Abstract
Objective
To estimate whether prenatal treatment with neuroprotective peptides prevent the developmental delay and the glial deficit in the Ts65Dn mouse model for Down syndrome, and to explore the peptides effects on achievement of normal development.
Methods
Pregnant Ts65Dn females were randomly assigned to NAPVSIPQ+ SALLRSIPA or control and were treated by investigators blinded to treatment and genotype on gestational days 8–12. Offspring were tested from postnatal day (P) 5 to 21 for motor and sensory milestones with standardized tests by operators blinded to the pup’s treatment and genotype. The pup’s genotype was determined after completion of all tests. Activity-dependent-neurotrophic-factor, glial fibrillary acidic protein, and vasoactive intestinal peptide expression were determined using real time polymerase chain reaction.
Results
Trisomic (Ts) mice achieved milestones with a significant delay in 4 of 5 motor and sensory milestones. Ts mice that were prenatally exposed to NAPVSIPQ+SALLRSIPA achieved developmental milestones at the same time as the controls in 3 of 4 motor and 1 of 4 sensory milestones (p<0.01). Euploid pups prenatally treated with NAPVSIPQ+SALLRSIPA achieved developmental milestones significantly earlier than the euploid pups prenatally treated with placebo. Activity-dependent-neurotrophic-factor, expression was significantly downregulated in the Ts65Dn brains versus the controls, prenatal treatment with NAPVSIPQ+SALLRSIPA prevented the activity-dependent-neurotrophic-factor, decrease in the Ts65Dn brains, and the expression was not different from the controls. The glial marker glial fibrillary acidic protein demonstrated the known glial deficit in the Ts65Dn mice, and treatment with NAPVSIPQ+SALLRSIPA prevented its downregulation. Lastly, vasoactive intestinal peptide levels were increased in the Ts brains, while treatment with NAPVSIPQ+SALLRSIPA did not prevent its upregulation.
Conclusions
Prenatal treatment with NAPVSIPQ and SALLRSIPA prevented developmental delay and the glial deficit in Down syndrome. These findings highlight a possibility for the prevention of developmental sequelae in Down syndrome and suggest a potential intervention during pregnancy that may improve the outcome.
Down Syndrome (DS) is the most common genetic cause of mental retardation due to triplication of all or part of chromosome 21(1) and occurs in 1 in 800 live births. In the newborn period, infants with DS have hypotonia and delay in achievement of developmental motor(2) and sensory milestones as well as in olfactory, auditory and visual sensitivity(3,4). At the neuropathological level, neonates with DS have a smaller brain, delayed myelination of neurons and glial alterations(5). These early developmental anomalies may predispose to abnormalities in adulthood including mental retardation and early onset of Alzheimer disease.
The Ts65Dn mouse is a well-characterized model for DS (6), with triplication of a segment of chromosome 16 which includes over 55% of the genes present on human chromosome 21. In the newborn period, Ts65Dn mice mimic the human condition presenting developmental delay in motor and sensory milestones (7). Microscopically, Ts65Dn neonates have fewer granule cells in the hippocampus, reduced Long Term Potentiation (LTP) and abnormal synaptic plasticity.(8, 9 and 10) In adulthood, Ts65Dn mice have a deficit in short and long term memory and learning, and early onset of the neuropathology of Alzheimer disease (7).
Vasoactive intestinal peptide (VIP) levels are altered in DS. Human DS newborns have increased VIP blood levels (11); Ts65Dn mice have elevated brain VIP mRNA (12), and cortical astrocytes from postnatal day 8 brains show a defect in the signal transduction mechanism of the VIP receptor VPAC-1 with astrocyte dysfunction (13). Furthermore, blockade of VIP during embryogenesis is followed by hypotonia, growth and developmental delays (14) in both human DS and Ts65Dn mice.
VIP stimulation of astrocytes results in the release of numerous neurotrophic factors, including Activity Dependent Neuroprotective Protein (ADNP) and Activity Dependent Neurotrophic Factor (ADNF), which have demonstrated neuroprotective proprieties (15). Active fragments of ADNP and ADNF, NAPVSIPQ (NAP) and SALLRSIPA (SAL) respectively, have shown therapeutic potential for developmental delay and learning deficit. Treatment of DS cortical neurons with SAL or NAP resulted in a 2-fold increase in neuronal survival as well as reduction of degenerative morphological changes (16). NAP+SAL are neuroprotective in vivo against diverse neuronal insults, including excitotoxicity, closed head injury, ischemic brain injury, apoplipoprotein E deficiency and alcohol-induced microcephaly, fetal death, growth restriction and learning deficits in fetal alcohol syndrome (15, 17 and 18).
To date, there is no therapy for the prevention of developmental delays in DS (MEDLINE, 1966-June 1, 2008; Keywords: Down syndrome, treatment, development, fetus; all languages). Our hypothesis was that prenatal treatment with NAP+SAL may prevent the developmental delay in the Ts65Dn mouse model for Down syndrome. In addition, since ¼ of the progeny inherits the trisomy, ¾ of the pups treated with the peptides are controls thus, we also sought to explore the peptides effects on achievement of normal development.
Methods
Pregnant Ts65Dn females were randomly assigned to NAP+SAL or control and were treated by investigators blinded to treatment and genotype on gestational days 8–12. This time period was chosen based on previous studies that showed that this is a critical time for VIP action during in utero development (19). Offspring were weighed and tested from postnatal day (P) 5 to 21 for motor and sensory milestones with standardized tests (7) by operators blinded to the pup’s treatment and genotype. The pup’s genotype was determined after completion of all tests.
Ts65Dn female mice (The Jackson Laboratory, Bar Harbor, ME) were kept in a 12-hour light/12-hour dark regimen, with food and water available at all times with 6% fat diet. The mice received humane animal care in compliance with the National Institutes of Health (NIH) guidelines for care and use of experimental animals. The protocol was approved by National Institute of Child Health and Human Development (NICHD) Animal Care and Use Committee. Females were mated with B6EiC3SnFi male mice; the presence of vaginal plug was checked twice daily and the day of its appearance was considered day0 (E0) of pregnancy.
Ts65Dn pregnant females were treated (intraperitoneal) on days E8,9,10,11 and 12 (a typical mouse gestation is 18–21 days long) with saline (placebo; n=6) or peptides(n=4). The researchers providing the treatments and performing the developmental assessments were blinded to the treatments and to the genotyping. The female breeders were eartagged. Treatments were made by a single investigator (CYS) and were labelled with the eartag number. Treatments were given by other investigators (LT and DA). After delivery, the litters were coded and each animal was labelled with standard markings. After completion of the developmental tests, tail tips were collected for genotyping and were sent to Jackson laboratory for genotyping. Treatment was performed with VIP-derived peptides from activity-dependent-neurotrophic-protein (ADNP) and activity-dependent-neurotrophic-factor (ADNF), whose active fragments are, respectively, NAP (20 μg) and SAL (20 μg) (SynPep, Dublin, CA). NAP was diluted in 50 μL of dimethyl sulfoxide and diluted in filtered Dulbecco’s phosphate-buffered saline (DPBS); ADNF-9 was dissolved and diluted in filtered DPBS.
Six Trisomic (Ts)+peptides from 4 litters, 14 Ts+placebo from 5 litters, 18 control+placebo from 6 litters and 9 control+peptides pups from 3 litters, were tested.
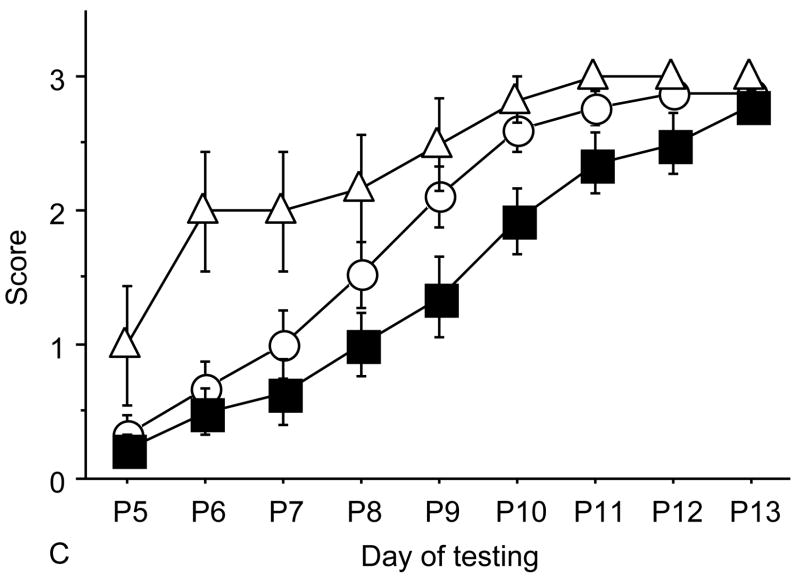
From neonatal days 5 to 21 newborn pups were weighed and tested daily for developmental milestones as previously published(7) by the same operators blinded both to the genotype and the treatment group (Figure 1). These standardized tests assess the achievement of motor and sensory behavior that would be expected for age. Tests were performed on the days behavior was expected to appear based on developmental curves from normal mice(20). A score was given every day for each behavior as follows: 0=behavior not present, 1=behaviour weakly present, 2=first day of behavior presence and 3=second day of behavior presence. Tests included: weak tactile stimulation, assesses the head turning response triggered by the application of tactile stimuli (cotton swab) in the perioral area on both sides of the head and it is a sensory response mediated by the 5th cranial nerve; righting reflex assessess the abilty of the pup to right itself when placed upside down and it is principally a test for labyrintine and body righting mechanism; vibrissa placing reflex, when the mouse is suspended by the tail and lowered so that the vibrissae make contact with a solid object the pup raises its head and the forelimbs to grasp the object, this is principally a measure of neck and forelimbs tone and strenght; forelimb grasp measures the the pup’s ability to flex its forelimbs and grasp a blunt instrument that strikes its fore feet, thus it’s a measure of fore limbs muscle tone and strenght; cliff aversion is the ability of the mouse to turn and crawl away when placed on the edge of a clliff with the fore paws and faces over the edge, this milestone requires intact feet sensitivity and muscle strenght tu turn its body; in audio startle response a loud sharp noise causes an immediate startle response, this is a test for auditory sensisitivity; in eye opening, the day in which the pups open their eyes is recorded; in forelimb placing, contact of the dorsum of the foot against the edge of an object will cause the foot to be raised and placed on the surface of that object when the animal is suspended and no other foot is in contact with a solid surface, this is a test on the placing reflex development with sensory and motor components; ear twitch, tests the twitching of the pup’s ears at the touch with a cotton swab and is a sensitivity test; in screen climbing test pups climb a vertical wire-mesh (5×5mm) screen using both fore- and hind-paws, this is a motor test (7, 20,21) (Figure 4)
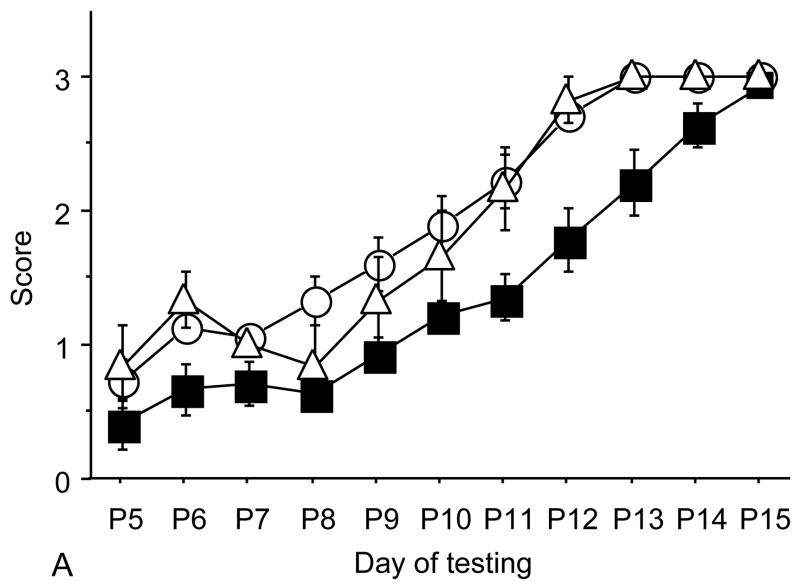
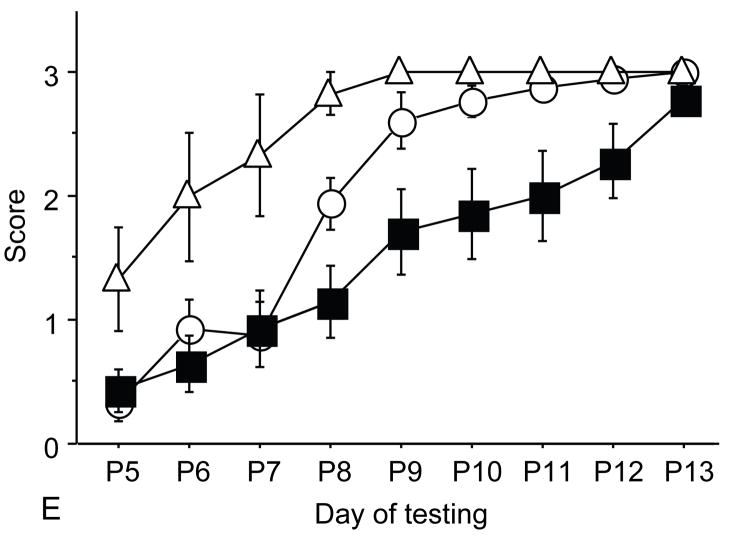
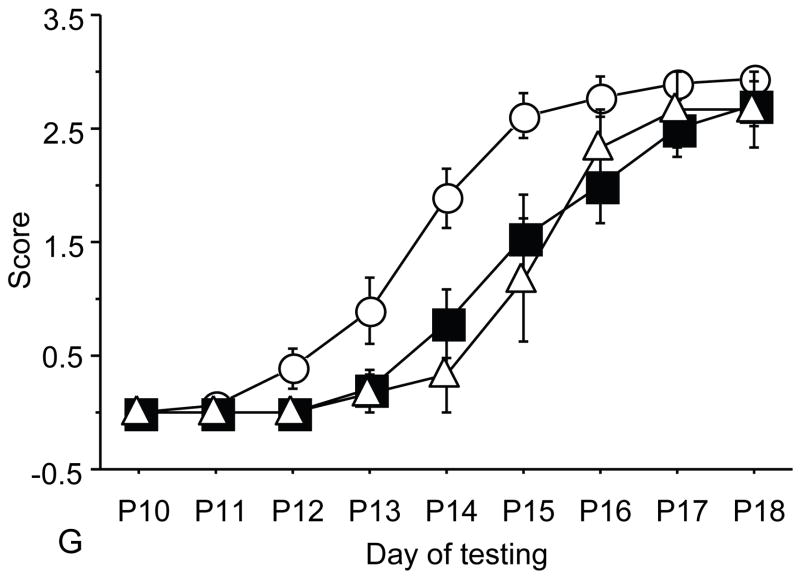
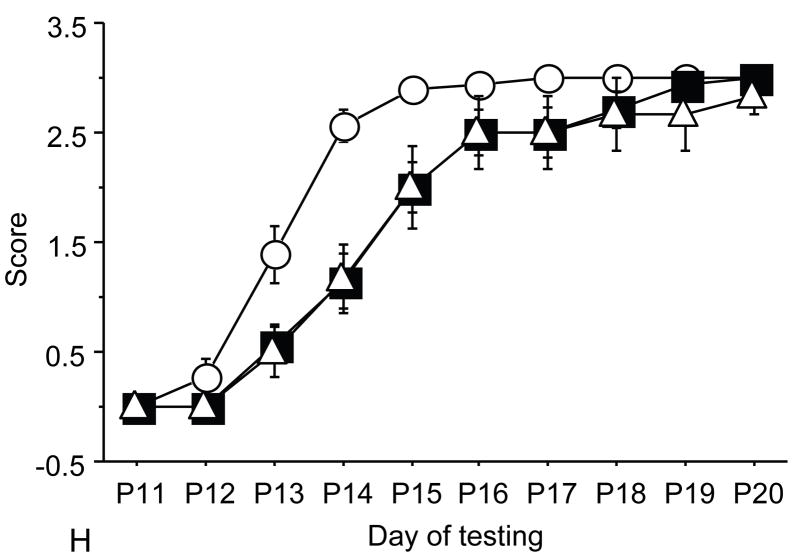
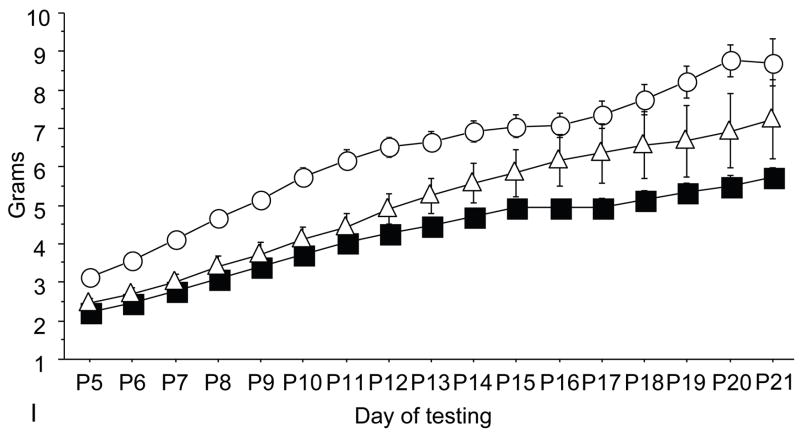
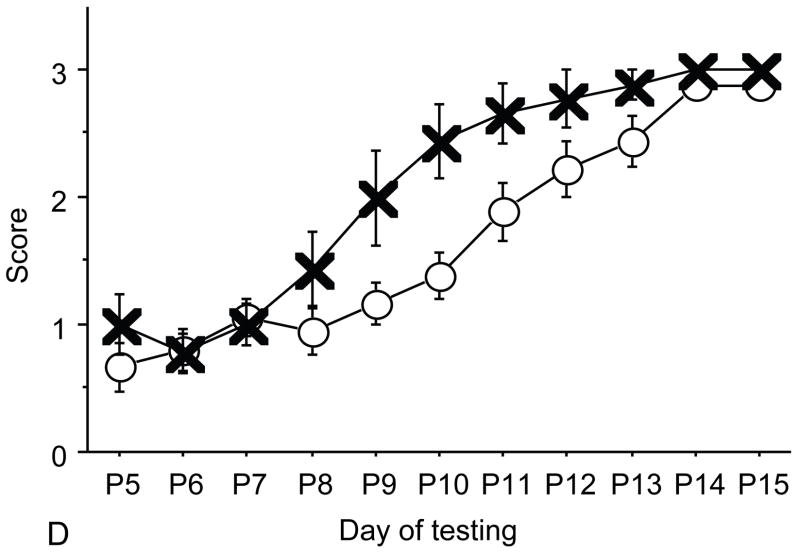
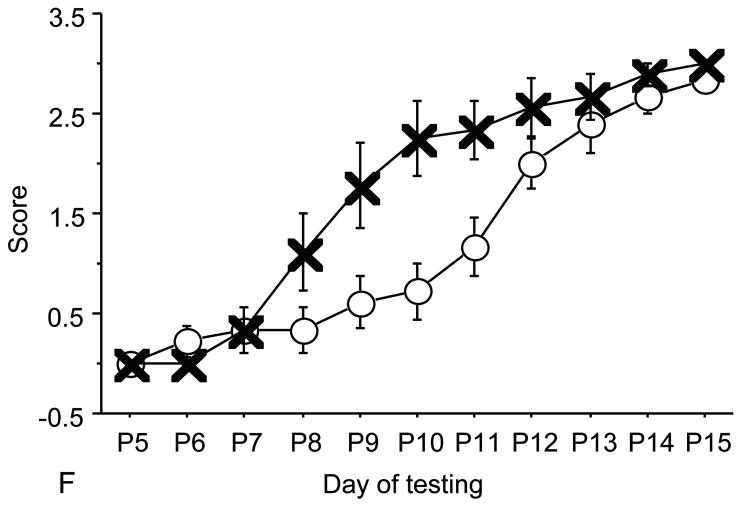
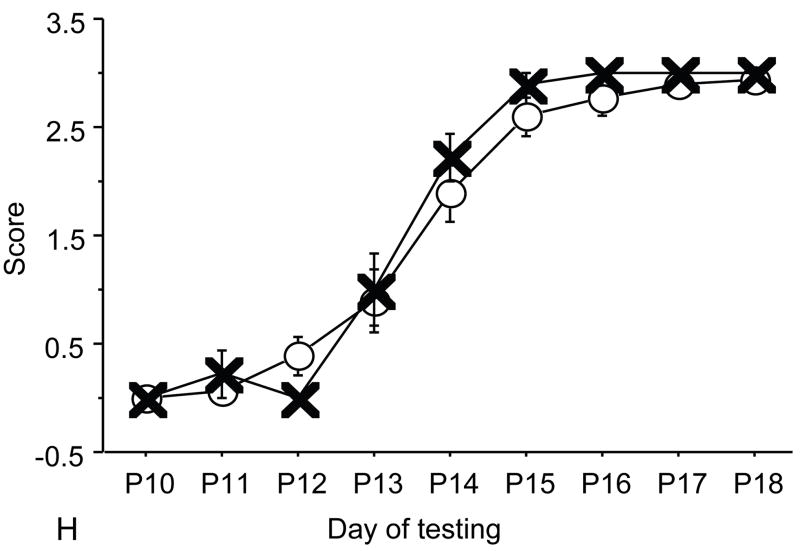
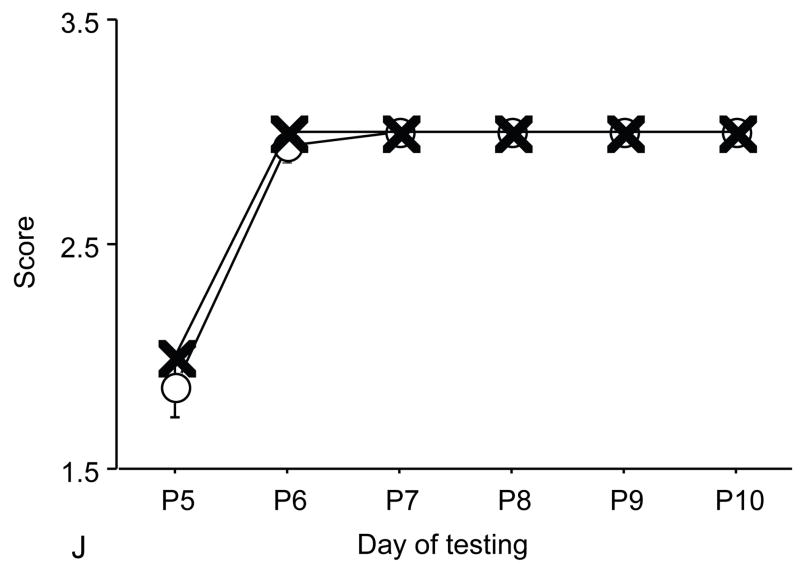
Figure 1.
A: motor milestones, B: sensory milestones. Circle: control, square: trisomic (Ts), triangle: Ts+peptides The x axes indicates the days of testing and y axis the score from 0 to 1 on the behavior performance, with higher score indicating achievement of the developmental milestone. While Ts mice (n=14 from 5 litters) were significantly delayed in achieving the milestones showed as compared to the controls (n=18 from 6 litters), prenatal treatment with NAP+SAL (n=6 from 4 litters) significantly prevented the delay in 3/4 motor and in 1/4 sensory milestones; it also induced a trend towards the prevention of deficit in forelimb placing and growth delay. Each point indicates mean score±s.e.m.
Figure 4.


Neonatal tests. Behavioral tests performed to assess sensory-motor development in newborn pups. A: weak tactile stimulation, assesses the head turning response triggered by the application of tactile stimuli (cotton swab) in the perioral area on both sides of the head and it is a sensory response mediated by the 5th cranial nerve; B: ear twitch, tests the twitching of the pup’s ears at the touch with a cotton swab and is a sensitivity test; in C: screen climbing test pups climb a vertical wire-mesh (5×5mm) screen using both fore- and hind-paws, this is a motor test; in D: vibrissa placing reflex, when the mouse is suspended by the tail and lowered so that the vibrissae make contact with a solid object, the pup raises its head and the forelimbs to grasp the object, this is principally a measure of neck and forelimbs tone and strength.
Statistical anlysis of behavior included post-hoc comparisons using paired t tests with conservative Bonferroni-Dunn’s correction, with significance level: 5%.
Genotyping of the Ts65Dn mice
Mouse genotyping revealed that in the neonatal tests were included 14 Ts pups from 5 litters, 18 control from 6 litters, 6 Ts+peptides from 4 litters and 9 control+peptides pups from 3 litters.
For genotyping, tail tips (~2mm/sample) from newborn pups from 7 to 21 days of age were collected for genotyping. A previously published method was used (22). Briefly, tail tips were digested using an alkali method(23), samples were neutralized and diluted with Tris, to about 0.1 ng/L based on an expected yield of approximately1 to 2g DNA from a 1- to 2-mm tail sample. For genotyping we used Real-Time Quantitative PCR to amplify myxovirus (influenza virus) resistance 1 (Mx1) gene, which is close to the distal end of the Chromosome 16 segment in the trisomic chromosome. For Mx1 (Gen-Bank accession no. M21117), exon 14 sequence was used, and the PCR product size is 74 bp. The sequences for primers and probe are: forward primer 5′-TCTCCGATTAACCAGGCTAGCTAT-3′, reverse primer 5′-GACATAAGGTTAGCAGCTAAAGGATCA-3′, and probe 5′-FAM-TGGCTTTCCTGGTCGCTGTGCA-TAMRA-3′. The apolipoproteinB gene (Apob; GenBank accession no. X15191) was used as an internal control to normalize variations of the amount of input DNA. The product size is 73 bp. Here are the primer and probe sequences: forward primer 5′-CACGTGGGCTCCAGCATT-3′, reverse primer 5′-TCACCAGTCATTTCTGCCTTTG- 3′, and probe 5′-VIC-CCAATGGTCGGGCACTGCTCAA-TAMRA-3′. The TaqMan probes for target gene Mx1 and the control gene Apob were labeled with two different fluorescent reporters (FAM and VIC) so that a multiplexed PCR could be used. The PCR was set up as follows: 12.5 μL of 2× TaqMan universal PCR master mix (Applied Biosystems), 0.25 μL of each primer (40 μM), 0.75 μL of each probe (5 μM), and 10 μL of diluted template DNA (about 0.1 ng/μL). The reaction was carried out at 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min on an ABI PRISM® 7000 sequence detection system (Applied Biosystems) with a 96-well format. For each sample, triplicate PCRs were carried out.
After real-time PCRs, the average change (Δ) in cycle threshold (CT) of target gene Mx1 from that of the internal control Apob was calculated (ΔCT = CT for App or Mx1-CT for Apob) and analyzed. For each PCR experiment, we included a standard curve. These were done by using four 1:2 serial dilutions over a range of 8-fold for one sample, including a dilution of about 0.1 ng/μL DNA. Also, known trisomic and wild type samples were included in each run. Since the Ts65Dn mouse has three copies of the Mx1 gene, the expected difference between the ΔCT for Ts65Dn mice and the ΔCT for diploid control mice should be 0.585 (2−0.585 = 1.5, where 1.5 is the fold difference between three copies of Mx1 in trisomic mice versus two copies in wild-type mice). The CT threshold was adjusted so that wild type samples would show an average ΔCT close to 0 while Trisomic show an average ΔCT close to −0.6. Anything less obvious would suggest a failure, and the whole experiment would need to be repeated. For the trisomic and wild type samples the 95% confidence interval was determined, which then was used as criteria to genotype whether a given unknown sample falls into the range of the diploid or trisomic samples. All genotyping samples were run after the completion of all testing.
Gene expression
In order to better understand NAP+SAL mechanism of action we explored whether NAP+SAL prevented the glial deficit in Ts65Dn mice by evaluating expression of VIP, the glial marker Glial fibrillary acidic protein (GFAP), and ADNP. To evaluate long-term alterations in gene expression, adult brains were removed and immediately frozen in dry ice. Four Ts, 4 control and 3 Ts+peptides adult brains were collected separately and immediately frozen in dry ice. Each group represented 3 litters and mice were 40 weeks old.
For RNA isolation, samples were homogenized by a sonicator (Janke & Kunkel, Wilmington, NC), and processed with SV Total RNA Isolation System (Promega, Madison, WI). A 5-μL aliquot was taken for spectrophotometeric determination of RNA content. The remaining sample was stored at −80°C. Using 5 μg of total RNA, the reverse transcriptase (RT) reaction was performed (Applied Biosystems, Roster City, CA) in a final volume of 150 μL. Each RNA sample was run in duplicate.
For PCR, Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were synthesized by IDT (Integrated DNA Technologies, Coralville, IA), GFAP primer pair was synthethized by Superarray (Frederick, MD) while ADNP primer pair was synthesized by Biosynthesis (Louisville, Tex), and VIP by TIB Molbiol (Adelphia, NJ). The GAPDH gene, a housekeeping gene located on mouse chromosome 6, is frequently used as a normalized gene due to its stability in cells. In our experiments and in experiments reported by other groups (Reeves et al., 1995) we did not detect a significant difference in GAPDH expression between the trisomic and wild type samples. The gene for the GFAP subunit is located on chromosome 11, VIP is located on chromosome 10, and ADNP on chromosome 2. With the use of the FastStart DNA Master SYBR Green 1 dye-base detection (Roche, Indianapolis, IN), VIP, ADNP, GFAP and GAPDH expression were measured by real-time PCR using the LightCycler with relative quantification software (Roche) and melting point analysis to assess the specificity of the amplified genes. Optimization for VIP, ADNP, GFAP and GAPDH-specific primers was performed with separate runs by varying magnesium chloride and primer concentrations, amount of template, and annealing temperature. The presence and purity of target gene sequence expression in the RT-PCR reaction were confirmed by gel electrophoresis. Samples were run in duplicate and relative quantification was performed by using calibrator-normalized data with efficiency correction. Results are presented as the normalized ratio of VIP, GFAP and ADNP-to-GAPDH expression.
ANOVA test was performed for overall comparisons between the treatment groups, and Fisher PLSD was used for post-hoc analysis, with P <0.05 considered significant (StatView v5.0.1, SAS, Cary, NC).
Results
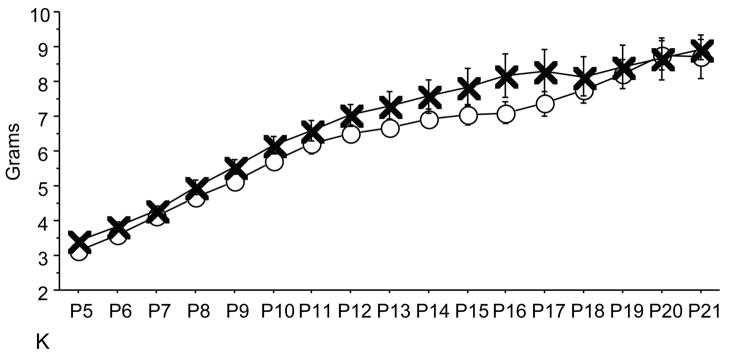
Trisomic (Ts) mice achieved milestones with a significant delay in 4 of 5 motor (forelimb grasp, vibrissa placing, righting and cliff aversion) and 4 of 5 sensory (eye open, ear open, weak tactile stimulation, forelimb placing) milestones and showed growth delay as compared to the controls. These results confirm a previous study that showed developmental delay in the Ts mice and is consistent with human findings(7). Ts mice that were prenatally exposed to NAP+SAL achieved developmental milestones at the same time as the controls in 3 of 4 motor (righting, vibrissa placing and forelimb grasp) and 1 of 4 sensory (weak tactile stimulation) milestones (p<0.01) and there was a trend towards better performance in the Ts+peptides compared to the Ts group in forelimb placing and weight (figure 1).
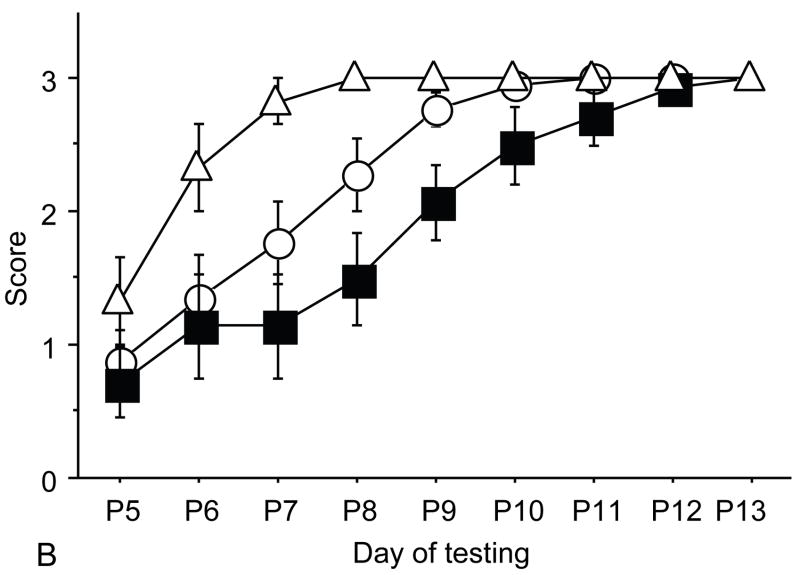
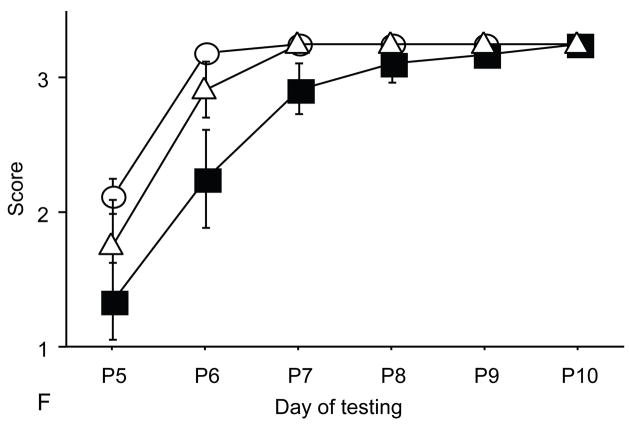
Control pups prenatally treated with NAP+SAL achieved developmental milestones significantly earlier than the controls treated with placbo in 4 of 5 motor (righting, vibrissa placing, cliff aversion and screen climbing) and in 2 of 5 sensory (ear twitch and weak tactile stimnulation) milestones (all p<0.01; figure 2 and Figure 4).
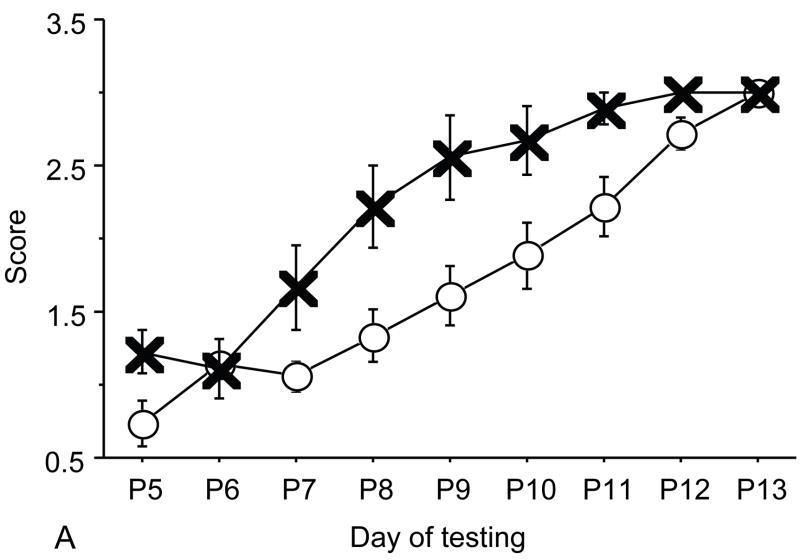
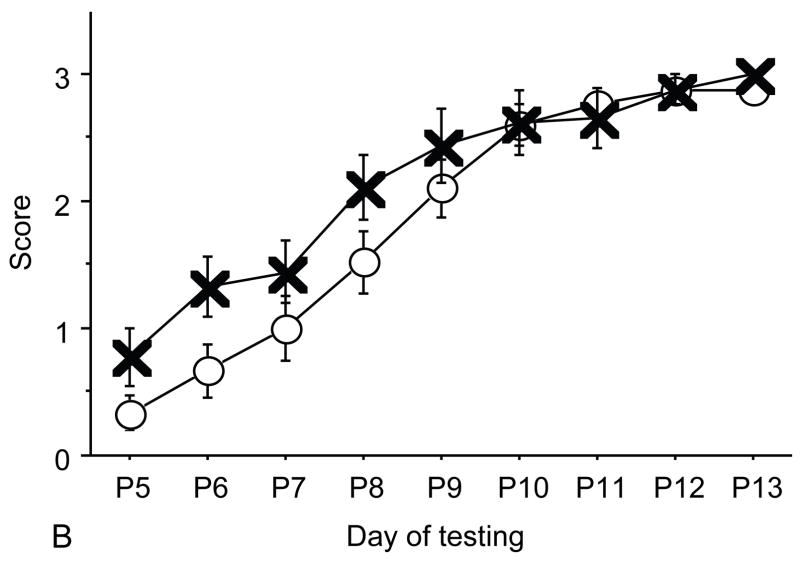
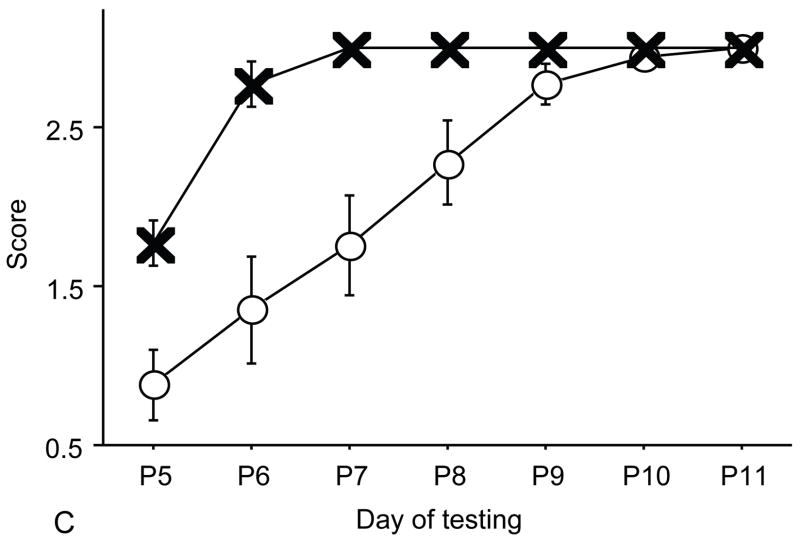
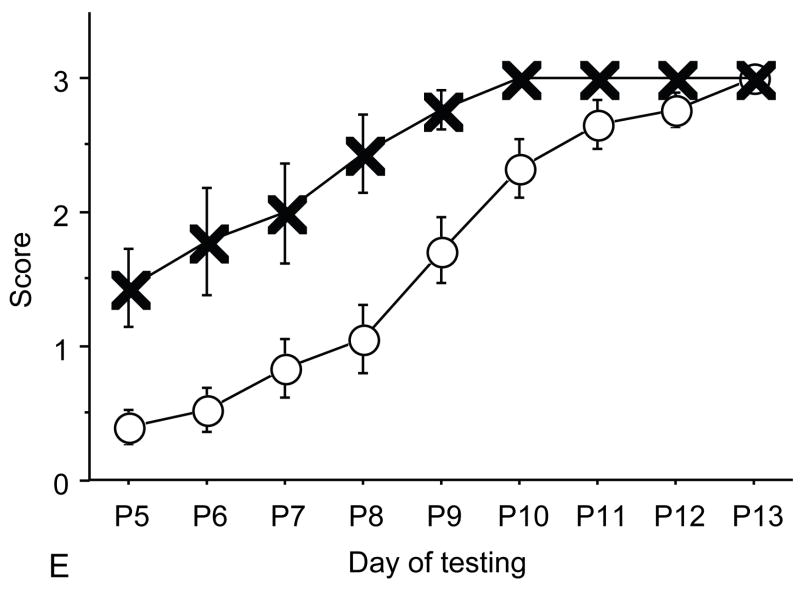
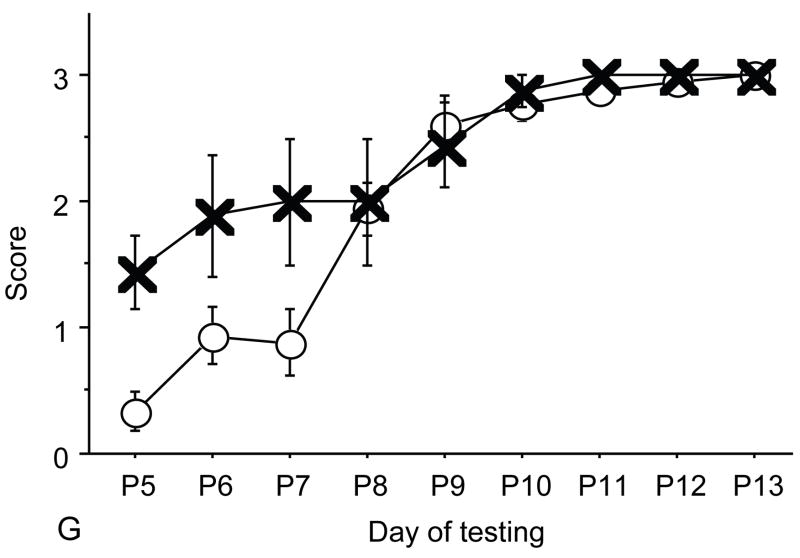
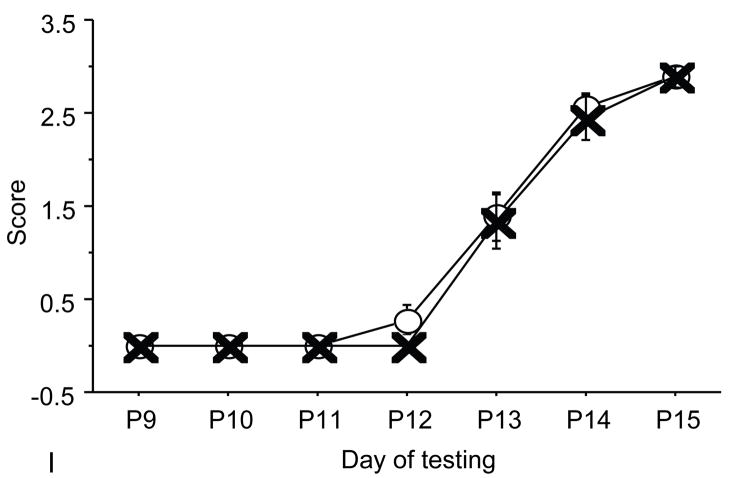
Figure 2.
A: motor milestones, B: sensory milestones. Circle: control, cross: control+peptides. Prenatal treatment with the peptides resulted in developmental enhancement of the control pups. The x axes indicate the days of testing and y axis the score from 0 to 1 on the behavior performance, with higher score indicating achievement of the developmental milestone. The milestones that were reached significantly earlier in the control pups treated with NAP+SAL (n=9 from 3 litters) as compared to the controls (n=18 from 6 litters) treated with placebo were righting, vibrissa placing, cliff aversion, screen climbing, ear twitch and weak tactile stimulation (4/5 motor and 2/5 sensory). Each point indicates mean score±s.e.m.
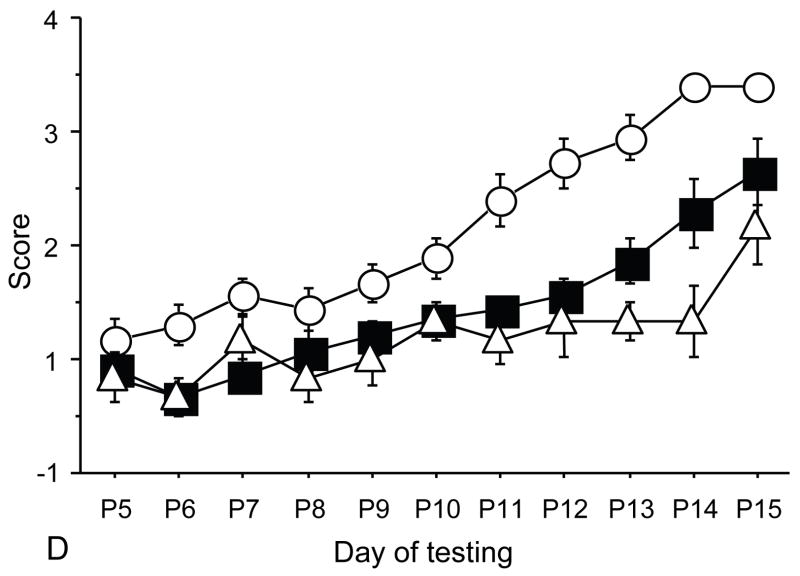
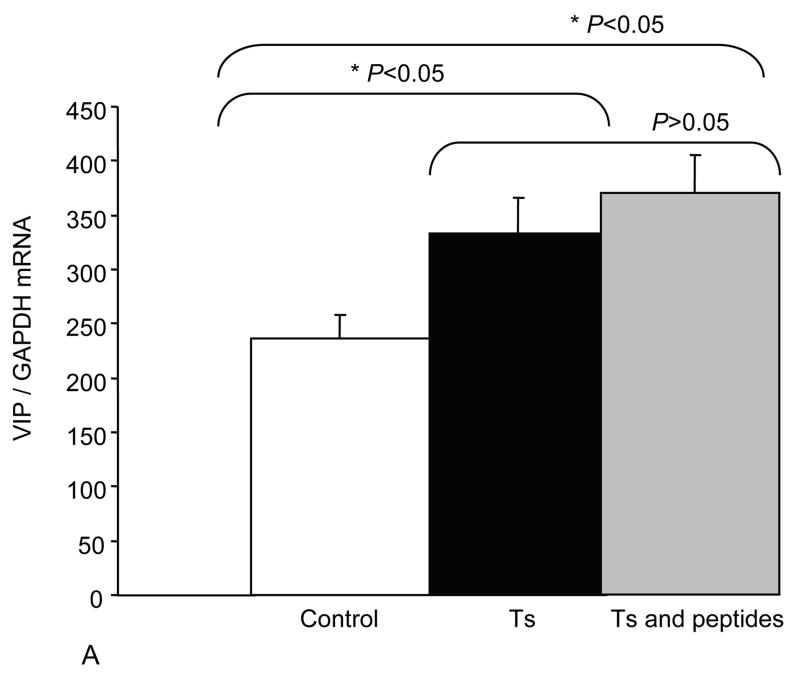
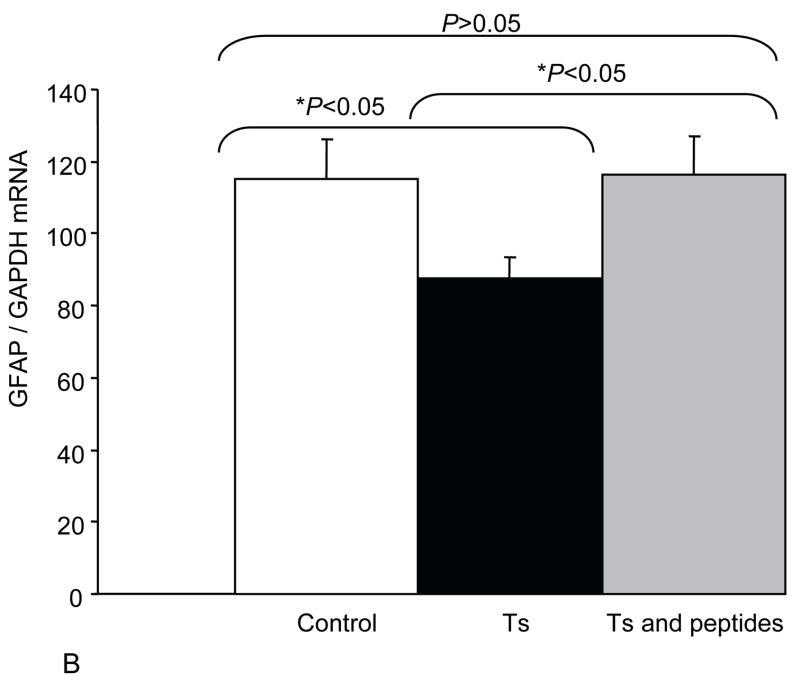
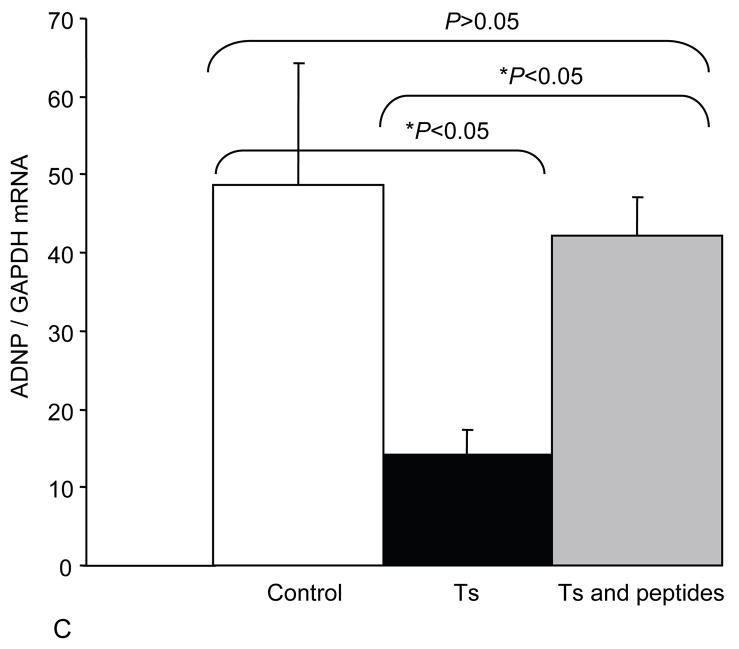
In order to better understand NAP+SAL mechanism of action we explored whether NAP+SAL prevented the glial deficit in Ts65Dn mice by evaluating expression of VIP, the glial marker Glial fibrillary acidic protein (GFAP), and ADNP. PCR analysis showed that, while ADNP was significantly downregulated in the Ts65Dn brains versus the controls, prenatal treatment with NAP+SAL prevented the ADNP decrease in the Ts65Dn brains and the expression was not different from the controls. The glial marker GFAP demonstrated the known glial deficit in the Ts65Dn mice, and treatment with NAP+SAL prevented its downregulation. Lastly, VIP levels were increased in the Ts brains, while treatment with NAP+SAL did not prevent its upregulation (Figure 3).
Figure 3.
The figures show the levels of expression for VIP (A), GFAP (B) and ADNP (C) in adult brains for controls, Ts65Dn and Ts65Dn+peptides. Bars represent relative mRNA expression ±s.e.m.
Discussion
We found that prenatal treatment with VIP-regulated peptides prevented developmental delay in the neonatal period and resulted in prevention of the glial deficit in adulthood. In the neonatal period Ts65Dn pups were delayed in achieving important milestones; as is seen in the human condition. These developmental milestones are ultimately achieved, the delay may represent a nervous system deficit which may result in neurodevelopmental and learning deficits in adulthood. We hypothesize that by preventing the developmental delay with the peptides we may be able to prevent longer term neurodevelopmental and learning abnormalities in the adult offspring. One of the neuropathologic characteristics of DS is a glial deficit which likely induced alterations in VIP and its related neuropeptides.
In cortical neurons of human DS brains ADNP and VIP receptor VPAC-1 expression have shown to be decreased (16), while VIP levels were found elevated in the blood of DS neonates (11), possibly, as a feed-back mechanism to overcome the glial deficit. In the Ts65Dn mouse also, we and others (13) show a decrease in ADNP and an upregulation of VIP, changes that are present already in postnatal day 8 neonates. Astrocytes in adult Ts65Dn brains are abnormal, as indicated by the downregulation of the glial marker GFAP. Here we show that prenatal treatment with peptides derived from ANDP and ADNF, resulted in a normalization of ADNP and of the glial marker GFAP in adult brains: treatment with the peptides may have overcome the glial deficit by restoring the appropriate neuropeptides. Nonetheless, treatment did not prevent VIP up-regulation; it is possible that other mechanisms regulate VIP release (more comments on NAP+SAL mechanism of action are in the Appendix, available online at www.greenjournal.org/cgi/content/full/112/6/XX/DC1.). These findings may explain, at least in part, NAP+SAL prevention of glial deficit, developmental delay in the Ts65Dn pups and developmental enhancement in the wild type mice.
In this study, we also show that control animals prenatally treated with NAP+SAL had accelerated development. Previously, we have shown learning enhancement after treatment with NAP+SAL in adulthood (18,24), and these results suggest that the adult enhancement observed might be a consequence of faster developmental milestones achievement.
Since Down syndrome can be diagnosed prenatally it is possible that an intervention during pregnancy may improve the outcome. The prevention of developmental delays in DS patients may improve both the neonatal period as well as affect learning skills in adulthood. We are currently studying the effects of prenatal treatment with NAP+SAL on prevention of learning deficit in adult mice. These findings highlight a possibility for the prevention of developmental sequelae in Down Syndrome and other conditions with developmental delay and learning deficit.
Acknowledgments
Supported by the Division of Intramural Research Program, NIH, NICHD and NIAAA. Cecilia Schmidt, The Jackson Laboratory, Bar Harbor, ME, for animal genotyping.
Footnotes
Financial Disclosure: The authors have no potential conflicts of interest to disclose.
References
- 1.Dolk H, Loane M, Garne E, De Walle H, Queisser-Luft A, De Vigan C, et al. Trends and geographic inequalities in the prevalence of Down syndrome in Europe, 1980–1999. Rev Epidemiol Sante Publique. 2005;53:2S87–2S95. [PubMed] [Google Scholar]
- 2.Vicari S. Motor development and neuropsychological patterns in persons with Down syndrome. Behavior Genetics. 2006;36:355–364. doi: 10.1007/s10519-006-9057-8. [DOI] [PubMed] [Google Scholar]
- 3.Chen YJ, Fang PC. Sensory evoked potentials in infants with Down syndrome. Acta Pediatrica. 2005;94:1615–1618. doi: 10.1080/08035250500252609. [DOI] [PubMed] [Google Scholar]
- 4.Toledo C, Alembik Y, Aguirre JA, Stoll C. Growth curves of children with Down syndrome. Ann Genet. 1999;42(2):81–90. [PubMed] [Google Scholar]
- 5.Nadel L. Down’s syndrome: a genetic disorder in biobehavioral perspective Genes. Brain and Behavior. 2003;2:156–166. doi: 10.1034/j.1601-183x.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 6.Davisson MT, Schmidt C, Akeson EC. Segmental trisomy of murine chromosome 16: a new model system for studying Down syndrome. Prog Clin Biol Res. 1990;360:263–280. [PubMed] [Google Scholar]
- 7.Holtzman DM, Santucci D, Kilbridge J, Chua-Couzens J, Fontana DJ, Daniels SE, et al. Developmental abnormalities and age-related neurodegeneration in a mouse model of Down syndrome. Proc Natl Acad Sci. 1996;93:13333–13338. doi: 10.1073/pnas.93.23.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belichenko PV, Masliah E, Kleschevnikov AM, Villar AJ, Epstein CJ, Salehi A, et al. J Comp Neurol. 2004;480:281–298. doi: 10.1002/cne.20337. [DOI] [PubMed] [Google Scholar]
- 9.Contestabile A, Fila T, Ceccarelli C, Bonasoni P, Bonapace L, Santini D, et al. Cell cycle alteration and decreased cell proliferation in the hippocampal dentate gyrus and in the neocortical germinal matrix of fetuses with Down syndrome and in Ts65Dn mice. Hippocampus. 2007;17(8):665–78. doi: 10.1002/hipo.20308. [DOI] [PubMed] [Google Scholar]
- 10.Best TK, Siarey RJ, Galdzicki Z. Ts65Dn, a mouse model of Down syndrome, exhibits increased GABAB-induced potassium current. J Neurophisiol. 2007;97:892–900. doi: 10.1152/jn.00626.2006. [DOI] [PubMed] [Google Scholar]
- 11.Nelson PG, Kuddo T, Song EY, Dambrosia JM, Kohler S, Satyanarayana G, et al. Selected neurotrophins, neuropeptides, and cytokines: developmental trajectory and concentrations in neonatal blood of children with autism or Down syndrome. Int J Dev Neurosci. 2006;24(1):73–80. doi: 10.1016/j.ijdevneu.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Hill JM, Ades AM, McCune SK, Sahir N, Moody EM, Abebe DT, et al. Exp Neurol. 2003;183(1):56–65. doi: 10.1016/s0014-4886(03)00164-x. [DOI] [PubMed] [Google Scholar]
- 13.Sahir N, Brenneman DE, Hill JM. Neonatal mice of the Down syndrome model, Ts65Dn, exhibit upregulated VIP measures and reduced responsiveness of cortical astrocytes to VIP stimulation. J Mol Neurosci. 2006;30(3):329–40. doi: 10.1385/JMN:30:3:329. [DOI] [PubMed] [Google Scholar]
- 14.Wu JY, Henins KA, Gressens P, Gozes I, Fridkin M, Brenneman DE, et al. Neurobehavioral development of neonatal mice following blockade of VIP during the early embryonic period. Peptides. 1997;18(8):1131–7. doi: 10.1016/s0196-9781(97)00146-0. [DOI] [PubMed] [Google Scholar]
- 15.Brenneman DE, Spong CY, Hauser JM, Abebe D, Pinhasov A, Golian T, et al. Protective peptides that are orally active and mechanistically nonchiral. JPET. 2004;309:1190–1197. doi: 10.1124/jpet.103.063891. [DOI] [PubMed] [Google Scholar]
- 16.Busciglio J, Pelsman A, Helguera P, Ashur-Fabian O, Pinhasov A, Brenneman DE, et al. NAP and ADNF-9 protect normal and Down’s syndrome cortical neurons from oxidative damage and apoptosis. Curr Pharm Des. 2007;13(11):1091–8. doi: 10.2174/138161207780618957. [DOI] [PubMed] [Google Scholar]
- 17.Spong CY, Abebe D, Gozes I, Brenneman DE. Prevention of fetal demise and growth restriction in a mouse model of fetal alcohol syndrome. J Pharmacol Exp Ther. 2001;297(2):774–9. [PubMed] [Google Scholar]
- 18.Vink J, Auth J, Abebe, Brenneman DE, Spong CY. Novel peptides prevent alcohol-induced spatial learning deficits and proinflammatory cytokine release in a mouse model of fetal alcohol syndrome. Am J Obstet Gynecol. 2005;193(3 Pt 1):825–9. doi: 10.1016/j.ajog.2005.02.101. [DOI] [PubMed] [Google Scholar]
- 19.Spong CY, Lee SJ, McCune SK, Gibney G, Abebe DT, Alvero R, et al. Endocrinology. 1999;140(2):917–24. doi: 10.1210/endo.140.2.6481. [DOI] [PubMed] [Google Scholar]
- 20.Fox WM. Reflex-ontogeny and behavioural development of the mouse. Anim Behav. 1965;13:234–241. doi: 10.1016/0003-3472(65)90041-2. [DOI] [PubMed] [Google Scholar]
- 21.Santucci D, Calamandrei G, Alleva E. Neonatal exposure to bFGF exerts NGF-like effects on mouse behavioral development. Neurotox Teratol. 1993;15:131–7. doi: 10.1016/0892-0362(93)90071-u. [DOI] [PubMed] [Google Scholar]
- 22.Liu DP, Schmidt C, Billings T, Davisson MT. Quantitative PCR genotyping assay for the Ts65Dn mouse model of Down syndrome. Biotechniques. 2003;35(6):1170–1179. doi: 10.2144/03356st02. [DOI] [PubMed] [Google Scholar]
- 23.Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT) BioTechniques. 2000;29:52–54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- 24.Toso L, Endres M, Vink J, Abebe DT, Brenneman DE, Spong CY. Learning enhancement with neuropeptides. Am J Obstet Gynecol. 2006;194(4):1153–8. doi: 10.1016/j.ajog.2005.12.023. discussion 1158–9. [DOI] [PubMed] [Google Scholar]