPs, e.g. in the pronator quadratus tendons (Fig. 6H, black arrows).
The combined expression of Tgfb2 or Tgfb3 in the stages relevant for the tendon phenotype therefore encompasses the TNPs, the muscles and the prechondrogenic skeletal condensations, suggesting a possible role for TGFβ signaling in the interaction that is established at this stage between the forming tendons and their musculoskeletal counterparts. In an attempt to identify the cells that can activate TGFβ signaling, we examined the expression of Tgfbr2, finding low and very broad expression in the undifferentiated mesenchyme (Fig. 6G). Interestingly, Tgfbr2 was not expressed at the distal parts of the limb bud, the site of the most recent induction of TNPs at this stage (Fig. 6C,G, white arrows).
Robust induction of tendon markers by TGFβ signaling
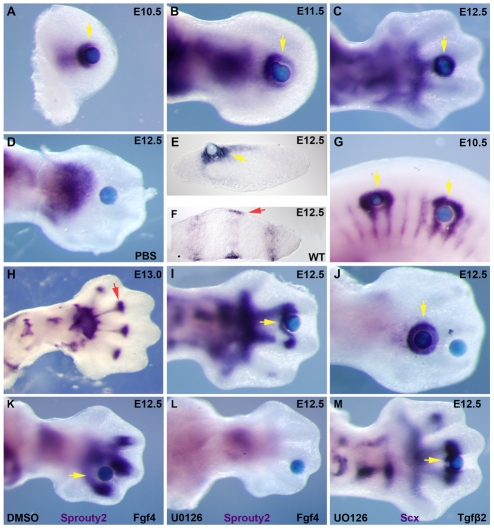
The abrupt loss of Scx expression in TGFβ signaling mutants prompted us to test whether TGFβ signaling has a direct effect on Scx expression. Affigel beads loaded with recombinant TGFβ2 protein were grafted into limb buds in organ culture and caused a robust and highly reproducible induction of Scx expression at all stages from E10.5 to E13.5 (Fig. 7A-D, yellow arrows; data not shown). Scx induction was detected already after 1.5-2 hours of incubation and up to 16 hrs of incubation (not shown), and Scx expression was also induced by beads loaded with recombinant TGFβ3 protein (not shown). Moreover, Scx expression was also induced by grafting TGFβ2-loaded beads in somites of E10.5 embryos (Fig. 7G, yellow arrows).
Fig. 7.
Induction of Scx by TGFβ signaling in organ culture. (A-J) Whole mount ISH for Scx after 4-6 hours of incubation with Affigel beads saturated with 0.02 mg/ml TGFβ2 protein or PBS. Yellow arrows, induced Scx expression; red arrows, endogenous Scx expression. (A-C) TGFβ2 beads in forelimb buds from embryos at E10.5 (A), E11.5 (B) and E12.5 (C). (D) PBS control beads in a forelimb from an embryo at E12.5. (E) Transverse cryosection after Scx whole-mount ISH for an E12.5 limb incubated with a TGFβ2 bead. (F) Section ISH for Scx in a transverse section from an E12.5 wild-type forelimb in a position corresponding to the section in E. (G) TGFβ2 beads in the trunk of an E10.5 embryo. (H) Normal expression of Scx at E13.0. The red arrow highlights the sharp boundary for Scx expression at the metacarpal-phalangeal joint. (I) TGFβ2 beads at the level of the metacarpal-phalangeal joint at E12.5. (J) Distal and proximal TGFβ2 beads in an E12.5 limb. (K,L) Whole-mount ISH for Sprouty2 after a 6-hour incubation with an FGF4-loaded bead in the presence of 50 μM UO126 dissolved in DMSO (L), or just DMSO as control (K). (M) Whole-mount ISH for Scx after a 6-hour incubation with a TGFβ2 bead in the presence of 50 μM UO126.
In limb buds, endogenous Scx expression is induced in mesenchymal cells directly under the ectoderm (Schweitzer et al., 2001), but Scx expression induced by a TGFβ2 bead extended much deeper in the mesenchyme (Fig. 7E,F). Interestingly, Scx induction was limited to undifferentiated mesenchyme and Scx expression was never detected in the ectoderm or prechondrogenic condensations. Endogenous expression of Scx is also constrained in the proximodistal axis, extending at E12.5 only up to the forming metacarpal-phalangeal joint (Fig. 7H, red arrow). Interestingly, a TGFβ2 bead grafted at the level of the metacarpal-phalangeal joint resulted in induction of Scx expression proximal but not distal to the bead (Fig. 7I, yellow arrow), and the introduction of two TGFβ2 beads resulted in robust and symmetric induction of Scx around the proximal bead, but Scx was not induced around the distal bead. The distal restriction of Scx induction by TGFβ-loaded beads is likely to be related to the fact that expression of Tgfbr2 could not be detected in the distal mesenchyme at this stage (Fig. 6G, white arrows).
Scx induction has been previously associated with FGF signaling and has been shown to be regulated by modulation of the MAP kinase cascade (Brent et al., 2003; Brent and Tabin, 2004; Edom-Vovard and Duprez, 2004; Smith et al., 2005). We therefore used UO126, a specific inhibitor of ERK1/2 phosphorylation (Favata et al., 1998; Yamamoto et al., 2003), to investigate whether Scx induction by TGFβ signaling occurred through an indirect activation of the MAP kinase cascade. To verify inhibition of the MAPK cascade, we tested induction of Sprouty2 (Spry2-Mouse Genome Informatics), a common target of FGF signaling (Minowada et al., 1999), and found that the induction of Sprouty2 by a bead loaded with FGF4 protein was completely blocked by the addition of 50 μM UO126 to the medium (Fig. 7K,L). However, induction of Scx by a TGFβ2 bead was not affected in the presence of the same concentration of UO126 (Fig. 7M), demonstrating a MAPK-independent pathway for Scx induction by TGFβ signaling.
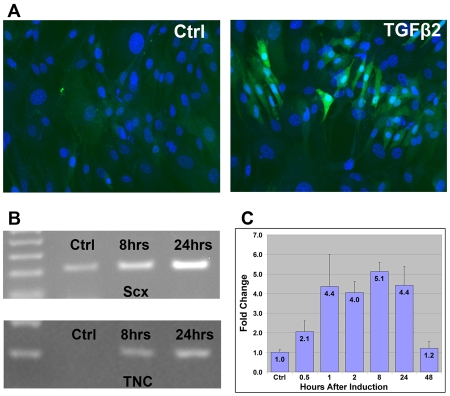
Robust induction of Scx in organ culture led us to test the capacity of TGFβ signaling to induce Scx expression in cultured cells as well. Induction was initially tested in mouse embryonic fibroblasts (MEFs) extracted from ScxGFP embryos. Propagation of ScxGFP MEFs in culture resulted in complete loss of the ScxGFP signal (Fig. 8A), but incubation with 0.3 nM TGFβ2 in the medium for 24 hours resulted in a considerable induction of ScxGFP (Fig. 8A). The ScxGFP signal level was variable in the induced MEFs, highlighting the importance of cellular context for Scx induction. To examine the induction in a more homogenous system, we next tested the effects of TGFβ signaling in C3H10T1/2 cells, a murine cell line considered to represent a mesenchymal progenitor state (Pinney and Emerson, 1989).
Fig. 8.
Induction of early tendon markers by TGFβ signaling in tissue culture. (A) Mouse embryonic fibroblasts (MEFs) from ScxGFP embryos after a 24-hour incubation in culture medium alone (control) or supplemented with 0.3 nM TGFβ2 protein, counterstained with DAPI to detect the cell nuclei (blue). (B) Induction of Scx and tenascin C in C3H10T1/2 cells by TGFβ signaling. C3H10T1/2 cells were incubated in six-well plates with culture medium supplemented with 0.3 nM TGFβ2 protein for 8 hours or 24 hours. Semi-quantitative RT-PCR amplification (25 cycles) was performed to detect the relative levels of mRNA for Scx and tenascin C. (C) Changes in the levels of Scx transcript following a pulse of TGFβ activation. C3H10T1/2 cells incubated in six-well plates were supplemented with 0.3 nM TGFβ2 protein for one hour, after which the cells were washed and returned to regular medium. Cell were harvested at the indicated times after the initiation of the induction. Levels of Scx mRNA were determined by QRT-PCR and normalized to the levels of GAPDH, results of four separate experiments were averaged. Levels of Scx transcript are represented as fold change relative to non-induced cells.
Non-induced C3H10T1/2 cells expressed Scx at a moderate level, and in semi-quantitative RT-PCR we found that addition of TGFβ2 to the medium resulted in a significant increase in Scx mRNA that continued to accumulate up to 24 hours after induction (Fig. 8B). To gain a better appreciation for the dynamics of Scx induction, we next exposed the cells to TGFβ2 just for one hour and monitored Scx expression for up to 48 hours (Fig. 8C). Quantitative RT-PCR of these samples shows induction of Scx expression already 30 minutes after the addition of TGFβ2 and maintenance of high levels of Scx up to 24 hours after induction. The moderate measure of the `fold change' of Scx expression in this experiment is likely to be a numeric reflection of the significant expression of Scx in non-induced cells and not a reflection of absolute levels of Scx induction.
To distinguish between a simple induction of Scx and an induction of TNPs, we wanted to evaluate the expression of other tendon markers following TGFβ activation. Tenascin C, an extracellular matrix protein, is expressed distinctly in tendons (Fig. 2I) (Chiquet-Ehrismann et al., 1991), and the expression has previously been used as a good marker for early tendon cells (Edom-Vovard et al., 2002; Kardon, 1998). The induction of tenascin C by TGFβ signaling has been previously reported (Pearson et al., 1988), and was also detected in our TGFβ2-induced C3H10T1/2 cells (Fig. 7B), but interestingly we did not detect induction of tenascin C by TGFβ beads in organ cultures (not shown), highlighting the importance of the cellular context for these activities.
A small number of other tendon markers have been identified, including Tnmd (Brandau et al., 2001), collagen XII and collagen XIV (Walchli et al., 1994; Young et al., 2000), and mohawk (Anderson et al., 2006). None of these genes has been linked to a progenitor state and some clearly represent a later stage of tendon differentiation (Murchison et al., 2007; Shukunami et al., 2006). The induction of any of these genes by TGFβ signaling could not be detected in organ culture or in C3H10T1/2 cells (not shown), suggesting that additional molecular events following the activation by TGFβ might be required for tendon differentiation.
DISCUSSION
We show that TGFβ signaling plays a major role in the genesis of tendons and ligaments. TGFβ signaling is a potent inducer of tendon markers in mesenchymal cells, and tendons and ligaments are entirely missing in embryos in which TGFβ signaling is disrupted. Our data provide insight but no conclusive answer to two major aspects of TGFβ signaling in tendon genesis. Are the essential TGFβs secreted by the TNPs or from the muscle and cartilage? And does TGFβ signaling promote just maintenance of existing TNPs or also recruitment of new TNPs? The analysis presented below of the phenotypic series of Tgfb2 and Tgfb3 null alleles in the context of the expression of these genes provides a strong argument for an essential role for TGFβs that emanate from the muscles and cartilage, and a more minor role for the TGFβs that originate in the TNPs. Whereas the induction and loss of TNPs in mutant embryos highlights an obvious role for TGFβ signaling in maintenance of TNPs, the involvement of TGFβs that emanate from the interacting tissues implies that other mesenchymal cells are inevitably also exposed to TGFβ signaling and thus are likely to be recruited to the growing tendon primordium, supporting its attachment to the muscles and cartilage (Fig. 9). These findings provide a new framework for understanding the integration and attachment of the forming tendons with their respective muscles and cartilage elements, and present TGFβ signaling as an obvious candidate in efforts to manipulate tendons and ligaments for experimental and clinical purposes.
Fig. 9.
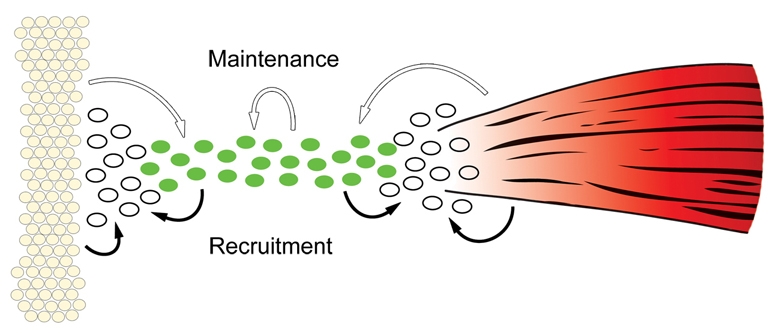
TGFβ signaling promotes maintenance and recruitment of tendon progenitors. Tendon progenitors (green cells) that align between the cartilage condensations (yellow cells) and differentiating muscles (red) at E12.5 are dependent at this stage on TGFβ signaling (white arrows). This essential role for TGFβs from the muscles and cartilage (e.g. TGFβ2 which is expressed exclusively in these tissues in the somites) suggests also an affect on adjacent mesenchymal cells (white), possibly by recruiting them to the tendon cell fate (black arrows). Autocrine activity of TGFβs from the tendon progenitors plays a more minor role in progenitor maintenance. Although the loss of TGFβ3, which is expressed exclusively in tendon progenitors in the somites, does not result in tendon loss, the enhanced phenotype in double mutant Tgfb2-/-;Tgfb3-/- embryos shows that autocrine activity does contribute to progenitor maintenance (white arrow). It is also possible that TGFβs from the tendon progenitors contribute to the recruitment of neighboring mesenchymal cells (black arrows).
Maintenance of the TNP cell fate is dependent on TGFβ signaling
TNPs, identified as Scx-expressing cells, are induced between E9.5 and E12.5 in the syndetome and limb bud mesenchyme, and later differentiate to overtly distinct tendons by E13.5 (Brent et al., 2003; Schweitzer et al., 2001). The tendon phenotype in Tgfb2-/-;Tgfb3-/- and Tgfbr2Prx1Cre embryos highlights a crucial stage in tendon development between E11.5 and E12.5; in mutant embryos, the TNPs appeared normal up to E11.5 and were then lost by E12.5, demonstrating a role for TGFβ signaling in maintenance of the tendon cell fate at this stage. Significantly, the loss of Scx expression was not accompanied by cell death, suggesting that in the absence of TGFβ signaling the progenitors assumed a different cell fate.
Loss of TNPs in slightly later stages has recently been associated with the tendon phenotype in two other mutations. In Scx-/- embryos, TNPs appeared normal up to E12.5, but the progenitors were lost by E13.5 in tendons that failed to differentiate (Murchison et al., 2007). TNP loss was also seen in muscleless limbs in chick embryos (Edom-Vovard and Duprez, 2004; Kardon, 1998), and in muscleless limbs of mouse embryos homozygous for the splotch delayed (Spd) mutation, in which Scx expression in the stylopod and zeugopod was reduced by E12.5 but lost only at E13.5 (Bonnin et al., 2005; Tremblay et al., 1998) (T. J. Riordan and R.S., unpublished). TNP loss in all of these cases was not associated with cell death. Taken together, these results imply that early Scx expression does not represent a commitment to the tendon cell fate and that putative TNPs are dependent on endogenous and exogenous activities to persist on the path of tendon differentiation, including TGFβ signaling at E11.5, a signal from muscles by E12.5 and the transcriptional activities of Scx by E13.5. The emergence of overtly distinct tendons at E13.5 is likely to be associated with differentiation of TNPs to committed tenocytes.
TGFβs from the muscles and cartilage are essential for tendon formation
The robust induction of tendon markers by TGFβ signaling represents an obvious mechanism for maintenance of the tendon cell fate. However, although expression of Tgfb2 or Tgfb3 in the TNPs suggests an autocrine TGFβ function, expression of these genes in the muscles and cartilage suggests an alternative in which TGFβ signaling plays a role in communication between the differentiating tissues of the musculoskeletal system. In somites, Tgfb2 is expressed in the differentiating muscles and cartilage, and Tgfb3 is expressed in the TNPs. The extensive loss of TNPs in the somites and tail of Tgfb2-/- embryos therefore demonstrates that the essential signal for maintenance of the TNPs comes from the muscles and cartilage. Interestingly, although Tgfb3 expression in the TNPs is not sufficient for their maintenance, the accelerated loss of TNPs in Tgfb2-/-;Tgfb3-/- embryos shows that Tgfb3, and by inference signals from the TNPs, also plays a maintenance role (Fig. 9, white arrows).
A similar analysis cannot be applied to the tendon phenotypes in limb buds because of the overlap between the expression domains of TGFβ2 and TGFβ3, but a comparison of the tendon phenotypes in the limbs of TGFβ signaling mutants with the tendon phenotype in muscleless limbs highlights a similar paradigm. In Spd embryos, Scx expression in the limb bud is normal at E11.5, but expression in the presumptive stylopod and zeugopod is decreased at E12.5 and lost completely by E13.5 (Bonnin et al., 2005) (T. J. Riordan and R.S., unpublished). A signal from the muscles, probably TGFβ, is therefore essential for maintenance of the TNPs. The loss of TNPs is, however, accelerated in TGFβ signaling mutants, with complete loss of TNPs already at E12.5, showing that TGFβs from sources other than the muscles, probably the expression of Tgfb2 and Tgfb3 in the TNPs, also contribute to the maintenance of TNP cell fate. We therefore conclude that TGFβs from the muscles and cartilage are essential for tendon formation at E12.5, and that TGFβs from the TNPs also contribute to the maintenance of tendon markers in these cells (Fig. 9, white arrows).
Recruitment of new tendon cells by TGFβ signaling
The robust induction of tendon markers by TGFβ signaling suggests also a possible role in the recruitment of new tendon cells. By E12.5, the TNPs align between the differentiating muscles and cartilage as tendon primordia that later connect with these tissues (Brent et al., 2003) (T. J. Riordan and R.S., unpublished). Dependence of tendon formation on TGFβ signaling is thus concurrent with the integration of the musculoskeletal system, and the essential role of TGFβs from the muscles and cartilage implicates TGFβ signaling in the cross talk between the tissues of the musculoskeletal system. TGFβs from the muscles and cartilage thus inevitably affect adjacent mesenchymal cells as well and are likely to recruit these cells to the tendon cell fate, leading to the generation of a continuous tendon primordium between the muscles and cartilage (Fig. 9, black arrows).
An important implication of this model is the notion that tendons may not be derived exclusively from committed early progenitors. The assertion that early Scx-expressing cells are tendon progenitors was based on the continuity of Scx expression, but a direct lineage from early Scx-expressing cells to all of the tenocytes in mature tendons has not been shown to date (Brent et al., 2003; Schweitzer et al., 2001; Tozer and Duprez, 2005). The results in this study suggest a wave of tendon progenitor recruitment between E11.5 and E12.5, and dynamic expression of the TGFβ genes in tendons through embryogenesis (not shown) suggests that TGFβ signaling may be involved in the recruitment of tendon cells in later stages as well. Continuous recruitment of tendon cells is further supported by the recent identification of progenitor or stem cells for tendons that can be isolated from human and mouse tendons (Bi et al., 2007).
Induction of TNPs by FGF and TGFβ signaling
A pulse of TGFβ signaling in C3H10T1/2 cells led to an early induction of Scx expression that occurred within 30 minutes and which therefore was most likely caused by direct mediators of TGFβ signaling. Elevated levels of Scx persisted for 24 hours after activation, suggesting that Scx expression might also be regulated secondarily by early transcriptional targets of TGFβ signaling. Interestingly, previous studies have shown a role for FGF signaling in the induction of TNPs. Both FGF and TGFβ signaling might thus be involved in tendon induction, and because TGFβ signaling is not essential for the early induction of TNPs, it is possible that the two cascades are essential in complementary phases of tendon induction - early progenitors being induced by FGF signaling and later recruitment of tendon cells being mediated by TGFβ signaling.
Combined functions of TGFβ and FGF signaling that manifest in synergism or epistasis of the two signaling cascades have been reported in a number of developmental and disease processes, including survival of dopaminergic neurons (Roussa et al., 2004), development of lens cataracts (Cerra et al., 2003), chondrocyte proliferation (Mukherjee et al., 2005) and the development of calvarial bones (Sasaki et al., 2006). As both FGF and TGFβ signaling have now been shown to induce early tendon markers, it will be important to establish in future studies the relationships between these signaling cascades in tendon induction.
The role of TGFβ signaling in differentiation of the connective tissues
The connective tissues comprise a heterogeneous group of tissues that combine to generate complex ECM structures, and that have so far received limited attention at the cellular and molecular levels. The fate of connective tissues in mutant or manipulated embryos has not often been addressed, largely owing to the paucity of distinct molecular markers. A possible role for TGFβ signaling in the formation of these tissues was suggested previously because of the capacity of TGFβ signaling to induce the accumulation of ECM proteins (Mauviel, 2005). Indeed, a recent study shows expression of TGFβ isoforms in healing tendons and their capacity to promote tendon healing (Chan et al., 2008). The tendons and ligaments are classified as dense regular connective tissues, and a recent study has demonstrated a disruption of another of these tissues, the annulus fibrosus of the intervertebral disc, when Tgfbr2flox was targeted using the Col2Cre mouse (Baffi et al., 2006). TGFβ signaling might also be involved in the induction or differentiation of other connective tissues. For example, the less compact appearance of muscles in mutant embryos suggests a partial disruption of the connective tissues of the muscles. Finally, the demonstration that the skeletal phenotype in the deltoid tuberosity of Tgfb2-/- mutants was a secondary consequence of the loss of limb tendons and biomechanical stimulation, suggests that unrecognized effects on the development of connective tissues may underlie other phenotypes identified in TGFβ signaling mutants.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/8/1351/DC1
Supplementary Material
The authors thank Tom Doetschman and Harold Moses for mice. This work was supported by a grant from the Shriners Hospitals and by NIH grant R01 AR055640 from NIAMS. Deposited in PMC for release after 12 months.
References
- Anderson, D. M., Arredondo, J., Hahn, K., Valente, G., Martin, J. F., Wilson-Rawls, J. and Rawls, A. (2006). Mohawk is a novel homeobox gene expressed in the developing mouse embryo. Dev. Dyn. 235, 792-801. [DOI] [PubMed] [Google Scholar]
- Baffi, M. O., Slattery, E., Sohn, P., Moses, H. L., Chytil, A. and Serra, R. (2004). Conditional deletion of the TGF-beta type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev. Biol. 276, 124-142. [DOI] [PubMed] [Google Scholar]
- Baffi, M. O., Moran, M. A. and Serra, R. (2006). Tgfbr2 regulates the maintenance of boundaries in the axial skeleton. Dev. Biol. 296, 363-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin, M., Kaiser, E. and Milz, S. (2008). Structure-function relationships in tendons: a review. J. Anat. 212, 211-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, Y., Ehirchiou, D., Kilts, T. M., Inkson, C. A., Embree, M. C., Sonoyama, W., Li, L., Leet, A. I., Seo, B. M., Zhang, L. et al. (2007). Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 13, 1219-1227. [DOI] [PubMed] [Google Scholar]
- Bonnin, M. A., Laclef, C., Blaise, R., Eloy-Trinquet, S., Relaix, F., Maire, P. and Duprez, D. (2005). Six1 is not involved in limb tendon development, but is expressed in limb connective tissue under Shh regulation. Mech. Dev. 122, 573-585. [DOI] [PubMed] [Google Scholar]
- Brandau, O., Meindl, A., Fassler, R. and Aszodi, A. (2001). A novel gene, tendin, is strongly expressed in tendons and ligaments and shows high homology with chondromodulin-I. Dev. Dyn. 221, 72-80. [DOI] [PubMed] [Google Scholar]
- Brent, A. E. and Tabin, C. J. (2004). FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development 131, 3885-3896. [DOI] [PubMed] [Google Scholar]
- Brent, A. E., Schweitzer, R. and Tabin, C. J. (2003). A somitic compartment of tendon progenitors. Cell 113, 235-248. [DOI] [PubMed] [Google Scholar]
- Cerra, A., Mansfield, K. J. and Chamberlain, C. G. (2003). Exacerbation of TGF-beta-induced cataract by FGF-2 in cultured rat lenses. Mol. Vis. 9, 689-700. [PubMed] [Google Scholar]
- Chai, Y., Jiang, X., Ito, Y., Bringas, P., Jr, Han, J., Rowitch, D. H., Soriano, P., McMahon, A. P. and Sucov, H. M. (2000). Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127, 1671-1679. [DOI] [PubMed] [Google Scholar]
- Chan, K.-M., Fu, S.-C., Wong, Y.-P., Hui, W.-C., Cheuk, Y.-C. and Wong, M. W.-N. (2008). Expression of transforming growth factor β isoforms and their roles in tendon healing. Wound Rep. Reg. 16, 399-407. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann, R., Matsuoka, Y., Hofer, U., Spring, J., Bernasconi, C. and Chiquet, M. (1991). Tenascin variants: differential binding to fibronectin and distinct distribution in cell cultures and tissues. Cell Regul. 2, 927-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chytil, A., Magnuson, M. A., Wright, C. V. and Moses, H. L. (2002). Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis 32, 73-75. [DOI] [PubMed] [Google Scholar]
- Cserjesi, P., Brown, D., Ligon, K. L., Lyons, G. E., Copeland, N. G., Gilbert, D. J., Jenkins, N. A. and Olson, E. N. (1995). Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development 121, 1099-1110. [DOI] [PubMed] [Google Scholar]
- Dudas, M., Kim, J., Li, W. Y., Nagy, A., Larsson, J., Karlsson, S., Chai, Y. and Kaartinen, V. (2006). Epithelial and ectomesenchymal role of the type I TGF-beta receptor ALK5 during facial morphogenesis and palatal fusion. Dev. Biol. 296, 298-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker, N. and Krieglstein, K. (2000). Targeted mutations of transforming growth factor-beta genes reveal important roles in mouse development and adult homeostasis. Eur. J. Biochem. 267, 6982-6988. [DOI] [PubMed] [Google Scholar]
- Dysart, P. S., Harkness, E. M. and Herbison, G. P. (1989). Growth of the humerus after denervation. An experimental study in the rat. J. Anat. 167, 147-159. [PMC free article] [PubMed] [Google Scholar]
- Edom-Vovard, F. and Duprez, D. (2004). Signals regulating tendon formation during chick embryonic development. Dev. Dyn. 229, 449-457. [DOI] [PubMed] [Google Scholar]
- Edom-Vovard, F., Bonnin, M. and Duprez, D. (2001). Fgf8 transcripts are located in tendons during embryonic chick limb development. Mech. Dev. 108, 203-206. [DOI] [PubMed] [Google Scholar]
- Edom-Vovard, F., Schuler, B., Bonnin, M. A., Teillet, M. A. and Duprez, D. (2002). Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev. Biol. 247, 351-366. [DOI] [PubMed] [Google Scholar]
- Favata, M. F., Horiuchi, K. Y., Manos, E. J., Daulerio, A. J., Stradley, D. A., Feeser, W. S., Van Dyk, D. E., Pitts, W. J., Earl, R. A., Hobbs, F. et al. (1998). Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273, 18623-18632. [DOI] [PubMed] [Google Scholar]
- Kardon, G. (1998). Muscle and tendon morphogenesis in the avian hind limb. Development 125, 4019-4032. [DOI] [PubMed] [Google Scholar]
- Kontges, G. and Lumsden, A. (1996). Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development 122, 3229-3242. [DOI] [PubMed] [Google Scholar]
- Kulkarni, A. B., Huh, C. G., Becker, D., Geiser, A., Lyght, M., Flanders, K. C., Roberts, A. B., Sporn, M. B., Ward, J. M. and Karlsson, S. (1993). Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA 90, 770-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan, M., Martin, J. F., Nagy, A., Lobe, C., Olson, E. N. and Tabin, C. J. (2002). Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 33, 77-80. [DOI] [PubMed] [Google Scholar]
- Massague, J., Blain, S. W. and Lo, R. S. (2000). TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 103, 295-309. [DOI] [PubMed] [Google Scholar]
- Mauviel, A. (2005). Transforming growth factor-beta: a key mediator of fibrosis. Methods Mol. Med. 117, 69-80. [DOI] [PubMed] [Google Scholar]
- Merino, R., Ganan, Y., Macias, D., Economides, A. N., Sampath, K. T. and Hurle, J. M. (1998). Morphogenesis of digits in the avian limb is controlled by FGFs, TGFbetas, and noggin through BMP signaling. Dev. Biol. 200, 35-45. [DOI] [PubMed] [Google Scholar]
- Minowada, G., Jarvis, L. A., Chi, C. L., Neubuser, A., Sun, X., Hacohen, N., Krasnow, M. A. and Martin, G. R. (1999). Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development 126, 4465-4475. [DOI] [PubMed] [Google Scholar]
- Mukherjee, A., Dong, S. S., Clemens, T., Alvarez, J. and Serra, R. (2005). Coordination of TGF-beta and FGF signaling pathways in bone organ cultures. Mech. Dev. 122, 557-571. [DOI] [PubMed] [Google Scholar]
- Murchison, N. D., Price, B. A., Conner, D. A., Keene, D. R., Olson, E. N., Tabin, C. J. and Schweitzer, R. (2007). Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 134, 2697-2708. [DOI] [PubMed] [Google Scholar]
- Oka, K., Oka, S., Hosokawa, R., Bringas, P., Jr, Brockhoff, H. C., 2nd, Nonaka, K. and Chai, Y. (2008). TGF-beta mediated Dlx5 signaling plays a crucial role in osteo-chondroprogenitor cell lineage determination during mandible development. Dev. Biol. 321, 303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima, M., Oshima, H. and Taketo, M. M. (1996). TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev. Biol. 179, 297-302. [DOI] [PubMed] [Google Scholar]
- Pearson, C. A., Pearson, D., Shibahara, S., Hofsteenge, J. and Chiquet-Ehrismann, R. (1988). Tenascin: cDNA cloning and induction by TGF-beta. EMBO J. 7, 2977-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney, D. F. and Emerson, C. P., Jr (1989). 10T1/2 cells: an in vitro model for molecular genetic analysis of mesodermal determination and differentiation. Environ. Health Perspect. 80, 221-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proetzel, G., Pawlowski, S. A., Wiles, M. V., Yin, M., Boivin, G. P., Howles, P. N., Ding, J., Ferguson, M. W. and Doetschman, T. (1995). Transforming growth factor-beta 3 is required for secondary palate fusion. Nat. Genet. 11, 409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce, B. A., Brent, A. E., Murchison, N. D., Tabin, C. J. and Schweitzer, R. (2007). Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev. Dyn. 236, 1677-1682. [DOI] [PubMed] [Google Scholar]
- Roussa, E., Farkas, L. M. and Krieglstein, K. (2004). TGF-beta promotes survival on mesencephalic dopaminergic neurons in cooperation with Shh and FGF-8. Neurobiol. Dis. 16, 300-310. [DOI] [PubMed] [Google Scholar]
- Sanford, L. P., Ormsby, I., Gittenberger-de Groot, A. C., Sariola, H., Friedman, R., Boivin, G. P., Cardell, E. L. and Doetschman, T. (1997). TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 124, 2659-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, T., Ito, Y., Bringas, P., Jr, Chou, S., Urata, M. M., Slavkin, H. and Chai, Y. (2006). TGFbeta-mediated FGF signaling is crucial for regulating cranial neural crest cell proliferation during frontal bone development. Development 133, 371-381. [DOI] [PubMed] [Google Scholar]
- Schweitzer, R., Chyung, J. H., Murtaugh, L. C., Brent, A. E., Rosen, V., Olson, E. N., Lassar, A. and Tabin, C. J. (2001). Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128, 3855-3866. [DOI] [PubMed] [Google Scholar]
- Seo, H. S. and Serra, R. (2007). Deletion of Tgfbr2 in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints. Dev. Biol. 310, 304-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra, R. and Chang, C. (2003). TGF-beta signaling in human skeletal and patterning disorders. Birth Defects Res. C Embryo Today 69, 333-351. [DOI] [PubMed] [Google Scholar]
- Shi, Y. and Massague, J. (2003). Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113, 685-700. [DOI] [PubMed] [Google Scholar]
- Shinohara, H. (1999). The musculature of the mouse tail is characterized by metameric arrangements of bicipital muscles. Okajimas Folia Anat. Jpn 76, 157-169. [DOI] [PubMed] [Google Scholar]
- Shukunami, C., Takimoto, A., Oro, M. and Hiraki, Y. (2006). Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev. Biol. 298, 234-247. [DOI] [PubMed] [Google Scholar]
- Smith, T. G., Sweetman, D., Patterson, M., Keyse, S. M. and Munsterberg, A. (2005). Feedback interactions between MKP3 and ERK MAP kinase control scleraxis expression and the specification of rib progenitors in the developing chick somite. Development 132, 1305-1314. [DOI] [PubMed] [Google Scholar]
- Spagnoli, A., O'Rear, L., Chandler, R. L., Granero-Molto, F., Mortlock, D. P., Gorska, A. E., Weis, J. A., Longobardi, L., Chytil, A., Shimer, K. et al. (2007). TGF-beta signaling is essential for joint morphogenesis. J. Cell Biol. 177, 1105-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozer, S. and Duprez, D. (2005). Tendon and ligament: development, repair and disease. Birth Defects Res. C Embryo Today 75, 226-236. [DOI] [PubMed] [Google Scholar]
- Tremblay, P., Dietrich, S., Mericskay, M., Schubert, F. R., Li, Z. and Paulin, D. (1998). A crucial role for Pax3 in the development of the hypaxial musculature and the long-range migration of muscle precursors. Dev. Biol. 203, 49-61. [DOI] [PubMed] [Google Scholar]
- Walchli, C., Koch, M., Chiquet, M., Odermatt, B. F. and Trueb, B. (1994). Tissue-specific expression of the fibril-associated collagens XII and XIV. J. Cell Sci. 107, 669-681. [DOI] [PubMed] [Google Scholar]
- Yamamoto, T., Cui, X. M. and Shuler, C. F. (2003). Role of ERK1/2 signaling during EGF-induced inhibition of palatal fusion. Dev. Biol. 260, 512-521. [DOI] [PubMed] [Google Scholar]
- Young, B. B., Gordon, M. K. and Birk, D. E. (2000). Expression of type XIV collagen in developing chicken tendons: association with assembly and growth of collagen fibrils. Dev. Dyn. 217, 430-439. [DOI] [PubMed] [Google Scholar]
- Zuniga, A., Haramis, A. P., McMahon, A. P. and Zeller, R. (1999). Signal relay by BMP antagonism controls the SHH/FGF4 feedback loop in vertebrate limb buds. Nature 401, 598-602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.