Abstract
Limited data suggest that athletes may have a higher risk of developing atrial fibrillation (AF); however there has been no large prospective assessment of the relationship between vigorous exercise and AF. Logistic regression analyses stratified by time were utilized to assess the association between frequency of vigorous exercise and risk of developing AF among 16,921 apparently healthy men in the Physicians’ Health Study. During 12 years of follow-up, 1,661 men reported developing AF. With increasing frequency of vigorous exercise (0, <1, 1−2, 3−4, 5−7 days per week), the multivariate relative risks for the full cohort were 1.0 (referent), 0.90, 1.09, 1.04, 1.20 (p=0.04). This risk was not significantly elevated when exercise habits were updated or in models excluding variables that may be in the biologic pathway through which exercise influences AF risk. In sub-group analyses, this elevated risk was observed only in men below age 50 [1.0, 0.94, 1.20, 1.05, 1.74 (p<0.01)] and joggers [1.0, 0.91, 1.03, 1.30, 1.53 (p<0.01)], where risks remained elevated in all analyses. In conclusion, frequency of vigorous exercise was associated with an increased risk of developing AF among young men and joggers. This risk diminished as the population aged and was offset by known beneficial effects of vigorous exercise on other AF risk factors.
Keywords: Atrial fibrillation, exercise, arrhythmia
Although vigorous exercise has numerous health benefits, case reports and limited data suggest that male elite athletes engaging in endurance exercise that enhances parasympathetic tone, particularly jogging, may be at higher risk for the development of atrial fibrillation (AF).1,2,3 There are limited data on the role of vigorous exercise in the development of AF among men participating in exercise at a less competitive level, where the known beneficial effects of exercise may counter balance this potential risk. We hypothesized that young men in whom parasympathetic tone is most pronounced may be at highest risk of developing AF. To further define the risks and benefits of exercise on AF risk, we prospectively examined the relationship between amount and type of vigorous exercise and subsequent development of AF among men in the Physicians’ Health Study.
Methods
The methods of the Physicians’ Health Study have been described in detail elsewhere.4 The study complies with the Declaration of Helsinki, was approved by the Institutional Review Board of Brigham and Women's Hospital and the participants gave informed consent. Briefly, 22,071 male physicians 40 − 84 years old in 1982 with no history of myocardial infarction, stroke, transient ischemic attacks, or cancer were randomized to aspirin and/or beta-carotene using a double-blind, placebo-controlled, two-by-two factorial design. Information on health status, risk factors for cardiovascular disease and AF, along with dietary and lifestyle factors was collected via questionnaires. Participants who failed to complete the 3-year follow-up questionnaire regarding exercise habits (n=678) and those who reported a diagnosis of myocardial infarction, angina, percutaneous coronary artery intervention, coronary artery bypass surgery, transient ischemic attack, cerebrovascular disease, claudication, peripheral vascular surgery, congestive heart failure or cancer prior to the 3-year questionnaire (n=1,572) were excluded from the analyses.
Physicians were asked at 15, 17, 18 and 19 years after enrollment if they had ever been diagnosed with AF and the date of diagnosis. In addition, participants were asked annually to report any new medical conditions. Participants were excluded from the analyses if they did not return at least 1 questionnaire regarding AF diagnosis (n=2,352) and had not reported AF on 1 of the annual questionnaires. As AF onset often precedes diagnosis by months, and AF often leads to fatigue and exercise intolerance that might effect exercise habits, participants who reported developing AF prior to or within 3 years after the 3-year exercise questionnaire were excluded (n=548).5 After these exclusions, 16,921 participants were available for this study.
Three and 9 years after enrollment, information on exercise habits was collected. The questionnaires asked, “Do you engage in a regular program of exercise vigorous enough to work up a sweat? - Yes/No.” The use of “exercise vigorous enough to break a sweat” has been validated to correlate with maximal oxygen uptake capacity and maximum exercise capacity on treadmill testing.6,7 Individuals who responded “Yes” were asked questions characterizing their exercise pattern. Exercise frequency was reported with response options of “less than 1 day per week, 1−2 days, 3−4 days, 5−7 days.” The duration of each exercise episode was asked with responses of “10 mins or less, 11−24 mins, 25−40 mins, 41+ mins.” The 3-year questionnaire asked, “What types of vigorous exercise do you engage in? Racquet sports, swimming, jogging/running, cycling (including indoor), other.” This was followed with, “If you jog/run, how long is your usual distance? 1 mile or less, 1.1−2 miles, 2.1−3 miles, 3.1−4 miles, more than 4 miles.”
AF cases were assessed by self-report. Although physicians have been documented to reliably self-report other cardiovascular endpoints such as angina and coronary revascularization, we performed a validation study on 400 randomly selected participants who reported AF on the 15-year questionnaire to determine the reliability of a self-reported diagnosis.8 These men were sent supplementary questionnaires with detailed questions regarding their diagnosis and treatment of AF, and a request for permission to review AF documentation.
Of these men, 352 (88%) provided information regarding the previous self-report of AF. Nine of the 48 non-respondents were deceased. Of these respondents, 39% had paroxysmal, 32% had persistent and 29% had permanent AF. Medical records were available for 225 men, confirming the self-reported diagnosis of AF in 99% (n=223). Another 101 men reported that medical record documentation existed, but was currently unavailable. In only 26 of the respondents (7%) was the diagnosis of AF not further supported. In addition to the 2 cases disconfirmed by medical record review, 12 reported that they had never had a history of AF (misclassification on the 15-year survey) and 14 reported that no medical records or electrocardiographic data ever existed to corroborate the diagnosis of AF (self-diagnosis). Although the participant's report of AF was found to be reliable, the date of AF diagnosis was not reliably reported. The mean difference between patient reported diagnosis dates and medical records diagnosis dates was 4.5 ± 7.8 years.
Means or proportions of baseline risk factors and treatment group assignment were computed for the 5 categories of vigorous exercise reported on the 3-year questionnaire. The significance of associations was tested using the Mantel-Haenszel Chi-square test for trend for categorical and linear regression for continuous variables. In the primary analyses, we examined whether frequency of participation in a regular program of vigorous exercise, as reported on the 3-year questionnaire, was associated with the development of AF. Secondary analyses examined the relationship between duration and type of vigorous exercise and AF development. To examine associations specific to each type of vigorous exercise, participants who reported engaging in more than 1 exercise type were excluded from the analyses, and participants who did not exercise regularly and did not engage in any exercise types served as the reference group.
Because exercise habits change over time and because the effects of exercise on the development of AF may change as the population ages, our prespecified analysis plan included a secondary analysis using updated exercise data from 9 years for cases of AF that developed 3 years after the 9-year exercise questionnaire (1,059 of 1,661 AF cases). Since the date of AF onset was often assessed retrospectively, and our validation study suggested that the participant's reported date of diagnosis might be unreliable, it was prespecified to use logistic regression stratified by time based upon the dates of the AF questionnaires, rather than Cox regression, to obtain adjusted estimates of AF risk.
Three separate models were developed. Model 1 controlled for age and treatment assignment (aspirin or placebo, beta carotene or placebo), and Models 2 and 3 simultaneously controlled for additional AF risk factors. These 2 multivariable models were constructed to control for confounding while considering the effects of exercise on biological processes within the potential causal pathway for AF development. For example, participants with higher body mass index (BMI) may be less likely to exercise, and since BMI has been previously shown to independently predict the development of AF, it is a possible confounder. However, exercise reduces BMI, thus BMI is possibly part of the means through which exercise may affect AF development. The first multivariable model (Model 2) considers all AF risk factors influenced by exercise, like BMI, as lying completely within the causal pathway. Model 2 therefore excludes these variables. In contrast, to adjust for possible confounding by a variable such as BMI, the variable must be included in the model. Model 3 treats risk factors as being solely confounders.
Because of the long follow-up, these variables were updated at various timepoints. To test for a trend between either exercise frequency or duration and development of AF, frequency and duration were redefined continuously by assigning each participant the midpoint value of the appropriate response category. To test for plausible effect modification by age, cross-product terms between age and exercise frequency at 3 years were added to all 3 models. Analyses were also repeated after stratification by age at the time when exercise habits were reported.
Results
At 3 years, 63% of participants reported engaging in a regular program of vigorous exercise and 13% reported exercising 5−7 times/week (Table 1). Among regular exercisers, the mean total amount of time spent exercising per week was 108 ± 78 minutes (Figure 1). Frequency of vigorous exercise was inversely associated with age, BMI, smoking, diabetes and hypertension; and directly associated with alcohol, fish, multivitamin, vitamin C and vitamin E intake.
Table 1.
Relation of frequency of vigorous exercise to baseline clinical characteristics and risk factors for atrial fibrillation
| Characteristic | Non-Exercisers | <1 day/week | 1−2 days/week | 3−4 days/week | 5−7 days/week | P Value |
|---|---|---|---|---|---|---|
| Total cohort | 6321, (37.4%) | 762, (4.5%) | 3215, (19.0%) | 4496, (26.6%) | 2127, (12.6%) | |
| Age, years (mean)* | 52.6 | 50.0 | 51.1 | 51.1 | 52.1 | <0.01 |
| Body Mass Index (mean) (kg/m2)*, | 25.1 | 24.8 | 24.7 | 24.4 | 24.0 | <0.01 |
| Parental myocardial infarction before age 60* | 8.9% | 10.4% | 10.2% | 9.6% | 10.0% | 0.10 |
| Smoking status* | ||||||
| Never | 49.0% | 55.7% | 53.6% | 54.8% | 51.9% | <0.01 |
| Past smoker | 37.4% | 35.4% | 37.0% | 38.8% | 43.1% | <0.01 |
| Current smoker | 13.8% | 9.1% | 9.4% | 6.5% | 5.0% | <0.01 |
| Medical Conditions | ||||||
| Diabetes Mellitus† | 3.1% | 2.2% | 2.5% | 1.6% | 1.9% | <0.01 |
| High cholesterol level†,‡ | 14.0% | 11.5% | 14.1% | 14.2% | 13.3% | 0.93 |
| Hypertension†,§ | 29.5% | 23.5% | 25.1% | 23.4% | 21.9% | <0.01 |
| Left ventricular hypertrophy∥ | 0.28% | 0.26% | 0.16% | 0.22% | 0.28% | 0.62 |
| Episodes of alcohol intake* | ||||||
| <1 time/week | 28.9% | 24.2% | 22.7% | 23.6% | 24.7% | <0.01 |
| 1−6 times/week | 46.9% | 55.3% | 54.1% | 54.3% | 50.9% | <0.01 |
| 1 or more times/day | 24.2% | 20.5% | 23.2% | 22.2% | 24.4% | 0.25 |
| Fish consumption: ≥1 meal per week# | 89.5% | 91.0 | 92.1% | 92.2% | 90.9% | <0.01 |
| Multivitamin consumption* | 32.1% | 32.6% | 34.4% | 35.7% | 39.2% | <0.01 |
| Vitamin C consumption* | 19.5% | 23.2% | 23.3% | 24.7% | 26.7% | <0.01 |
| Vitamin E consumption* | 8.3% | 8.4% | 9.2% | 11.1% | 12.6% | <0.01 |
Information ascertained on baseline questionnaire
Information ascertained on baseline and 24 month questionnaire
The diagnosis of high cholesterol level was based upon self-report, a total cholesterol level ≥ 260 mg/dl (6.7 mmol/liter) or the use of cholesterol lowering medications.
Hypertension was diagnosed based on a self-reported systolic blood pressure ≥ 160mm Hg, a diastolic blood pressure ≥ 90 or the use of antihypertensive medications.
Information ascertained on 15-year questionnaire
Information ascertained on 12-month questionnaire
Figure 1.
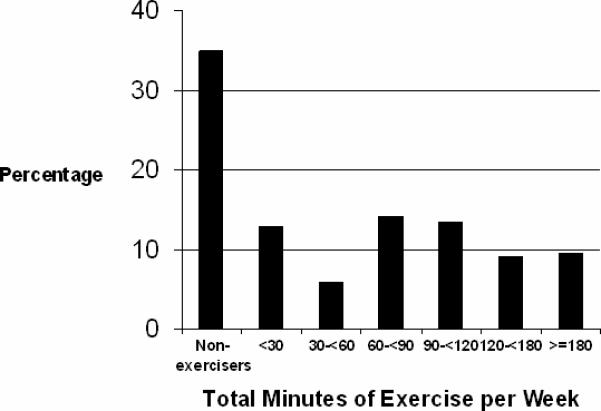
Distribution of total exercise time per week (minutes).
Frequency of vigorous exercise at 3 years was not associated with the subsequent development of AF in age-adjusted models or in Model 2 which excluded covariates that were medical conditions and/or risk factors that could be influenced by exercise. After controlling for all potential confounding variables and medical conditions associated with AF (Model 3), increasing frequency of vigorous exercise at 3 years was associated with a small increased risk of developing AF (for variables in each model see legend, Table 2). Men who exercised 5−7 times/week had the greatest risk of developing AF compared to those who did not exercise (p=0.025). The above analyses were repeated using exercise habits updated at 9 years. In the maximally adjusted model, the most frequent exercisers still had a marginal, but not significantly increased risk of developing AF (RR 1.16; 95% CI, 0.99 to 1.36, p=0.076); however, the trend over categories was no longer statistically significant. Duration of exercise was not related to the development of AF in any of the 3-year or 9-year updated models (data not shown).
Table 2.
Relative risk of atrial fibrillation according to frequency of habitual vigorous exercise
| Variable: Frequency of Habitual Vigorous Exercise | Non-Exercisers | <1 day/wk | 1−2 days/wk | 3−4 days/wk | 5−7 days/wk | P Value for Trend |
|---|---|---|---|---|---|---|
| Exercise at 3 Years | ||||||
| Age-Adjusted (Model 1)* | ||||||
| Relative risk | 1.0 | 0.90 | 1.04 | 0.99 | 1.15 | |
| 95% confidence interval | 0.69−1.18 | 0.91−1.20 | 0.87−1.12 | 0.98−1.33 | 0.17 | |
| Multivariable Model 2† | ||||||
| Relative risk | 1.0 | 0.87 | 1.05 | 1.00 | 1.13 | |
| 95% confidence interval | 0.66−1.15 | 0.91−1.22 | 0.87−1.14 | 0.97−1.33 | 0.19 | |
| Multivariable Model 3‡ | ||||||
| Relative risk | 1.0 | 0.90 | 1.09 | 1.04 | 1.20 | |
| 95% confidence interval | 0.68−1.20 | 0.95−1.26 | 0.91−1.19 | 1.02−1.41 | 0.040 | |
| Updated Exercise | ||||||
| Age-Adjusted (Model 1)* | ||||||
| Relative risk | 1.0 | 1.09 | 1.02 | 0.96 | 1.08 | |
| 95% confidence interval | 0.83−1.43 | 0.88−1.18 | 0.85−1.09 | 0.93−1.26 | 0.69 | |
| Multivariable Model 2† | ||||||
| Relative risk | 1.0 | 1.09 | 1.02 | 0.96 | 1.07 | |
| 95% confidence interval | 0.82−1.44 | 0.88−1.18 | 0.84−1.09 | 0.92−1.26 | 0.76 | |
| Multivariable Model 3‡ | ||||||
| Relative risk | 1.0 | 1.14 | 1.06 | 1.01 | 1.16 | |
| 95% confidence interval | 0.86−1.51 | 0.91−1.23 | 0.89−1.16 | 0.99−1.36 | 0.21 | |
Model 1 controlled for age and treatment assignment (aspirin or placebo, beta carotene or placebo).
Model 2 excluded possible physiologic intermediaries between exercise and AF. Model 2 controlled for age, treatment assignment (aspirin or placebo, beta carotene or placebo), parental history of premature myocardial infarction, alcohol intake, smoking habits, fish consumption, multivitamin intake, vitamin C intake and vitamin E intake.
Model 3 controlled for age, treatment assignment (aspirin or placebo, beta carotene or placebo), BMI, history of diabetes, history of hypertension, history of hyperlipidemia, parental history of premature myocardial infarction, alcohol intake, smoking habits, fish consumption, multivitamin intake, vitamin C intake, vitamin E intake, left ventricular hypertrophy, congestive heart failure, and evidence of cardiovascular disease.
Since prior associations between exercise and AF had been reported primarily among young men, and the relationship observed here diminished over the course of the study, we hypothesized that the effects of vigorous exercise on the incidence of AF may diminish as the population ages. To test this hypothesis, it was prespecified to stratify men by their age (<50, 50−65, >65) at the time of the 3-year exercise questionnaire. Among men under age 50, increasing frequency of exercise was associated with an increased risk of developing AF across all 3 models (Table 3). The elevation in risk again appeared limited to those who exercised 5−7 times/week. In contrast, there was no association among men over age 50, and the test for an interaction between exercising 5−7 times/week and age above or below 50 was significant in the full multivariate model (p=0.02). When the age stratified analyses were repeated using updated exercise habits at 9 years, exercise frequency across the 5 categories was not significantly associated with AF in any age group; however, the risk of AF remained significantly elevated among men under age 50 at the time of the 9-year questionnaire who exercised 5 or more times per week (RR, 2.08; 95% CI, 1.16 to 3.71, p=0.014). The test for interaction between age above and below 50 and this category of exercise was not statistically significant in the updated model (p=0.12).
Table 3.
Relative risk of atrial fibrillation according to frequency of habitual vigorous exercise at 3 years, stratified by age group
| Variable: Frequency of Habitual Vigorous Exercise | Non-Exercisers | <1 day/week | 1−2 days/week | 3−4 days/week | 5−7 days/week | P Value for Trend |
|---|---|---|---|---|---|---|
| Age Less than 50 Years | ||||||
| Age-Adjusted (Model 1)* | ||||||
| Relative risk | 1.0 | 0.96 | 1.15 | 0.95 | 1.69 | |
| 95% confidence interval | 0.56−1.63 | 0.84−1.57 | 0.71−1.28 | 1.22−2.33 | 0.014 | |
| Multivariable Model 2† | ||||||
| Relative risk | 1.0 | 0.95 | 1.22 | 1.00 | 1.69 | |
| 95% confidence interval | 0.55−1.66 | 0.88−1.68 | 0.73−1.36 | 1.21−2.37 | 0.015 | |
| Multivariable Model 3‡ | ||||||
| Relative risk | 1.0 | 0.94 | 1.20 | 1.05 | 1.74 | |
| 95% confidence interval | 0.53−1.67 | 0.87−1.66 | 0.76−1.43 | 1.23−2.47 | <0.01 | |
| Age 50 Years or Greater, but Less than Age 65 Years | ||||||
| Age-Adjusted (Model 1)* | ||||||
| Relative risk | 1.0 | 0.87 | 1.01 | 0.97 | 1.09 | |
| 95% confidence interval | 0.61−1.24 | 0.85−1.21 | 0.82−1.14 | 0.88−1.34 | 0.57 | |
| Multivariable Model 2† | ||||||
| Relative risk | 1.0 | 0.88 | 1.02 | 0.97 | 1.07 | |
| 95% confidence interval | 0.61−1.27 | 0.85−1.22 | 0.82−1.15 | 0.87−1.33 | 0.65 | |
| Multivariable Model 3‡ | ||||||
| Relative risk | 1.0 | 0.93 | 1.07 | 1.03 | 1.16 | |
| 95% confidence interval | 0.65−1.35 | 0.89−1.29 | 0.86−1.22 | 0.93−1.44 | 0.25 | |
| Age 65 Years or Greater | ||||||
| Age-Adjusted (Model 1)* | ||||||
| Relative risk | 1.0 | 1.00 | 1.06 | 1.15 | 0.94 | |
| 95% confidence interval | 0.51−1.95 | 0.77−1.46 | 0.88−1.49 | 0.67−1.31 | 0.97 | |
| Multivariable Model 2† | ||||||
| Relative risk | 1.0 | 0.78 | 1.03 | 1.12 | 0.92 | |
| 95% confidence interval | 0.37−1.64 | 0.75−1.43 | 0.86−1.47 | 0.66−1.29 | 0.99 | |
| Multivariable Model 3‡ | ||||||
| Relative risk | 1.0 | 0.81 | 1.10 | 1.12 | 0.97 | |
| 95% confidence interval | 0.39−1.71 | 0.79−1.53 | 0.85−1.48 | 0.69−1.37 | 0.86 | |
Model 1 controlled for age and treatment assignment (aspirin or placebo, beta carotene or placebo).
Model 2 excluded possible physiologic intermediaries between exercise and AF. Model 2 controlled for age, treatment assignment (aspirin or placebo, beta carotene or placebo), parental history of premature myocardial infarction, alcohol intake, smoking habits, fish consumption, multivitamin intake, vitamin C intake and vitamin E intake.
Model 3 controlled for age, treatment assignment (aspirin or placebo, beta carotene or placebo), BMI, history of diabetes, history of hypertension, history of hyperlipidemia, parental history of premature myocardial infarction, alcohol intake, smoking habits, fish consumption, multivitamin intake, vitamin C intake, vitamin E intake, left ventricular hypertrophy, congestive heart failure, and evidence of cardiovascular disease.
Participants who exercised regularly were then subdivided based on the type of vigorous exercise reported at 3-years (Figure 2). In the maximally adjusted model, men who regularly and exclusively jogged (9.6% of the cohort) were at increased risk of developing AF (p<0.01), while no significant elevation in risk was found with regular cycling, swimming or racquet sports. Increased frequency of jogging was associated with an increased risk of developing AF in all 3 previously defined models (Figure 3). Men who jogged exclusively 5−7 times/week had a significantly increased risk of developing AF (RR, 1.53; 95% CI, 1.12 to 2.09, p<0.01) compared to men who did not exercise vigorously after controlling for multiple cardiovascular risk factors. There was also a direct statistically significant relationship between miles jogged per episode and the development of AF across all 3 models. In the maximally adjusted model, men who jogged < 4 miles were at the highest risk (RR, 1.38; 95% CI, 1.06 to 1.79, p=0.016). When the primary analyses were repeated in the subgroup of participants who did not jog regularly (n=12,721), no association between frequency of vigorous exercise and the development of AF was found in the age-adjusted or the multivariate models (data not shown).
Figure 2.
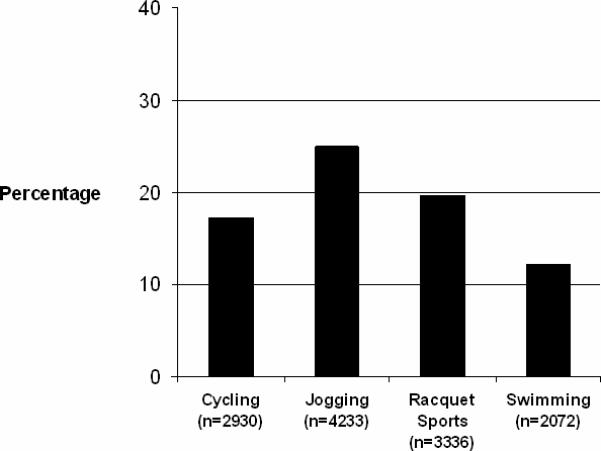
Frequency of participation in different sports activities.
Figure 3.
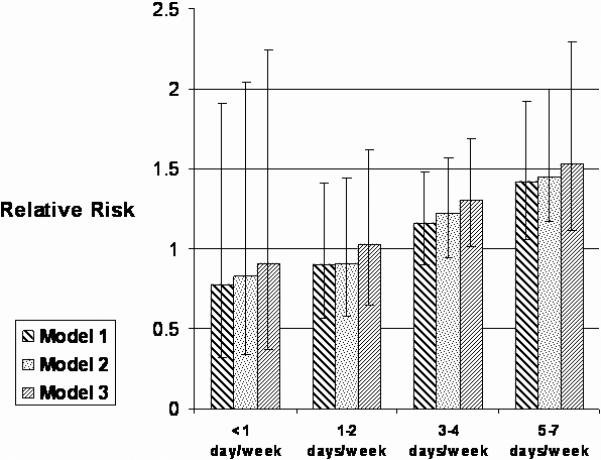
Relative risk of AF according to jogging frequency at 3 years.
Discussion
In this large prospective cohort study of apparently healthy men, a complex association between exercise and the development of AF was observed. After adjustment for multiple potentially confounding lifestyle factors and health conditions, higher frequency of participation in a regular program of vigorous exercise at 3 years was associated with a modestly increased risk of developing AF. This elevated risk was primarily among those who exercised 5−7 times weekly. These men had a 20% increased risk of developing AF compared to those who did not exercise. The relationship between vigorous exercise and AF was no longer significant when exercise habits were updated at 9 years or in models excluding possible biologic intermediaries. The increase in risk diminished with increasing age. In men 50 years or older, no significant association was found; while in men younger than 50 years who exercised 5−7 times/week, the association was statistically significant in all analyses.
Secondary analyses found that frequency of jogging was most strongly associated with the development of AF. Men who jogged 5 or more days per week, had a 53% increased risk of developing AF compared to men who did not exercise, after controlling for multiple risk factors. When joggers were excluded, the relationship between frequency of vigorous exercise and AF was no longer present, suggesting that jogging may account for much of the association.
These results expand on prior epidemiologic data regarding the relationship between exercise and AF, which is limited to case series and small case control studies. Case series have suggested that the incidence of AF may be higher in athletes.1,2,9 A retrospective case-control study found a higher incidence of long-term sport activity among men with lone AF compared to controls.10,11,12,13 In a small prospective study, the 10-year cumulative incidence of lone AF in 300 top-ranked runners was 5.3% compared to 0.9% in 495 healthy controls.5 Although limited, these data are consistent with our findings that frequent endurance exercise, particularly jogging, may increase the risk of developing AF. In addition, the mean age of the exercisers in all studies was < 60, consistent with our finding that the association is strongest in younger populations.13
There are several mechanisms through which frequent exercise might influence risk of AF, including left atrial enlargement, left ventricular hypertrophy, left ventricular dilation, and the most commonly cited mechanism, an increase in parasympathetic tone.2,14,15 Left atrial size is significantly increased in competitive athletes, and left atrial size is a strong and independent risk factor for AF.16,17 However, left atrial size does not appear to explain the entire association between exercise and AF. In a case-control study including 107 lone AF patients, greater participation in cumulative moderate and heavy physical activity was significantly associated with the development of AF even after controlling for left atrial size.11 Part of this unexplained risk may be due to an increase in parasympathetic activity among habitual exercisers. Jogging in particular result in a greater enhancement of the parasympathetic nervous system compared to other exercise types.18,19 Heightened parasympathetic tone has been associated with AF onset in patients with structurally normal hearts, and in both animal and human studies, parasympathetic stimulation frequently induces and maintains AF, while vagal denervation prevents AF. 20,21,22
Aging results in reduced parasympathetic activity.23 Therefore, chronic exercise in older individuals may lead to a less significant enhancement of parasympathetic activity. Also, lone AF comprises a smaller proportion of AF cases among older individuals where underlying structural heart disease is more common. This may explain why no association between exercise and AF in older men was found. Alternatively, men susceptible to AF as a result of exercise may have developed AF at a younger age and therefore would be excluded from analyses of older populations. It is also possible that participants exercised less vigorously as they aged, diminishing the power to detect an association between exercise and AF.
In addition to the pro-fibrillatory effects, exercise has multiple beneficial effects on cardiovascular health that may lower AF risk. Exercise lowers blood pressure, improves lipid profile and glucose control, and reduces risk of cardiovascular disease. Removal of the potentially inappropriate control of these intermediaries (Model 2) either eliminated or significantly attenuated the association between exercise and AF when compared to maximally adjusted analyses (Model 3). This suggests that any pro-fibrillatory effect of exercise is counterbalanced by additional anti-fibrillatory effects in the majority of, but not the entire population.
A number of limitations warrant discussion. First, our measure of physical activity, although correlated with VO2 max, is limited compared to more objective measures of physical fitness. Although we assessed exercise habits at 2 time points, physician habits may have further changed over time. If such misclassification of exercise habits were random, this may have reduced our ability to detect associations between exercise habits and AF. AF can be occult and serial electrocardiograms were not available for the entire cohort, thus under-detection may exist in these analyses, although less likely in a cohort of physicians. As AF was self-reported, men who exercise may be more likely to notice they are in AF and seek medical attention. It is possible that participants who exercised frequently developed undiagnosed AF resulting in reduced exercise tolerance that could have influenced exercise habits leading to an underestimation of the association between exercise and AF. The limited number of men under age 50 at the time of the 9-year exercise questionnaire (n=949, 6.3%) may have limited our power to detect an association in the updated analysis.
With respect to generalizability, our findings are limited by the selective nature of the cohort, healthy male physicians free of known cardiovascular disease at baseline. It is unclear if this same relationship between exercise and AF extends to women or less healthy populations. Any participant who died prior to 1997 (n= 1,713) did not complete 1 of the AF questionnaires. As a result, participants with healthy lifestyle habits, such as exercise, may have been over-represented.
Although the primary, secondary, and subgroup analyses were pre-specified, concern for multiple comparisons is warranted. However the consistency and strength of the association of exercise with the development of AF among men age < 50 and joggers, and prior epidemiologic and physiologic studies supporting these findings, supports the validity of these results. Finally, as with any observational study, our study cannot prove causality and the associations observed here could be due, at least in part, to residual confounding. However, vigorous exercise was directly associated with several AF risk factors, and therefore, it is also possible that more complete control for risk factors would have strengthened the inverse associations observed.
Acknowledgments
Supported by grants from the NIH (CA-47988 and HL-43851).
References
- 1.Coelho A, Palileo E, Ashley W, Swiryn S, Petropoulos AT, Welch WJ, Bauernfeind RA. Tachyarrhythmias in young athletes. J Am Coll Cardiol. 1986;7:237–243. doi: 10.1016/s0735-1097(86)80287-x. [DOI] [PubMed] [Google Scholar]
- 2.Hood S, Northcote RJ. Cardiac assessment of veteran endurance athletes: a 12 year follow up study. Br J Sports Med. 1999;33:239–243. doi: 10.1136/bjsm.33.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karjalainen J, Kujala UM, Kaprio J, Sarna S, Viitasalo M. Lone AF in vigorously exercising middle aged men: case-control study. BMJ. 1998;316:1784–1785. doi: 10.1136/bmj.316.7147.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Steering Committee of the Physicians’ Health Study Research Group Final report in the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129–335. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 5.Page RL, Tilsch TW, Connolly SJ, Schnell DJ, Marcello SR, Wilkinson WE, Pritchett EL. Asymptomatic or “silent” atrial fibrillation: frequency in untreated patients and patients receiving azimilide. Circulation. 2003;107:1141–1145. doi: 10.1161/01.cir.0000051455.44919.73. [DOI] [PubMed] [Google Scholar]
- 6.Siconolfi SF, Lasater TM, Snow RCK, Carleton RA. Self-reported physical activity compared with maximal oxygen uptake. Am J Epidemiol. 1985;122:101–105. doi: 10.1093/oxfordjournals.aje.a114068. [DOI] [PubMed] [Google Scholar]
- 7.Kohl HW, Blair SN, Paffenbarger RS, Jr., Macera CA, Kronenfeld JJ. A mail survey of physical activity habits as related to measured physical fitness. Am J Epidemiol. 1988;127:1228–1239. doi: 10.1093/oxfordjournals.aje.a114915. [DOI] [PubMed] [Google Scholar]
- 8.Manson JE, Grobbee DE, Stampfer MJ, Taylor JO, Goldhaber SZ, Gaziano JM, Ridker PM, Buring JE, Hennekens CH. Aspirin in the primary prevention of angina pectoris in a randomized trial of United States physicians. Am J Med. 1990;89:772–776. doi: 10.1016/0002-9343(90)90220-8. [DOI] [PubMed] [Google Scholar]
- 9.Heidbuchel H, Anne W, Willems R, Adriaenssens B, Van de Werf F, Ector H. Endurance sports is a risk factor for atrial fibrillation after ablation of atrial flutter. Int J Cardiol. 2006;107:67–72. doi: 10.1016/j.ijcard.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 10.Mont L, Sambola A, Brugada J, Vacca M, Marrugat J, Elosua R, Pare C, Azqueta M, Sanz G. Long-lasting sports practice and lone atrial fibrillation. Eur Heart J. 2002;23:477–482. doi: 10.1053/euhj.2001.2802. [DOI] [PubMed] [Google Scholar]
- 11.Mont L, Tamborero D, Elosua R, Molina I, Coll-Vincent B, Sitges M, Vidal B, Scalise A, Tejeira A, Berruezo A, Brugada J. Physical activity, height, and left atrial size are independent risk factors for lone atrial fibrillation in middle-aged healthy individuals. Europace. 2008;11:15–20. doi: 10.1093/europace/eum263. [DOI] [PubMed] [Google Scholar]
- 12.Elosua R, Arquer A, Mont L, Sambola A, Molina L, Garcia-Moran E, Brugada J, Marrugat J. Sport practice and the risk of lone atrial fibrillation: A case-control study. Int J Cardiol. 2006;108:332–337. doi: 10.1016/j.ijcard.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Baldesberger S, Bauersfeld U, Candinas R, Sefiert B, Zuber M, Ritter M, Jenni R, Oechslin E, Luthi P, Scharf C, Marti B, Attenhofer Jost CH. Sinus node disease and arrhythmias in the long-term follow-up of former professional cyclists. Eur Heart J. 2008;29:71–78. doi: 10.1093/eurheartj/ehm555. [DOI] [PubMed] [Google Scholar]
- 14.Northcote R, McKillop G, Todd I, Canning G. The effect of habitual sustained endurance exercise on cardiac structure and function. Eur Heart J. 1990;11:17–22. doi: 10.1093/oxfordjournals.eurheartj.a059585. [DOI] [PubMed] [Google Scholar]
- 15.Kasikcioglu E, Oflaz H, Akhan H, Kayserilioglu A, Umman B, Bugra Z, Erzengin F. Left atrial geometric and functional remodeling in athletes. Int J Sports Med. 2006;27:267–271. doi: 10.1055/s-2005-865660. [DOI] [PubMed] [Google Scholar]
- 16.Pelliccia A, Maron MJ, Di Paolo FM, Biffi A, Quattrini FM, Pisicchio C, Roselli A, Caselli S, Culasso F. Prevalence and clinical significance of left atrial remodeling in competitive athletes. J Am Coll Cardiol. 2005;46:690–696. doi: 10.1016/j.jacc.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 17.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 18.Jost J, Weiss M, Weicker H. Comparison of sympatho-adrenergic regulation at rest and of the adrenoceptor system in swimmers, long-distance runners, weight lifters, wrestlers and untrained men. Eur J Appl Physiol Occup Physiol. 1989;58:596–604. doi: 10.1007/BF00418505. [DOI] [PubMed] [Google Scholar]
- 19.Talan DA, Bauernfeind RA, Ashley WW, Kanakis C, Jr., Rosen KM. Twenty-four hour continuous ECG recordings in long distance runners. Chest. 1982;1:19–24. doi: 10.1378/chest.82.1.19. [DOI] [PubMed] [Google Scholar]
- 20.Huang JL, Wen ZC, Lee WL, Chang MS, Chen SA. Changes in autonomic tone before the onset of paroxysmal atrial fibrillation. Int J Cardiol. 1998;66:275–283. doi: 10.1016/s0167-5273(98)00241-1. [DOI] [PubMed] [Google Scholar]
- 21.Schuaerte P, Scherlag BJ, Pitha J, Scherlag MA, Reynolds D, Lazzara R, Jackman WM. Catheter ablation of cardiac autonomic nerves for the prevention of vagal atrial fibrillation. Circulation. 2000;102:2774–2780. doi: 10.1161/01.cir.102.22.2774. [DOI] [PubMed] [Google Scholar]
- 22.Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 23.Oida E, Kannagi T, Moritani T, Yamori Y. Aging alteration of cardiac vagosympathetic balance assessed through tone-entropy analysis. J Gerontol A Biol Sci Med Sci. 1999;54:M219–224. doi: 10.1093/gerona/54.5.m219. [DOI] [PubMed] [Google Scholar]


