Abstract
Purpose
This study examined the associations of free testosterone and family environment with delinquent and aggressive behaviors among adolescent boys and girls with elevated low-density lipoprotein (LDL)-cholesterol levels.
Methods
Participants were 164 boys and 180 girls (ages 11–14 years). The female parent provided ratings of family cohesion and child aggressive and delinquent behaviors. Tanner ratings of pubertal development were obtained during physical examination, and a blood sample was drawn for assessment of serum levels of free testosterone.
Results
Hierarchical regression analyses revealed significant two-way interactions of free testosterone and family cohesion on delinquent behaviors among adolescent boys and girls. Specifically, under conditions of low family cohesion, free testosterone was positively associated with delinquent behaviors among boys, whereas no association between free testosterone and delinquent behavior was observed in families with high cohesion. In contrast, free testosterone was negatively associated with delinquent behaviors among adolescent girls in low cohesion families. For girls, family cohesion was negatively associated with aggressive behaviors; for boys, LDL-C was positively associated with aggressive behaviors.
Conclusions
Child gender and family environment factors appear to modify the associations between free testosterone and delinquent behaviors in adolescent boys and girls.
Keywords: testosterone, delinquent behaviors, aggression, family cohesion
Developing a greater understanding of the various factors that contribute to adolescent behavioral outcomes has been of extensive interest to clinicians and researchers. Many such studies have focused on factors that are influenced by puberty, as this represents a period of considerable biological and social change [1]. During puberty, secretion of androgens increases significantly as sexual maturation progresses. Boys and girls differ dramatically in the production of several key hormones, particularly testosterone, as adolescent girls and adult women produce far less testosterone than adolescent boys and adult men do. Testosterone, which is the most abundant active androgen and is distributed widely throughout the body including the central nervous system [2], has been associated with a variety of behaviors including antisocial behavior, aggression, and delinquency [3–5].
Empirical evidence generally supports a positive association between high levels of testosterone and aggressive and delinquent behaviors among boys [3, 5]. Specifically, high levels of testosterone were positively associated with aggressive and delinquent behaviors [3] and greater attention problems [6], although one early study reported no association between testosterone and aggressive behavior among boys [7].
Less is known, however, about the association between testosterone and behavioral problems among adolescent girls. In one study, girls who displayed symptoms of aggressive conduct disorder had significantly higher mean free testosterone than girls with non-aggressive conduct disorder [4]. However, adolescent girls with conduct disorder did not differ from girls without any psychiatric disorder on testosterone levels, and no association was observed generally between aggression and mean free testosterone levels [4]. The absence of an association between aggression and testosterone is consistent with the few studies that have been conducted, which have reported no direct association between absolute testosterone levels and aggression or delinquency among adolescent girls [6, 7]. Because testosterone levels follow a diurnal pattern, with the highest levels in the morning followed by declining levels throughout the remainder of the day and evening, one study assessed the association between diurnal variation in testosterone and behavioral problems [6]. Results from that study indicated that steeper declines in testosterone levels during the day were related to more disruptive behavior problems among girls [6].
The somewhat inconsistent findings reported between testosterone and behavioral problems may be due, in part, to the contribution of external factors that influence adolescent adjustment. The biological changes that arise during puberty do not take place in a social void, but rather occur in the context of socioemotional environments that can either exacerbate or attenuate the effects of biological influences. Indeed, a number of studies have documented the contribution of family factors to adolescent behavioral outcomes [8].
The role of family environment
Social factors, such as the quality of familial relationships or the general social environment of the family, have been shown to be highly correlated with a child’s vulnerability to delinquent behavior [9]. High levels of family cohesion are protective against behavioral problems [8] and moderated the use of aggressive tactics during conflict [10], further illustrating the relevance of family relationships for adolescent behaviors.
While the singular effects of family environment and pubertal maturation on adolescent outcomes have been widely investigated, few studies have examined their potential interactive effects, even though empirical data support using such a biosocial approach [11, 12]. For example, Booth and colleagues found that the quality of the child-parent relationship moderated the association of testosterone with risk behaviors [12]. Among adolescent boys, testosterone was not significantly associated with outcomes when the quality of the parent-child relationship was high. As the quality of the parent-child relationship decreased, however, associations between testosterone and risk behaviors emerged [12]. Likewise, the quality of the child-parent relationship also moderated the testosterone-behavior relationship for girls, as low testosterone was associated with more risky behaviors (e.g., delinquency, substance use) but only among girls with poor quality mother-child relations. The inverse association between testosterone and risk behaviors observed among girls was attributed to the role of gender socialization and other societal factors that discourage such behaviors among girls [12]. Indeed, gender differences in the development of delinquent activities during adolescence is a well-established finding [13].
Thus, the primary purpose of the present study was to utilize a biosocial approach to investigate associations between testosterone and family environment with behavioral outcomes (i.e. delinquent and aggressive behaviors) among adolescent boys and girls identified as having unfavorable serum cholesterol profiles. These outcomes may be particularly relevant in this population given that some previous studies have reported positive relations between cholesterol and aggression [14]. Thus, based on prior empirical studies, it is hypothesized that under conditions of low family cohesion, testosterone will be positively associated with behavioral problems among adolescent boys. Among adolescent girls, it is hypothesized that an inverse association between testosterone and behavioral problems will be observed, but only under conditions of low family cohesion. In an environment of high family cohesion, it is hypothesized that associations between testosterone and adolescent adjustment will be less evident.
Methods
Participants
Participants were 164 boys and 180 girls enrolled in a multi-center Dietary Intervention Study in Children (DISC). The design and results of this randomized, controlled clinical trial have been published elsewhere [15]. Informed consent was obtained from study participants and their parents prior to enrollment in the study. In brief, children with elevated low-density lipoprotein (LDL)-cholesterol were randomly assigned to receive usual care or a dietary intervention to reduce total fat and saturated fat intake [15]. Children were recruited at six clinical centers: Children’s Hospital, New Orleans, LA; Johns Hopkins University Hospital, Baltimore, MD; Kaiser Permanente Center for Health Research, Portland, OR; University of Medicine and Dentistry of New Jersey, Newark, NJ; Northwestern University Medical School, Chicago, IL; and University of Iowa Hospital and Clinics, Iowa City, IA. Data for the present analyses were drawn from the children’s third annual visit (i.e. 3 years following baseline) at which time all key psychosocial, hormonal, and serum lipid measurements were assessed. The children were, on average, 12.6 years of age (SD = 0.72; range = 11 to 14), with a mean body mass index (BMI) of 20.01 (SD = 3.47). Eighty-two percent of participants were non-Hispanic white, 9% were African American, 6% were Hispanic/Latino, and 2% were Asian.
Measures
Demographic information
Demographic information, including child age, race and ethnicity, was obtained from the child’s parent or guardian. Parental education level and income level were assessed.
Pubertal development
Pubertal development was assessed by trained examiners during physical examinations. Using a standardized protocol based on the Tanner method [16], breast and pubic hair development were assessed among girls, and menarcheal age was determined. For boys, genital and pubic hair development were evaluated and testicular volume was measured using orchidometers.
Delinquent and aggressive behaviors
Delinquent and aggressive behaviors were assessed using the Delinquent Behavior and Aggressive Behavior subscales of the Child Behavior Checklist (CBCL)[17], which was completed by the female parent or guardian. The parent or guardian was asked to rate the degree to which various child behaviors had occurred in the past 6 months. Ratings ranged from 0 (not true), 1 (somewhat or sometimes true), to 2 (very true or often true). The age-standardized scores (T scores) for the Delinquent Behavior and Aggressive Behavior scales were computed and can be used to compare with scores obtained from normative samples. Given that T scores are not truncated, it has been recommended to use T scores in statistical analyses of these scales [17].
Family cohesion
Family cohesion was rated by the female parent or guardian using the cohesion subscale of the Family Environment Scale (FES) [18]. The cohesion subscale consists of 9 items designed to measure the degree of commitment and support family members provide for one another. Each item is rated as “True” or “False” by the respondent. Responses are summed to create the raw score, which is then converted to a standard score using the scale’s conversion table [18]. The content, construct, and predictive validity of this instrument have been demonstrated across a number of studies [18].
Free testosterone
Total testosterone concentrations were determined by radioimmunoassay (RIA) using a modification of the procedure developed by Furuyama and colleagues [19]. Serum samples were extracted with hexane:ethyl acetate, 90:10 (vol/vol), and the extracts were applied to aluminum oxide micro columns. The columns were washed with hexane containing 0.55% ethanol, and testosterone was specifically eluted using hexane containing 1.4% ethanol. Testosterone in eluates was quantified in duplicate by RIA using antiserum raised to a testosterone-3-oxime-BSA conjugate. Testosterone -1,2,6,7-[3H] was obtained from New England Nuclear-Dupont (now PerkinElmer Life and Analytical Sciences, Inc., Waltham, MA). The limit of sensitivity was 3.0 ng/dl.
Levels of free testosterone were then calculated using the methods of Sodergard and colleagues [20] and Vermeulen and colleagues [21] as detailed by Rinaldi and colleagues [22]. Levels of free testosterone are reported in ng/dL. For girls, we used the two equation model [22] to estimate free testosterone from total assay measures of estradiol, total testosterone, sex hormone-binding globulin concentration (SHBG), and albumin concentration. For boys, we used the three equation model [22] for calculation of free testosterone because levels of three relevant hormones (5alpha-dihydrotestosterone or DHT, estradiol, and total testosterone) were available for boys [22]. DHT levels in girls are generally very low and were not assessed among the girls in the present study. SHBG was measured using an immunoradiometric assay (IRMA) developed at Esoterix, Inc. (Calabasas Hills, CA). The sensitivity of the IRMA assay was 10 nmol/L and intra-and inter-assay precision was high.
Measurement of serum lipids
In light of empirical data suggesting that serum cholesterol levels may be positively associated with aggression [14] in adults and adolescents, we included serum lipid levels in the multivariate analyses. Two fasting venipunctures for serum lipids were drawn for the assessment of total cholesterol, HDL-C, and LDL-C as previously described [15]. For each measure (total cholesterol, HDL-C, and LDL-C), the average of the two assessments was used in analyses.
Procedure
Data were collected by trained staff who were masked to the participants’ treatment assignment. During a scheduled clinic visit, the height and weight of each participant was measured and used to calculate body mass index (BMI; weight [in kg] divided by height2 [in m]). Each participant then underwent a brief physical examination that included Tanner staging. The female parent was asked to complete the FES and CBCL while waiting during the child’s examination. Blood samples were collected by venipuncture the morning of the clinic visit after an overnight fast. Serum was separated by centrifugation after the blood sample was kept at room temperature for at least 45 minutes to allow complete clotting. Serum was then aliquoted and stored in glass vials at −80°C until it was analyzed for hormone levels.
Statistical analysis
Data analyses were conducted using SPSS 14.0. First, preliminary analyses were conducted to characterize the sample in terms of the variables of interest. Second, correlational analyses and one-way analyses of variance (ANOVAs) were performed to examine potential sociodemographic covariates of delinquent and aggressive behaviors. Third, correlations among the independent variables (free testosterone and family cohesion) and dependent variables were examined.
Finally, separate hierarchical multiple regression analyses were conducted for boys and girls to examine associations of free testosterone and family cohesion with behavioral outcomes (delinquent and aggressive behaviors). Given that testosterone levels vary across the menstrual cycle and a subset of girls had reached menarche, we controlled for menstrual cycle phase in the regression analyses. Day of the menstrual cycle that corresponded to the date of blood collection was obtained from menstrual cycle calendars that were completed by the postmenarcheal girls for 6 weeks before and 6 weeks after their blood collections. Because the time from ovulation to the start of the next menses is more constant than the time from start of last menses to ovulation for postmenarcheal girls, we defined menstrual cycle days in relation to the start of next menses. Serum samples collected 1 through 14 days before the next menses were classified as luteal (n = 25), and samples collected15 through 33 days before or on the day of onset of the next menses were classified as follicular (n = 19). The majority of girls was pre-menarchal (n = 146). In the regression equation, four blocks of variables were entered sequentially. The blocks were comprised of: (a) potential covariates (i.e. parental income, developmental status, body mass index, serum lipid levels, and menstrual cycle phase [in girls only]); (b) family cohesion; (c) free testosterone; and (d) the product term representing the two-way interaction (i.e. free testosterone × cohesion). All tests of statistical significance were performed using two-tailed tests.
Results
Preliminary Analyses
Initial analyses were conducted to examine whether key study variables differed by treatment group (i.e. dietary intervention vs. usual care). Separate one-way ANOVAs conducted for boys and girls indicated no significant differences in family cohesion, free testosterone levels, or delinquent and aggressive behaviors by treatment group. Therefore, data were combined across treatment conditions for all subsequent analyses.
Table 1 summarizes the mean levels of free testosterone, family cohesion, and outcome variables by Tanner Stage for girls and boys. One-way ANOVAs indicated that mean levels of free testosterone increased significantly across Tanner Stage for both boys and girls. Mean levels of family cohesion and delinquent and aggressive behaviors did not differ across Tanner Stage among boys. For girls, delinquent behaviors significantly decreased across Tanner Stage.
Table 1.
Means and Standard Deviations of Variables by Tanner Stage among Adolescent Girls and Boys
| Tanner Stage | ||||||
|---|---|---|---|---|---|---|
| Variables | I | II | III | IV | V | F-value |
| Girls: | (n = 14) | (n = 43) | (n = 67) | (n = 47) | (n = 9) | |
| Boys: | (n = 6) | (n = 42) | (n = 43) | (n = 62) | (n = 11) | |
| Free testosterone, ng/dl | ||||||
| Girls | 0.06 (0.05) | 0.16 (0.13) | 0.27 (0.15) | 0.35 (0.16) | 0.33 (0.19) | 16.25*** |
| Boys | 0.19 (0.20) | 0.58 (0.55) | 2.03 (1.95) | 6.03 (3.03) | 7.68 (2.38) | 55.57*** |
| Cohesion | ||||||
| Girls | 55.00 (12.08) | 55.00 (12.89) | 59.90 (10.91) | 55.51 (16.15) | 61.33 (7.91) | 1.57 |
| Boys | 65.33 (4.13) | 57.40 (11.69) | 59.21 (8.54) | 56.94 (13.53) | 63.18 (6.95) | 1.45 |
| Delinquency (T scores) | ||||||
| Girls | 60.79 (4.21) | 57.44 (3.76) | 57.37 (3.70) | 57.87 (3.85) | 56.22 (1.72) | 2.95* |
| Boys | 55.00 (0.00) | 57.07 (3.30) | 56.63 (2.94) | 58.16 (5.41) | 58.91 (4.06) | 1.81 |
| Aggression (T scores) | ||||||
| Girls | 56.57 (3.23) | 56.42 (3.10) | 57.01 (5.04) | 57.26 (4.59) | 56.22 (3.67) | 0.29 |
| Boys | 55.00 (0.00) | 57.60 (4.44) | 56.98 (4.35) | 57.71 (5.02) | 58.09 (3.53) | 0.67 |
Note: p <.05;
p <.01;
p <.001
One-way ANOVAs revealed no significant differences in family cohesion or behavioral outcomes by gender or racial/ethnic group. Among boys, parental income was negatively correlated with delinquent behaviors, r(164) = −0.17, p <.05. Income was also positively associated with family cohesion, r(164) = 0.20, p < 0.01. Therefore, income was included as a covariate in multivariate analyses.
Correlations among free testosterone, family environment, and child outcome variables
Correlational analyses were performed to examine associations among free testosterone, family cohesion, delinquent and aggressive behaviors, and serum lipid levels by gender (see Table 2). Among girls, family cohesion was negatively correlated with delinquent (r= −0.20, p < 0.01) and aggressive behaviors (r = −0.34, p < 0.001). Levels of free testosterone were negatively associated with delinquent behaviors, r = −0.15, p < 0.05. Boys’ data are presented in the lower half of Table 3. Among boys, a similar pattern was observed with family cohesion being negatively related to delinquent (r = −0.35, p < 0.001) and aggressive behaviors, r = −0.29, p < 0.001. In contrast to girls, free testosterone was positively associated with delinquent behaviors among adolescent boys, r = 0.22, p < 0.01.
Table 2.
Intercorrelations of Study Variables for Girls and Boys
| Variable | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|
| Girls | |||||||
| 1. Free testosterone | −0.15 | −0.15* | 0.02 | 0.34*** | 0.08 | −0.15* | −0.06 |
| 2. Cohesion | -- | −0.20** | −0.34*** | −0.10 | −0.002 | 0.02 | 0.01 |
| 3. Delinquency | -- | 0.59*** | 0.02 | 0.10 | 0.17* | 0.21** | |
| 4. Aggression | -- | 0.13 | −0.03 | 0.07 | 0.07 | ||
| 5. Body mass index | -- | −0.29*** | 0.17* | 0.12 | |||
| 6. HDL-C | -- | −0.05 | 0.21** | ||||
| 7. LDL-C | -- | 0.85*** | |||||
| 8. Total cholesterol | -- | ||||||
|
| |||||||
| Boys | |||||||
| 1. Free testosterone | −0.03 | 0.22** | 0.12 | 0.09 | −0.21** | −0.43*** | −0.43*** |
| 2. Cohesion | -- | −0.35*** | −0.29*** | 0.09 | −0.05 | 0.11 | 0.03 |
| 3. Delinquency | -- | 0.74*** | 0.06 | 0.06 | −0.08 | −0.04 | |
| 4. Aggression | -- | 0.02 | 0.05 | 0.004 | −0.03 | ||
| 5. Body mass index | -- | −0.28*** | 0.16* | 0.08 | |||
| 6. HDL-C | -- | 0.06 | 0.36** | ||||
| 7. LDL-C | -- | 0.84*** | |||||
| 8. Total cholesterol | -- | ||||||
Note: p <.05;
p <.01;
p <.001.
Table 3.
Associations of Free Testosterone and Family Cohesion with Adolescent Boys’ and Girls’ Delinquent Behaviors
| Boys |
Girls |
|||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | β | ΔR2 | B | SE | β | ΔR2 | |
| Step 1 | 0.07 | 0.07* | ||||||
| Tanner stage | 0.28 | 0.44 | 0.07 | −0.10 | 0.33 | −0.03 | ||
| BMI | 0.07 | 0.10 | 0.06 | 0.41 | 0.09 | 0.04 | ||
| Parental income | −0.32 | 0.29 | −0.08 | −0.48 | 0.27 | −0.13 | ||
| HDL-C | 0.06 | 0.04 | 0.14 | 0.41 | 0.03 | 0.10 | ||
| LDL-C | 0.02 | 0.03 | 0.11 | 0.01 | 0.03 | 0.05 | ||
| Total cholesterol | −0.004 | 0.03 | −0.03 | 0.02 | 0.03 | 0.11 | ||
| Step 2 | 0.10*** | 0.04** | ||||||
| Family cohesion | −0.02 | 0.04 | −0.06 | −0.15 | 0.04 | −0.50*** | ||
| Step 3 | 0.02 | 0.02 | ||||||
| Free testosterone | 1.72 | 0.51 | 1.40*** | −22.36 | 8.21 | −0.97** | ||
| Step 4 | 0.04** | 0.03* | ||||||
| Testosterone × cohesion | −0.02 | 0.01 | −1.22** | 0.33 | 0.14 | 0.89* | ||
| Total R2 | 0.24*** | 0.15*** | ||||||
Note: p < 0.05;
p < 0.01;
p < 0.001. Beta estimates are shown for the final model only.
Associations of free testosterone and family cohesion with delinquent and aggressive behaviors
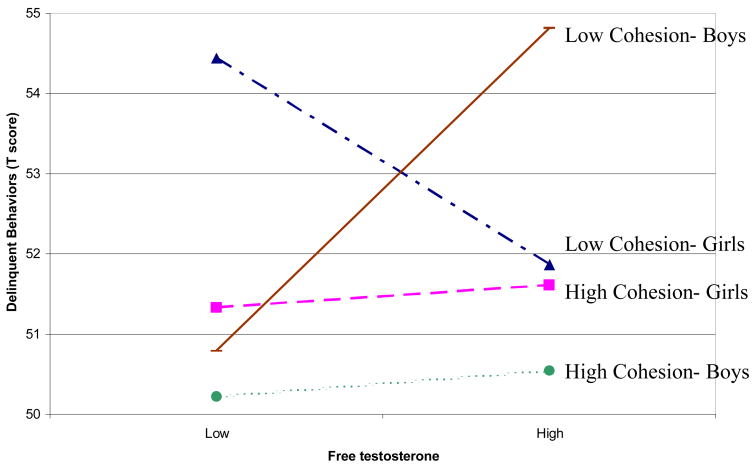
To evaluate the associations of free testosterone and family cohesion with behavioral outcomes, separate hierarchical multiple regression analyses were conducted for boys and girls. Among boys, free testosterone was positively associated with delinquent behaviors (see Table 3). This main effect was qualified by a two-way interaction of free testosterone and family cohesion. The interaction effect was interpreted according to the guidelines outlined by Aiken and West [23], which involves developing the simple regression equations where delinquent behaviors are regressed upon free testosterone at high and low levels of family cohesion. Low values of family cohesion were defined as one standard deviation below the sample mean, and high values as one standard deviation above the mean. As illustrated in Figure 1, free testosterone was positively associated with delinquent behaviors, but only among boys in low cohesion family settings. Under conditions of high family cohesion, no association between free testosterone and delinquent behaviors emerged.
Figure 1.
Family cohesion moderates the association between free testosterone and delinquent behaviors among adolescent boys and girls.
Among girls, free testosterone and family cohesion were negatively associated with delinquent behaviors (Table 3). These main effects were qualified by a two-way interaction, which was interpreted as described above. Specifically, under conditions of low family cohesion, free testosterone was negatively associated with delinquent behaviors (see Figure 1). Again, in highly cohesive families, no association between free testosterone and delinquent behaviors was observed.
For aggressive behaviors, only family cohesion was associated with aggressive behaviors among adolescent girls (β = −0.45, t = −3.00, p <.01). For boys, LDL-C was positively associated with aggressive behaviors (β = 0.37, t = 2.27, p <.05). Levels of HDL-C (β = 0.19, t = 1.91, p =.058) and free testosterone (β = 0.85, t = 1.92, p =.057) were also positively associated with aggressive behaviors, but these did not reach statistical significance at the two-tailed level. The interaction term (free testosterone × cohesion) was not associated with aggressive behavior in either boys or girls.
Discussion
In the present study, family cohesion moderated the relation between free testosterone and delinquent behaviors in adolescent boys and girls. Specifically, in highly cohesive families, no association between free testosterone and delinquent behaviors was observed; whereas under conditions of low family cohesion, increasing levels of free testosterone were associated with greater delinquent behaviors among boys. The opposite pattern was observed among adolescent girls. That is, under conditions of high family cohesion, no association between free testosterone and delinquent behaviors was observed (similar to the boys), but a negative association between free testosterone and delinquent behaviors was observed among girls in low cohesion family settings. The inverse association between testosterone and delinquent behaviors in girls may be attributed, in part, to societal pressures on girls to behave in gender-appropriate ways, particularly as they approach adolescence [24]. Indeed, our data suggest a linear negative association between delinquent behaviors and pubertal maturation (i.e. Tanner staging) in girls, which supports the proposition that, as girls get older, delinquent behaviors are viewed as inappropriate and are heavily sanctioned by society [13].
Our findings parallel those reported by Booth and colleagues [12], in which the association between testosterone and risk behaviors was conditional on the quality of the parent-child relationship. Indeed, a similar pattern of findings was reported in the earlier study, as testosterone was positively associated with risk behaviors among boys and negatively associated with risk behaviors among girls, but only among those adolescents with poor quality parent-child relations. Moreover, our findings are consistent with numerous previous studies that have reported positive family factors (e.g., parental involvement and warmth) to be predictive of lower rates of delinquent behavior during adolescence [8, 9]. Other studies have reported that adolescents from cohesive families, or those with close parent-child relations, are more likely to experience better self-esteem [25], which may then decrease the likelihood of engaging in delinquent behaviors [26].
In light of the cross-sectional nature of the present analyses, it is important to consider possible alternative explanations that may exist. For example, perhaps the performance of delinquent or risky behaviors leads to increases in testosterone levels among adolescent boys. Indeed early studies have reported that changes in dominance behaviors can cause changes in testosterone levels (for a review, see [27]). Thus, one alternative hypothesis is that delinquent boys are more likely to engage in greater risk taking behaviors, which increases their testosterone levels -- and that these behaviors are also more likely to occur in the context of low cohesion homes, where parental monitoring of adolescents may be less vigilant. Because the present study did not include assessments of parenting variables, this alternative hypothesis remains to be investigated in future studies.
High family cohesion was protective with respect to delinquent behaviors, but low family cohesion was associated with aggressive behaviors among adolescent girls. A number of prior studies have underscored the critical impact of family environment on the development of behavioral problems in children [8], and empirical data indicate that adverse conditions in the family (e.g., low family support) are key risk factors for poor adjustment outcomes [28]. These factors may be especially relevant for girls, as studies have demonstrated that adolescent girls are generally more affected by familial factors than are boys [29].
With regards to cholesterol and aggressive behaviors, LDL-C was significantly related to aggressive behaviors but only among adolescent boys. The present finding of a positive association between LDL-C and aggressive behaviors is in contrast to several previous studies that have documented negative associations between cholesterol and aggressive behaviors [30]. On the other hand, some early studies reported that increased levels of hostility were associated with elevated LDL-C levels [31]. In a study of participants in a cholesterol-lowering program, improvements in diet were associated with reductions in aggressive hostility and plasma cholesterol levels [32]. More recent studies have also reported a positive association of total serum cholesterol and non-physical forms of aggression (e.g., verbal aggression, hostility) among healthy adults [14]. In addition, total serum cholesterol was positively related to aggressiveness among college males who were not physically fit [33], which may reflect a similar sample as the adolescent boys in the current study who presented with unfavorable lipid profiles. Finally, one study reported higher levels of aggression to be associated with higher levels of LDL-C among young adult males who had a propensity toward aggressive behavior [34].
The biological pathways that may account for the association between cholesterol and aggressive behaviors have been proposed to involve stress and the catecholaminergic system [35]. In brief, this model proposes that activation of the sympathetic nervous system during stress results in increased production of catecholamines and cortisol and elevated blood pressure, which results in elevated blood lipid concentrations. In one study, middle-aged adults displayed significant increases in LDL-C following an acute laboratory stressor [36]. Further, exposure to chronic stress has been shown to result in greater overt aggressive responses among men, but not among women [37]. Thus, in the context of the present study, it may be that higher levels of LDL-C reflect greater exposure to stress and that it is this stress exposure that contributes to greater aggressive behaviors among the adolescent boys. Although the proposed pathways are biologically plausible and have been empirically demonstrated in adults, comparable studies have yet to be conducted in adolescent populations and thus, our explanation for the observed association between LDL-C and aggressive behavior remains speculative. Nonetheless, the present findings highlight potential associations among lipid levels and behavioral outcomes and suggest that such relationships are gender dependent.
In conclusion, the findings from the present study contribute to the growing literature on the interactions between biological and social processes and their associations with adolescent behavioral outcomes. A key strength of the study was the ability to assess pubertal development and physical growth independently from testosterone levels. However, there are also several limitations. First, ratings of family cohesion and behavioral problems were provided by the female parent. It is possible that the mother’s ratings could have differed from the child’s perceptions. Other studies, however, have demonstrated significant convergence across family members in reports of family environment, specifically using the FES [38]. Further, although parental characteristics could have influenced the reporting of behavioral problems, it appears that these characteristics do not lead to increased variability or changes in the relative ranking of specific problem behaviors [39]. Second, due to the small number of adolescent girls who had reached menarche, the implications of our findings apply primarily to pre-menarcheal adolescent girls. Associations between mean levels of free testosterone and behavioral problems may differ in post-menarchal girls. Third, due to the cross-sectional nature of the data, any causal role for the interaction between biological and social factors in contributing to adolescent behavioral outcomes cannot be determined. Prospective studies involving multiple assessments of such factors over time are needed to address these key questions.
In sum, the present findings suggest that child gender and family environment moderate the association between free testosterone and delinquent behaviors in adolescent boys and girls. Because adolescent behavioral problems are early indicators of long-term risk for poor adjustment, a comprehensive understanding regarding how social factors, particularly those inherent in the family, may modify the association between testosterone and delinquency is critical. Family-centered interventions can be effective in reducing problem behaviors and can be targeted to children and adolescents in at-risk settings.
Acknowledgments
Preparation of this manuscript was supported in part by National Cancer Institute grant K22CA107115 and P30CA006927. The Dietary Intervention Study in Children (DISC) Hormone Ancillary Study (HAS) was supported by Public Health Service cooperative agreements U01-HL37947, U01-HL37948, U01-HL37954, U01-HL37962, U01-HL37966, U01-HL37975, and U01-HL38110 from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), Department of Health and Human Services (DHHS), and supplemented by funding from the National Cancer Institute, NIH, DHHS. We thank the girls and boys who participated in the DISC and their families.
Contributor Information
Carolyn Y. Fang, Fox Chase Cancer Center
Brian L. Egleston, Fox Chase Cancer Center
Kathleen M. Brown, Maryland Medical Research Institute
John V. Lavigne, Children’s Memorial Hospital and Northwestern University Medical School.
Victor J. Stevens, Kaiser Permanente Center for Health Research
Bruce A. Barton, Maryland Medical Research Institute
Donald W. Chandler, Esoterix Endocrinology, Inc
Joanne F. Dorgan, Fox Chase Cancer Center.
References
- 1.Angold A, Costello EJ, Erkanli A, et al. Pubertal changes in hormone levels and depression in girls. Psychological Medicine. 1999 Sep;29(5):1043–1053. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- 2.Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol. 2006 Oct;101(4):1252–1261. doi: 10.1152/japplphysiol.01095.2005. [DOI] [PubMed] [Google Scholar]
- 3.Maras A, Laucht M, Gerdes D, et al. Association of testosterone and dihydrotestosterone with externalizing behavior in adolescent boys and girls. Psychoneuroendocrinology. 2003 Oct;28(7):932–940. doi: 10.1016/s0306-4530(02)00119-1. [DOI] [PubMed] [Google Scholar]
- 4.Pajer K, Tabbah R, Gardner W, et al. Adrenal androgen and gonadal hormone levels in adolescent girls with conduct disorder. Psychoneuroendocrinology. 2006 Nov;31(10):1245–1256. doi: 10.1016/j.psyneuen.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 5.van Bokhoven I, van Goozen SH, van Engeland H, et al. Salivary testosterone and aggression, delinquency, and social dominance in a population-based longitudinal study of adolescent males. Hormones & Behavior. 2006 Jun;50(1):118–125. doi: 10.1016/j.yhbeh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Granger DA, Shirtcliff EA, Zahn-Waxler C, et al. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: individual differences and developmental effects. Dev Psychopathol. 2003 Spring;15(2):431–449. [PubMed] [Google Scholar]
- 7.Susman EJ, Inoff-Germain G, Nottelmann ED, et al. Hormones, emotional dispositions, and aggressive attributes in young adolescents. Child Development. 1987;58(4):1114–1134. [PubMed] [Google Scholar]
- 8.Lucia VC, Breslau N. Family cohesion and children’s behavior problems: a longitudinal investigation. Psychiatry Res. 2006 Feb 28;141(2):141–149. doi: 10.1016/j.psychres.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Matherne MM, Thomas A. Family environment as a predictor of adolescent delinquency. Adolescence. 2001 Winter;36(144):655–664. [PubMed] [Google Scholar]
- 10.Johnson HD. Associations among family adaptability and cohesion, interparental conflict, and tactics used during young adults’ conflict with parents. Psychol Rep. 2002 Aug;91(1):315–325. doi: 10.2466/pr0.2002.91.1.315. [DOI] [PubMed] [Google Scholar]
- 11.Updegraff KA, Booth A, Thayer SM. The role of family relationship quality and testosterone levels in adolescents’ peer experiences: a biosocial analysis. Journal of Family Psychology. 2006 Mar;20(1):21–29. doi: 10.1037/0893-3200.20.1.21. [DOI] [PubMed] [Google Scholar]
- 12.Booth A, Johnson DR, Granger DA, et al. Testosterone and child and adolescent adjustment: the moderating role of parent-child relationships. Developmental Psychology. 2003 Jan;39(1):85–98. doi: 10.1037//0012-1649.39.1.85. [DOI] [PubMed] [Google Scholar]
- 13.Calvete E, Cardenoso O. Gender Differences in Cognitive Vulnerability to Depression and Behavior Problems in Adolescents. Journal of Abnormal Child Psychology. 2005;33(2):179–192. doi: 10.1007/s10802-005-1826-y. [DOI] [PubMed] [Google Scholar]
- 14.Hillbrand M, Waite BM, Rosenstein M, et al. Serum cholesterol concentrations and non-physical aggression in healthy adults. J Behav Med. 2005 Jun;28(3):295–299. doi: 10.1007/s10865-005-4665-y. [DOI] [PubMed] [Google Scholar]
- 15.DISC Collaborative Research Group. Dietary intervention study in children (DISC) with elevated low-density-lipoprotein cholesterol. Design and baseline characteristics. DISC Collaborative Research Group. Ann Epidemiol. 1993 Jul;3(4):393–402. doi: 10.1016/1047-2797(93)90067-e. [DOI] [PubMed] [Google Scholar]
- 16.Tanner JM. Growth at adolescence. 3. Oxford: Blackwell Scientific; 1962. [Google Scholar]
- 17.Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- 18.Moos Moos BS. Family Environment Scale. 2. Palo Alto, CA: Consulting Psychologists Press; 1986. [Google Scholar]
- 19.Furuyama S, Mayes DM, Nugent CA. A radioimmunoassay for plasma testosterone. Steroids. 1970 Oct;16(4):415–428. doi: 10.1016/s0039-128x(70)80124-6. [DOI] [PubMed] [Google Scholar]
- 20.Sodergard R, Backstrom T, Shanbhag V, et al. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982 Jun;16(6):801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 21.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999 Oct;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 22.Rinaldi S, Geay A, Dechaud H, et al. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev. 2002 Oct;11(10):1065–1071. [PubMed] [Google Scholar]
- 23.Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park: Sage; 1991. [Google Scholar]
- 24.Huston A, Alvarez M. The socialization context of gender role development in early adolescence. In: Montemayor R, Adams G, Gullotta T, editors. Advances in adolescent development: Vol 2. The transition from childhood to adolescnece. Beverly Hills, CA: Sage; 1990. pp. 156–179. [Google Scholar]
- 25.Laible DJ, Carlo G, Roesch SC. Pathways to self-esteem in late adolescence: the role of parent and peer attachment, empathy, and social behaviours. J Adolescence. 2004 Dec;27(6):703–716. doi: 10.1016/j.adolescence.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Donnellan MB, Trzesniewski KH, Robins RW, et al. Low self-esteem is related to aggression, antisocial behavior, and delinquency. Psychol Sci. 2005 Apr;16(4):328–335. doi: 10.1111/j.0956-7976.2005.01535.x. [DOI] [PubMed] [Google Scholar]
- 27.Mazur A, Booth A. Testosterone and dominance in men. The Behavioral and brain sciences. 1998 Jun;21(3):353–363. discussion 363–397. [PubMed] [Google Scholar]
- 28.Windle M. A longitudinal study of stress buffering for adolescent problem behaviors. Developmental Psychology. 1992;28:522–530. [Google Scholar]
- 29.Davies PT, Lindsay LL. Interparental conflict and adolescent adjustment: why does gender moderate early adolescent vulnerability? J Family Psychology. 2004 Mar;18(1):160–170. doi: 10.1037/0893-3200.18.1.160. [DOI] [PubMed] [Google Scholar]
- 30.Golomb BA. Cholesterol and violence: is there a connection? Ann Intern Med. 1998 Mar 15;128(6):478–487. doi: 10.7326/0003-4819-128-6-199803150-00009. [DOI] [PubMed] [Google Scholar]
- 31.Weidner G, Sexton G, McLellarn R, et al. The role of type A behavior and hostility in an elevation of plasma lipids in adult women and men. Psychosom Med. 1987;49(2):136–145. doi: 10.1097/00006842-198703000-00004. 1987 March 1. [DOI] [PubMed] [Google Scholar]
- 32.Weidner G, Connor SL, Hollis JF, et al. Improvements in hostility and depression in relation to dietary change and cholesterol lowering. The Family Heart Study. Ann Intern Med. 1992 Nov 15;117(10):820–823. doi: 10.7326/0003-4819-117-10-820. [DOI] [PubMed] [Google Scholar]
- 33.Greene RE, Jr, Houston BK, Holleran SA. Aggressiveness, dominance, developmental factors, and serum cholesterol level in college males. J Behav Med. 1995 Dec;18(6):569–580. doi: 10.1007/BF01857896. [DOI] [PubMed] [Google Scholar]
- 34.Troisi A, D’Argenio A. Apolipoprotein A-I/apolipoprotein B ratio and aggression in violent and nonviolent young adult males. J Psychiatr Res. 2006 Aug;40(5):466–472. doi: 10.1016/j.jpsychires.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Niaura R, Stoney CM, Herbert PN. Lipids in psychological research: the last decade. Biol Psychol. 1992 Oct;34(1):1–43. doi: 10.1016/0301-0511(92)90022-m. [DOI] [PubMed] [Google Scholar]
- 36.Stoney CM, Niaura R, Bausserman L, et al. Lipid reactivity to stress: I. Comparison of chronic and acute stress responses in middle-aged airline pilots. Health Psychol. 1999 May;18(3):241–250. doi: 10.1037//0278-6133.18.3.241. [DOI] [PubMed] [Google Scholar]
- 37.Verona E, Kilmer A. Stress exposure and affective modulation of aggressive behavior in men and women. J Abnorm Psychol. 2007 May;116(2):410–421. doi: 10.1037/0021-843X.116.2.410. [DOI] [PubMed] [Google Scholar]
- 38.Sheeber L, Sorensen E. Family relationships of depressed adolescents: a multimethod assessment. J Clin Child Psychol. 1998 Oct;27(3):268–277. doi: 10.1207/s15374424jccp2703_4. [DOI] [PubMed] [Google Scholar]
- 39.Youngstrom E, Loeber R, Stouthamer-Loeber M. Patterns and correlates of agreement between parent, teacher, and male adolescent ratings of externalizing and internalizing problems. Journal of Consulting & Clinical Psychology. 2000 Dec;68(6):1038–1050. doi: 10.1037//0022-006x.68.6.1038. [DOI] [PubMed] [Google Scholar]