Abstract
Purpose: To determine whether a type of contrast medium (CM), iodixanol, modifies outer medullary descending vasa recta (DVR) vasoreactivity and nitric oxide (NO) production in isolated microperfused DVR.
Materials and Methods: Animal handling conformed to the Animal Care Committee Guidelines of all participating institutions. Single specimens of DVR were isolated from rats and perfused with a buffered solution containing iodixanol. A concentration of 23 mg of iodine per milliliter was chosen to mimic that expected to be used in usual examinations in humans. Luminal diameter was determined by using video microscopy, and NO was measured by using fluorescent techniques.
Results: Iodixanol led to 52% reduction of DVR luminal diameter, a narrowing that might interfere with passage of erythrocytes in vivo. Vasoconstriction induced by angiotensin II was enhanced by iodixanol. Moreover, iodixanol decreased NO bioavailability by more than 82%. Use of 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (a superoxide dismutase mimetic) prevented both vasoconstriction with iodixanol alone and increased constriction with angiotensin II caused by CM.
Conclusion: Iodixanol in doses typically used for coronary interventions constricts DVR, intensifies angiotensin II–induced constriction, and reduces bioavailability of NO. CM-induced nephropathy may be related to these events and scavenging of reactive oxygen species might exert a therapeutic benefit by preventing the adverse effects that a CM has on medullary perfusion.
© RSNA, 2009
Contrast medium (CM)–induced nephropathy (CIN) is a life-threatening complication of radiologic examinations and procedures in which an iodinated CM is used (1). In Europe and the United States, CIN accounts for more than 10% of all causes of hospital-acquired acute renal failure (2).
Percutaneous coronary angiography is associated with a high risk of CIN in patients with preexisting chronic kidney disease: Roughly one-half of these patients develop acute renal failure (3) that is associated with a 25% 1-year mortality rate (4). Several prior investigators have studied whether infusing sodium bicarbonate or scavenging reactive oxygen species (ROS) with ascorbic acid or N-acetylcysteine can prevent CIN (5–10). The intrarenal events that underlie CIN remain controversial, and the mechanism by which scavenging ROS might exert a therapeutic benefit remains to be determined.
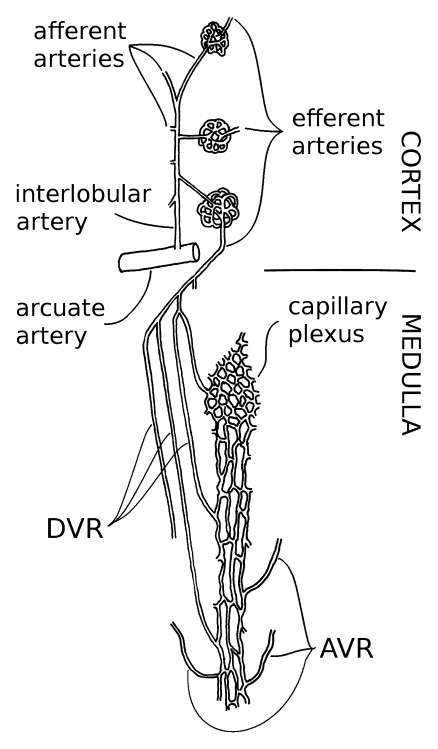
Evidence supports a high degree of vulnerability of the renal outer medulla to CM-induced damage (11). That propensity has been attributed to the nature of tubular-vascular relationships (12). Vulnerability of the thick ascending limbs to ischemic insult therefore may be an inescapable consequence of the anatomic arrangements in the outer medulla. In summary, descending vasa recta (DVR) are microvessels whose luminal diameters approach the dimensions of an erythrocyte and are anatomically positioned to regulate regional blood flow to the medulla (13) wherein they serve conflicting roles of tissue oxygenation and counter current trapping of solute (Fig 1). Enhancement of DVR contraction through endothelial dysfunction might therefore have dire pathologic consequences to yield acute renal dysfunction.
Figure 1:
Blood flow to renal medulla is supplied entirely by DVR, 12–18-μm-diameter microvessels that originate near the corticomedullary junction as branches of efferent arterioles of juxtamedullary glomeruli. DVR are lined by continuous endothelium and are surrounded by smooth muscle–like pericytes that impart contractility. DVR traverse outer medulla of kidney in vascular bundles, closely juxtaposed to ascending vasa recta (AVR) that return from inner medulla. Countercurrent exchange between DVR and ascending vasa recta traps and recycles NaCl and urea. That exchange is necessary to preserve corticomedullary gradients that enable urinary concentration but also results in shunting of oxygen from DVR to ascending vasa recta, thereby lowering oxygen tensions in medulla. DVR peel off from periphery of outer medullary vascular bundles to perfuse capillary plexus of interbundle region wherein salt-transporting, metabolically demanding thick ascending limbs of Henle loop reside (13).
Outer medullary DVR are inaccessible in vivo, so that technically demanding methods for isolation must be used to study their functional characteristics. Studies of angiotensin II and nitric oxide (NO) responses have revealed their respective capacities to constrict and dilate DVR (13), and evidence points to a tonic role for NO in preserving medullary blood flow and preventing ischemic injury to the medulla (13–15). NO also inhibits salt reabsorption, thereby reducing oxygen demands within the outer medulla (16). In contrast, angiotensin II constricts DVR (17), enhances sodium reabsorption (18), and favors formation of oxygen-free radicals (19).
The purpose of our study was to determine whether a type of CM, iodixanol, modifies DVR vasoreactivity and NO production in isolated microperfused DVR.
MATERIALS AND METHODS
Isolation and Perfusion of DVR
Animal handling conformed to the Animal Care Committee Guidelines of all participating institutions. Male Sprague-Dawley rats (n = 37) (Charles River Laboratories, Sulzfeld, Germany), with a weight range of 120–200 g, were sedated with intraperitoneally injected ketamine hydrochloride/xylazine hydrochloride solution (Sigma, Munich, Germany) and sacrificed by means of decapitation. The kidneys were removed, sliced along the corticomedullary axis, and placed in buffered solution at 4°C. The solution that was used for dissection and microperfusion of DVR and as the bath solution in the perfusion chamber contained 140 mmol/L NaCl, 10 mmol/L NaCH3COO, 5 mmol/L KCl, 1.2 mmol/L MgSO4, 1.2 mmol/L Na2HPO4, 1 mmol/L CaCl2, 5 mmol/L HEPES buffer, 5 mmol/L l-alanine, 0.1 mmol/L l-arginine, 5 mmol/L d-glucose, and 0.08 mmol/L albumin (all chemicals from Sigma, Munich, Germany). The pH was adjusted to 7.4 at 37°C. Single specimens of DVR from the renal outer medulla were dissected by using sharpened microforceps, transferred into a chamber on the stage of an inverted microscope, and maintained at 37°C. DVR were mounted on concentric pipettes, cannulated at one end, and held at the other end with a collection pipette in a manner that permitted free flow through the lumen, as previously described (20). Effectiveness of perfusion was tested throughout and at the end of experiments to ensure that changes in luminal diameter were not caused by technical problems.
Measurement of DVR Diameter
The luminal diameter of DVR was observed with a ×40 objective to yield a final magnification of ×1300. The experiments were recorded on digital video disks, and a computer with a frame grabber was used to acquire images during playback. Image analysis was performed with software (ImageJ; National Institutes of Health, Bethesda, Md) available at http://rsb.info.nih.gov/ij/, and luminal diameter was measured from acquired images. Vasoreactivity was quantified as the percentage of change of luminal diameter from the baseline measurement.
Fluorescent Detection of NO
To measure NO, 4-amino-5 methylamino-2, 7-difluorofluorescein (DAF-FM) diacetate (DAF-FM DA) (Sigma, Stockholm, Sweden) was used. DAF-FM DA is diacetylated intracellularly by esterases to DAF-FM. It reacts with NO in the presence of oxygen to form green-fluorescent triazolofluoresceins that provide a measure of intracellular NO bioavailability (21). DAF-FM DA was loaded into DVR by halting perfusion and adding it to the bath (2 × 10−5 mol/L, 30 minutes, room temperature). DAF-FM was excited at 480 nm, and the emission was isolated at 535 nm. Digital images were acquired every 30 seconds at a magnification of ×1300. Fluorescence intensity of DAF-FM was quantified as the percentage of change from the initial value.
Protocols
Vasoreactivity protocols.—After achieving stable perfusion, DVR were stabilized at 37°C for 10 minutes. Recording was then started, and after 1 minute of baseline image acquisition, the perfusate was exchanged to contain either iodixanol (Visipaque; GE Healthcare, Munich, Germany) with 23 mg of iodine per milliliter, a concentration chosen to mimic that expected in usual examinations in humans, or a vehicle (control). The constant flow of new solution inside the DVR, as well as the washing and income of new bath solution into the perfusion chamber, kept the differences between intra- and extravascular concentrations of iodixanol constant. In experiments where the NO synthase inhibitor N(G)-nitro-l-arginine methyl ester (l-NAME) (Sigma, Munich, Germany), at −10−4 mol/L, was used in the bath alone or a superoxide dismutase mimetic 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (Tempol; Sigma, Munich, Germany), at 10−3 mol/L, was used in the bath and perfusate, they were exchanged into the bath at the same time as the perfusate was exchanged. In all protocols, perfusion was maintained for 20 minutes. To test the effects of iodixanol on angiotensin II (Sigma, St Louis, Mo)–induced constriction, angiotensin II was exchanged at 5-minute intervals to concentrations of 10−10, 10−9, and 10−8 mol/L into the bath of DVR that had been exposed to iodixanol beforehand. Diameter measurements were performed during the last 5 seconds of each interval.
NO bioavailability protocols.—After achieving stable perfusion of DVR, flow was halted, and DAF-FM DA (2 × 10−5 mol/L) was added to the bath solution at room temperature for 30 minutes. At the end of that loading period, the perfusion chamber temperature was set to 37°C, and the perfusate was exchanged to contain iodixanol (23 mg of iodine per milliliter) or vehicle (control). When l-NAME (10−4 mol/L) was used, it was exchanged into the bath at the same time as the perfusate was exchanged. Perfusion was then restarted, providing flow as a stimulus to DVR endothelial production of NO (22). DAF-FM fluorescence images were captured at 30-second intervals throughout the 20-minute perfusion period. Luminal perfusion was then halted, and fluorescence was again recorded for 20 minutes.
Reagents
DAF-FM DA, angiotensin II, Tempol, and l-NAME were dissolved in aliquots, stored at −20°C, and thawed for daily use. Angiotensin II, l-NAME, and Tempol were dissolved in aliquots in water, and DAF-FM DA was dissolved in aliquots in dimethyl sulfoxide.
Statistical Analysis
Results were expressed as the mean ± standard error of the mean. The size of each sample is indicated for Figures 2–6. Data were compared by using the Brunner rank test (nonparametric analysis of variance–like test for repeated measurements) (23) (Appendix E1 [http://radiology.rsnajnls.org/cgi/content/full/2513081732/DC1]). A difference with P < .05 was used to reject the null hypothesis.
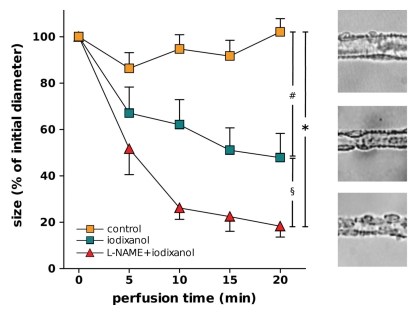
Figure 2:
Left: Luminal diameter of DVR treated with vehicle (control) perfusion (n = 10), iodixanol perfusion (n = 8), or l-NAME and iodixanol perfusion in bath (n = 6). Designations of significance are: * = P < .001, # = P < .005, § = P < .05. Right: Typical images of blood vessels from each of three groups.
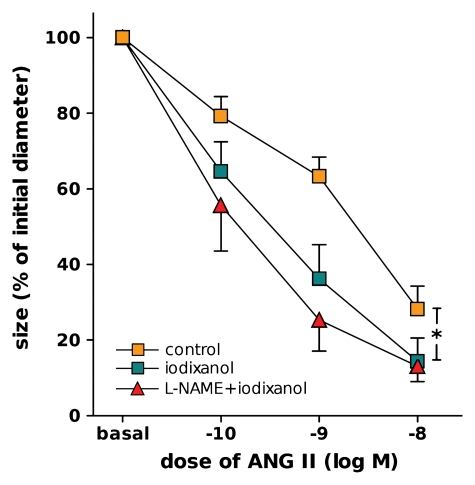
Figure 3:
Luminal diameter of DVR treated with vehicle (control) perfusion (n = 10), iodixanol perfusion (n = 8), or l-NAME and iodixanol perfusion in bath (n = 6) for 20 minutes, followed by exposure to log–mole-per-liter (M) increments of angiotensin II (ANG II) in bath (* = P < .05, control vs iodixanol).
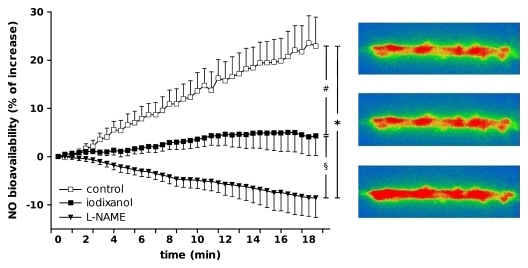
Figure 4:
Left: Relative changes of DAF-FM fluorescence over time as indicator of DVR NO bioavailability during perfusion with vehicle (control) (n = 9), iodixanol (n = 8), or l-NAME (n = 6). Designations of significance are * = P < .001, # = P < .01, § = P < .05. Right: Typical original images for vessels during experiment.
Figure 5:
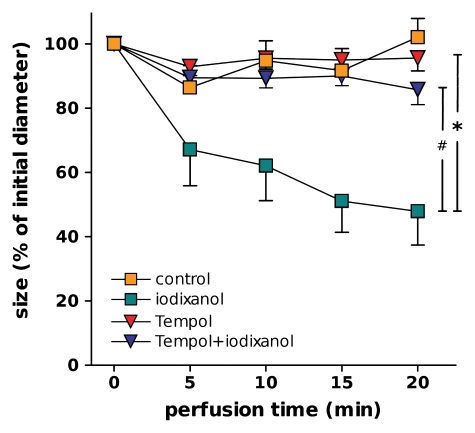
Luminal diameter of DVR perfused with vehicle (control) (n = 10), iodixanol (n = 8), Tempol (n = 6, perfusate and bath), or Tempol and iodixanol (n = 8). Designations of significance are * = P < .001, # = P < .01.
Figure 6:
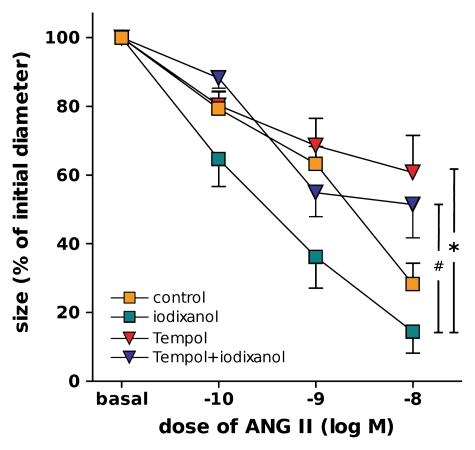
Luminal diameter of DVR during angiotensin II (ANG II) exposure. DVR were perfused with vehicle (control) (n = 10), iodixanol (n = 8), Tempol (n = 6, perfusate and bath), or Tempol and iodixanol (n = 8) for 20 minutes, followed by introduction of log–mole-per-liter (M) increments of angiotensin II into bath. Designations of significance are * = P < .005, # = P < .05.
RESULTS
Vasoreactivity during Perfusion of DVR with Iodixanol
As depicted in Figure 2, iodixanol alone significantly constricted DVR. After 20 minutes of perfusion with iodixanol, mean luminal diameter decreased to 48% of its initial value ± 10 (standard error of the mean), whereas that for the controls remained at a mean of 102% of the initial diameter ± 6. DVR constriction by using angiotensin II was also significantly enhanced by exposure to iodixanol beforehand (Fig 3).
Vasoreactivity during NO Synthase Blockade and Iodixanol Perfusion
The nonselective NO synthase inhibitor l-NAME was used to evaluate the contribution of NO bioavailability to the effects of iodixanol on DVR. As illustrated in Figure 2, compared with the effect with iodixanol alone, the combination of 20-minute exposure to iodixanol along with l-NAME enhanced DVR constriction (final mean diameter, 18% of initial diameter ± 5 for the combination vs 48% of initial diameter ± 10 for iodixanol alone).
The angiotensin II dose-response curves performed after exposure to iodixanol beforehand are shown in Figure 3. The combination of l-NAME and iodixanol did not significantly intensify angiotensin II–induced constriction when compared with iodixanol alone.
NO Bioavailability during and after Iodixanol Perfusion
Iodixanol significantly decreased NO bioavailability when compared with this factor in control buffer, as shown in Figure 4. At the end of 20-minute perfusion with iodixanol, the mean increase in DAF-FM fluorescence was 4.3% of the initial value ± 4.2, whereas the mean increase in controls was 23.9% of the initial value ± 6.3.
NO bioavalability in perfused DVR treated with l-NAME also is shown in Figure 4. Compared with controls, NO synthase blockade significantly reduced DAF-FM fluorescence (23.9% of initial value ± 6.3 vs −8.6% of initial value ± 3.2, respectively, at 20 minutes). Fluorescence measurements performed after flow cessation were not different between groups.
Vasoreactivity during ROS Scavenging and Iodixanol Perfusion
Because ROS may decrease NO bioavailability, we used the superoxide dismutase mimetic Tempol to test the contribution of ROS to iodixanol-induced DVR constriction. As shown in Figure 5, Tempol prevented the DVR constriction caused by iodixanol (final diameter, 85% of initial diameter ± 5 vs 48% of initial diameter ± 10, respectively).
The angiotensin II dose-response curves performed after iodixanol exposure beforehand are shown in Figure 6. Administration of Tempol and iodixanol prevented the changes in angiotensin II–induced constriction caused by iodixanol alone.
DISCUSSION
Our understanding of the mechanisms that underlie CIN is incomplete. Our study sheds new light on the intrarenal events: Iodixanol, a CM in a concentration chosen to mimic that expected to be used in usual examinations in humans, can constrict the blood vessels that supply blood flow to the outer medulla, the region of the kidney most commonly believed to be at risk for hypoxic damage (24). We showed that vasoconstriction induced by iodixanol may be related to reduction of NO bioavailability in DVR and that scavenging superoxide with a superoxide dismutase mimetic prevents iodixanol-induced vasoconstriction.
The injury caused by reduction of medullary blood flow resulting from CM exposure is generally confined to the outer medulla, as evidenced by necrosis of medullary thick ascending limbs, tubular collapse, and cast formation (15). Medullary blood flow is provided exclusively by highly specialized blood vessels, the vasa recta (13,14,25). These blood vessels transport solutes and water, express endothelial water channels and urea transporters, and are surrounded by pericytes that express α–smooth muscle actin (26), in the manner of other vascular smooth muscle cells. The area at risk for CIN (ie, the interbundle region of the inner stripe of the outer medulla) is supplied by a dense capillary plexus that arises from DVR on the periphery of vascular bundles. Vasoconstriction of those DVR may compromise the oxygen supply to this region, where the metabolically demanding process of salt transport takes place in the thick ascending limb.
In our study, we examined events that occur when a specific CM comes in contact with the DVR endothelium. Iodixanol, a dimeric late-generation nonionic CM, constricted isolated, microperfused DVR by roughly 50%. The DVR lumen is approximately 12–18 μm in diameter, compared with the 8-μm diameter of red blood cells. Were such constriction to occur in vivo, it might halt the flow of red blood cells. Evidence of red blood cell stasis in the medullary vasculature has been described in animal models of CIN, where it correlates with necrosis of thick ascending limbs (27). Similar evidence of complete obstruction of medullary vessels induced by CM was found by Liss et al (28).
The iodixanol-induced DVR vasoconstriction we observed also is consistent with prior observations of the reduction of medullary perfusion CM exposure (29–31). As previously shown, the viscous properties of some contrast media may underlie the reduction of medullary blood flow: Iodixanol, a hyperviscous CM, prolongs flow of blood through the renal medulla and reduces its oxygen supply (30). Our current observations in isolated DVR complement that whole animal study by demonstrating that exposure of the DVR endothelium to iodixanol causes direct vasoconstriction independent of its viscosity. The concentration used represents 13.9 times the dilution of the commercial solution, which brings the viscosity of the perfusing solution to values close to the control solution. This action could augment the effects of increased viscosity in reducing medullary blood flow observed in vivo.
Angiotensin II and NO are key controllers of DVR resistance (13,14). DVR may be exposed to varying levels of angiotensin II, depending on the activity of the renin-angiotensin system (32). In our study, iodixanol significantly augmented the vasoconstrictor response to angiotensin II. Hence, iodixanol may impair DVR blood flow by more than one route: through vasoconstriction of DVR and through enhancement of the vasoconstrictor response to angiotensin II. In the setting of CIN, angiotensin II might also stimulate sodium transport and enhance oxygen demand in the outer medulla (33).
Under control conditions, perfused DVR produce NO, which may be important to maintain luminal patency. During administration of iodixanol, basal NO production was markedly reduced, implying that blunted production of NO might favor DVR vasoconstriction during iodixanol exposure. This finding also is consistent with findings in experiments that show worsening of medullary perfusion induced by l-NAME after CM infusion in rats (34).
A competitive relationship may exist between NO formation and ROS generation (17). Angiotensin II promotes ROS generation, which can have deleterious effects, particularly when NO is limited (19,35). One consequence of increased ROS concentration is reduced NO bioavailability, which might enhance pericyte contraction, favoring DVR occlusion (35,36).
Taken together, iodixanol constricts DVR, reduces NO production by DVR, and augments angiotensin II–induced vasoconstriction. ROS may play a key role in mediating these effects, so that ROS scavenging could be an effective therapeutic target. Researchers in a great number of studies have set out to investigate the effectiveness of N-acetylcysteine and bicarbonate infusions in alleviating CIN (10,37,38). Our study may provide a basis for understanding the benefit of those therapies: Iodixanol-induced vasoconstriction of DVR was prevented by Tempol. Moreover, Tempol prevented iodixanol from augmenting vasoconstriction induced by angiotensin II.
Issues related to the clinical effectiveness of ROS scavenging remain to be resolved. For instance, it is unclear whether the doses used in the clinical trials are sufficient to effectively reduce ROS in outer medullary vascular bundles where DVR reside. Moreover, DVR are not the only sites where ROS are generated. The medullary thick ascending limb is another important source (35). Finally, N-acetylcysteine may not be as effective as Tempol in reducing superoxide concentrations.
Limitations of our study included the use of only one type of CM (ie, we do not know whether other contrast media, with different physicochemical properties, would have the same constrictory effect as iodixanol). Another limitation was the fact that Tempol cannot be used clinically, thus limiting the extrapolation of our findings to a clinical setting.
In conclusion, this study adds to our understanding of CIN by showing that the blood vessels that supply the vulnerable outer medulla of the kidney are significantly affected by a type of CM. DVR perfusion with iodixanol at a concentration similar to that which occurs in vivo during coronary angiography caused constriction, increased vasoconstrictor response to angiotensin II, and decreased NO bioavailability. A diminution of oxygen supply to the outer medulla seems a plausible consequence of those actions, which may ultimately result in tubular hypoxia and apoptosis. Superoxide scavenging effectively prevented the deleterious effect of iodixanol on DVR.
ROS may play a key role in mediating the effects of CM on DVR and on medullary perfusion, so that ROS scavenging might be an effective therapeutic target for CIN. Our study provides a basis for understanding the benefit of those therapies.
ADVANCES IN KNOWLEDGE
In isolated microperfused descending vasa recta (DVR), perfusion with iodixanol per se leads to constriction of DVR.
Iodixanol also decreased nitric oxide production and increased reactivity of DVR to angiotensin II.
The addition of scavenging superoxides in the perfusate and bath prevented changes in vasoreactivity owing to iodixanol.
IMPLICATIONS FOR PATIENT CARE
Our findings widen the current knowledge about the pathophysiology of contrast medium (CM)–induced nephropathy (CIN), and possible therapeutic approaches might be developed that are based on the findings.
This study adds to our understanding of CIN by showing that the blood vessels that supply the vulnerable outer medulla of the kidney are significantly affected by a type of CM.
Supplementary Material
Acknowledgments
We thank Dr Heimo Ehmke, Institute of Autonomic Physiology and Pathophysiology, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany, for thoughtful comments.
Abbreviations
CIN = CM-induced nephropathy
CM = contrast medium
DAF-FM = 4-amino-5 methylamino-2,7-difluorofluorescein
DAF-FM DA = DAF-FM diacetate
DVR = descending vasa recta
l-NAME = N(G)-nitro-l-arginine methyl ester
NO = nitric oxide
ROS = reactive oxygen species
Author contributions: Guarantors of integrity of entire study, M.S., A.P.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; manuscript final version approval, all authors; literature research, M.S., A.P., T.L.P., A.E.P., P.B.P.; experimental studies, M.S., A.P., T.L.P., C.C., A.E.P.; statistical analysis, M.S., A.P., T.L.P., P.B.P.; and manuscript editing, M.S., A.P., T.L.P., A.E.P., P.B.P.
Authors stated no financial relationship to disclose.
Funding: This research was supported by the National Institutes of Health (grants R37 DK42495 and R01 DK6762).
References
- 1.Wysowski DK, Nourjah P. Deaths attributed to x-ray contrast media on U.S. death certificates. AJR Am J Roentgenol 2006;186:613–615. [DOI] [PubMed] [Google Scholar]
- 2.Briguori C, Tavano D, Colombo A. Contrast agent–associated nephrotoxicity. Prog Cardiovasc Dis 2003;45:493–503. [DOI] [PubMed] [Google Scholar]
- 3.Liss P, Persson PB, Hansell P, et al. Renal failure in 57 925 patients undergoing coronary procedures using iso-osmolar or low-osmolar contrast media. Kidney Int 2006;70:1811–1817. [DOI] [PubMed] [Google Scholar]
- 4.Dangas G, Iakovou I, Nikolsky E, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol 2005;95:13–19. [DOI] [PubMed] [Google Scholar]
- 5.Schweiger MJ, Chambers CE, Davidson CJ, et al. Prevention of contrast induced nephropathy: recommendations for the high risk patient undergoing cardiovascular procedures. Catheter Cardiovasc Interv 2007;69:135–140. [DOI] [PubMed] [Google Scholar]
- 6.Fishbane S. N-acetylcysteine in the prevention of contrast-induced nephropathy. Clin J Am Soc Nephrol 2008;3:281–287. [DOI] [PubMed] [Google Scholar]
- 7.Stacul F, Adam A, Becker CR, et al. Strategies to reduce the risk of contrast-induced nephropathy. Am J Cardiol 2006;98:59K–77K. [DOI] [PubMed] [Google Scholar]
- 8.Spargias K, Alexopoulos E, Kyrzopoulos S, et al. Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation 2004;110:2837–2842. [DOI] [PubMed] [Google Scholar]
- 9.Tepel M, Aspelin P, Lameire N. Contrast-induced nephropathy: a clinical and evidence-based approach. Circulation 2006;113:1799–1806. [DOI] [PubMed] [Google Scholar]
- 10.Marenzi G, Assanelli E, Marana I, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med 2006;354:2773–2782. [DOI] [PubMed] [Google Scholar]
- 11.Heyman SN, Reichman J, Brezis M. Pathophysiology of radiocontrast nephropathy: a role for medullary hypoxia. Invest Radiol 1999;34:685–691. [DOI] [PubMed] [Google Scholar]
- 12.Brezis M, Rosen S. Hypoxia of the renal medulla: its implications for disease. N Engl J Med 1995;332:647–655. [DOI] [PubMed] [Google Scholar]
- 13.Pallone TL, Turner MR, Edwards A, et al. Countercurrent exchange in the renal medulla. Am J Physiol Regul Integr Comp Physiol 2003;284:R1153–R1175. [DOI] [PubMed] [Google Scholar]
- 14.Cowley AW Jr, Mori T, Mattson D, et al. Role of renal NO production in the regulation of medullary blood flow. Am J Physiol Regul Integr Comp Physiol 2003;284:R1355–R1369. [DOI] [PubMed] [Google Scholar]
- 15.Heyman SN, Brezis M, Reubinoff CA, et al. Acute renal failure with selective medullary injury in the rat. J Clin Invest 1988;82:401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrera M, Ortiz PA, Garvin JL. Regulation of thick ascending limb transport: role of nitric oxide. Am J Physiol Renal Physiol 2006;290:F1279–F1284. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Rhinehart K, Solis G, et al. Chronic ANG II infusion increases NO generation by rat descending vasa recta. Am J Physiol Heart Circ Physiol 2005;288:H29–H36. [DOI] [PubMed] [Google Scholar]
- 18.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol 2002;13:1131–1135. [DOI] [PubMed] [Google Scholar]
- 19.Mori T, O'Connor PM, Abe M, et al. Enhanced superoxide production in renal outer medulla of Dahl salt-sensitive rats reduces nitric oxide tubular-vascular cross-talk. Hypertension 2007;49:1336–1341. [DOI] [PubMed] [Google Scholar]
- 20.Pallone TL, Work J, Myers RL, et al. Transport of sodium and urea in outer medullary descending vasa recta. J Clin Invest 1994;93:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima H, Nakatsubo N, Kikuchi K, et al. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem 1998;70:2446–2453. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Pallone TL. Response of descending vasa recta to luminal pressure. Am J Physiol Renal Physiol 2004;287:F535–F542. [DOI] [PubMed] [Google Scholar]
- 23.Brunner E, Puri M. Nonparametric methods in factorial designs. Stat Papers 2001;42:1–52. [Google Scholar]
- 24.Brezis M, Epstein FH. A closer look at radiocontrast-induced nephropathy. N Engl J Med 1989;320:179–181. [DOI] [PubMed] [Google Scholar]
- 25.Mattson DL. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol 2003;284:R13–R27. [DOI] [PubMed] [Google Scholar]
- 26.Park F, Mattson DL, Roberts LA, et al. Evidence for the presence of smooth muscle alpha-actin within pericytes of the renal medulla. Am J Physiol 1997;273:R1742–R1748. [DOI] [PubMed] [Google Scholar]
- 27.Heyman SN, Brezis M, Epstein FH, et al. Early renal medullary hypoxic injury from radiocontrast and indomethacin. Kidney Int 1991;40:632–642. [DOI] [PubMed] [Google Scholar]
- 28.Liss P, Nygren A, Olsson U, et al. Effects of contrast media and mannitol on renal medullary blood flow and red cell aggregation in the rat kidney. Kidney Int 1996;49:1268–1275. [DOI] [PubMed] [Google Scholar]
- 29.Liss P, Nygren A, Hansell P. Hypoperfusion in the renal outer medulla after injection of contrast media in rats. Acta Radiol 1999;40:521–527. [DOI] [PubMed] [Google Scholar]
- 30.Seeliger E, Flemming B, Wronski T, et al. Viscosity of contrast media perturbs renal hemodynamics. J Am Soc Nephrol 2007;18:2912–2920. [DOI] [PubMed] [Google Scholar]
- 31.Lancelot E, Idee JM, Couturier V, et al. Influence of the viscosity of iodixanol on medullary and cortical blood flow in the rat kidney: a potential cause of nephrotoxicity. J Appl Toxicol 1999;19:341–346. [DOI] [PubMed] [Google Scholar]
- 32.Navar LG, Nishiyama A. Why are angiotensin concentrations so high in the kidney? Curr Opin Nephrol Hypertens 2004;13:107–115. [DOI] [PubMed] [Google Scholar]
- 33.Prieto-Carrasquero MC, Botros FT, Pagan J, et al. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip goldblatt hypertensive rats. Hypertension 2008;51:1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agmon Y, Peleg H, Greenfeld Z, et al. Nitric oxide and prostanoids protect the renal outer medulla from radiocontrast toxicity in the rat. J Clin Invest 1994;94:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mori T, Cowley AW Jr. Angiotensin II-NAD(P)H oxidase-stimulated superoxide modifies tubulovascular nitric oxide cross-talk in renal outer medulla. Hypertension 2003;42:588–593. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z, Rhinehart K, Kwon W, et al. ANG II signaling in vasa recta pericytes by PKC and reactive oxygen species. Am J Physiol Heart Circ Physiol 2004;287:H773–H781. [DOI] [PubMed] [Google Scholar]
- 37.Merten GJ, Burgess WP, Gray LV, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA 2004;291:2328–2334. [DOI] [PubMed] [Google Scholar]
- 38.Tepel M, van der GM, Schwarzfeld C, et al. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med 2000;343:180–184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.