Abstract
The five-year survival rate of patients after curative resection of hepatocellular carcinoma (HCC) has been reported to be 30 to 50%, however the actual survival rate may be different. We analyzed the actual 5-year survival rate and prognostic factors after curative resection of HCC. Retrospective analysis was performed on 63 HCC patients who underwent curative resection from 1998 to 1999. A total of 63 cases were reviewed, consisting of 53 men and 10 women, with a median age of 49 years. These cases included all four pathologic T stages (pT stage) and had the following representation: stage 1 (1 case), stage 2 (17 cases), stage 3 (38 cases), and stage 4 (7 cases). In our study, the actual 5-year survival rate was 57.0% and the median survival time was 60 months. In addition, the patients in our study had an actual 5-year disease-free survival rate of 50.2% and a median disease-free survival time of 46 months. Thirty-one patients had recurrences, with a majority occurring within one year (65%). These patients with early recurrences had a poor actual 5-year survival rate of 5%. A univariate analysis showed that the prognostic factors influencing survival rate were the presence of satellite nodules, increased pT stage, HCC recurrence, and the time to recurrence (within one year). Interestingly, microvascular invasion made a difference in survival rate but was not statistically significant (p = 0.08). Furthermore, factors influencing the disease free survival rate include the presence of satellite nodules, microvascular invasion, and pT stage. Multivariate analysis identified pT stage as the only statistically related factor in determining the disease-free survival rate. The most important prognostic factor of HCC is recurrence. Moreover, the major risk factor for recurrence is an advanced pT stage. Therefore, performing prospective studies of postoperative adjuvant therapy is necessary to prevent recurrences after hepatic resection. Furthermore, active preventative treatment and early diagnosis of recurrences should be of the highest priority in the care of high-risk patient groups that have an advanced pT stage.
Keywords: Hepatocellular carcinoma, hepatic resection, five-year survival rate
INTRODUCTION
The best therapeutic modality for hepatocellular carcinoma (HCC) is a curative resection. However, the percent of patients able to be resected is reported to be between 10 and 50% due to impaired liver function and delayed diagnosis.1-3 Recently, the incidence of hepatic resection has increased due to the development of imaging modalities, early diagnosis by screening high risk groups with serum alpha-fetoprotein (AFP) for protein-induced vitamin K absence or antagonist-II (PIVKA-II), increased understanding of liver anatomy, and the development of hepatic resection techniques. Due to the increasing rates of hepatic resection, many reports on results of these resections have been published. Such studies report that the five-year cumulative survival rate after hepatic resection for HCC is 55 to 60% in Korea,4-6 and 30 to 50% in other countries.7-11 While the actual 5-year survival rates are rarely reported limited studies have reported these rates to be to be about 30 to 50%.12-14 These statistics reveal a difference between the actual 5-year survival rate and the statistical survival rate. These differences are thought to result from factors affecting survival rates after hepatic resection. Even though a great number of studies have searched for prognostic factors of HCC after hepatic resection, the results of these studies vary according to how the study was performed.4-7,10-19
In this study, we analyzed the actual 5-year survival rates and prognostic factors for patients with HCC who underwent curative resection and had more than 5 years of follow-up care in the Severance Hospital of the Yonsei University College of Medicine.
MATERIALS AND METHODS
We examined the medical records of 66 HCC patients who underwent hepatic resection from January, 1998 to December, 1999 in our institution and who had more than 5 years of follow-up care. Three patients were excluded from the study. Two of the three excluded patients died of postoperative complications from hepatic failure, and the third had an obvious residual tumor at the time of surgery and underwent palliative resection after several treatments of transarterial chemoembolization (TACE) for multiple HCC. A retrospective analysis was performed using operation records and pathologic reports. Five years after hepatic resection, we investigated the survival status and evaluated both the long-term and disease-free survival rates in these patients.
The preoperative studies included screening for serum tumor markers using AFP and/or PIVKA-II), analysis of residual liver function using a liver function test and the indocyanine green retention rate at 15 minutes (ICG R15), and finally, radiological imaging studies by either ultrasonogram, computerized tomography scan (CT-scan), magnetic resonance imaging (MRI), or hepatic artery angiogram. Preoperative PIVKA-II levels were not determined during the study period.
The HCC pathologic stage, both pre- and postoperatively, was defined according to the modified UICC stages outlined by the Korean Liver Cancer Study Group (Table 1). All patients were monitored regularly for recurrences by serum AFP screening and CT-scans at the out-patient clinics. Recurrence was diagnosed when imaging studies showed a hypervascular nodule or was diagnosed as HCC by a radiologist.20,21 Follow-up periods lasted between 4 and 85 months with a median follow-up period of 62 months for the entire study.
Table 1.
The TNM Staging System of HCC as Defined by the Korean Liver Cancer Study Group
Tumor (T stage) factors are dependant on a single tumor with a maximum size of < 2 cm and no vascular invasion. T1, including 3; T2, including 2; T3, including 1; T4, including none.
Statistical analysis was performed using SPSS (Windows, version 11.5) and all calculations are reported as median values. Survival and disease-free survival rates were calculated by the Kaplan-Meier method. Univariate analysis was performed using a log-rank test, and multivariate analysis was performed using Cox's proportional hazard model. Statistical significance was defined as p < 0.05.
RESULTS
Clinical findings, preoperative study, and treatment
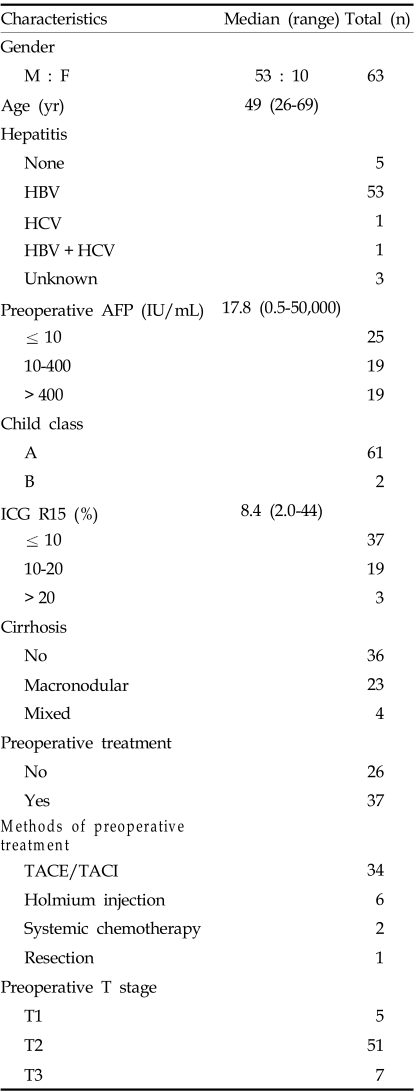
A total of 63 patients, 53 male and 10 female, were reviewed. The ages of the patients in this study ranged from 26 to 69 years with a median age of 49. Fifty-eight patients had chronic hepatitis, with 53 of these cases derived from B-viral infection. In addition, 27 patients had liver cirrhosis, while 23 cases had macronodular cirrhosis.
The median preoperative serum AFP level was 17.8 IU/mL (ranging from 0.5 to 50,000). Sixty-one patients were classified as Child-Pugh class A. The median preoperative ICG R15 was 8.4% (ranging from 2.0% to 44.4%).
Thirty-seven patients had received preoperative treatment. These treatments included: TACE (34 cases), transarterial or percutaneous holmium injection (6 cases), systemic chemotherapy (2 cases), and surgical resection (1 case).
Preoperative T stage was defined by imaging studies. The number of patients per stage was as follows: stage 1, 5 cases, stage 2, 51 cases, and stage 3, 7 cases (Table 2).
Table 2.
Patient Characteristics
HBV, hepatitis B Virus; HCV, hepatitis C virus; AFP, alpha-fetoprotein; ICG R15, indocyanine green retention rate at 15 minutes; TACE/TACI, transarterial chemoembolization/chemoinfusion.
Operation types and pathologic findings
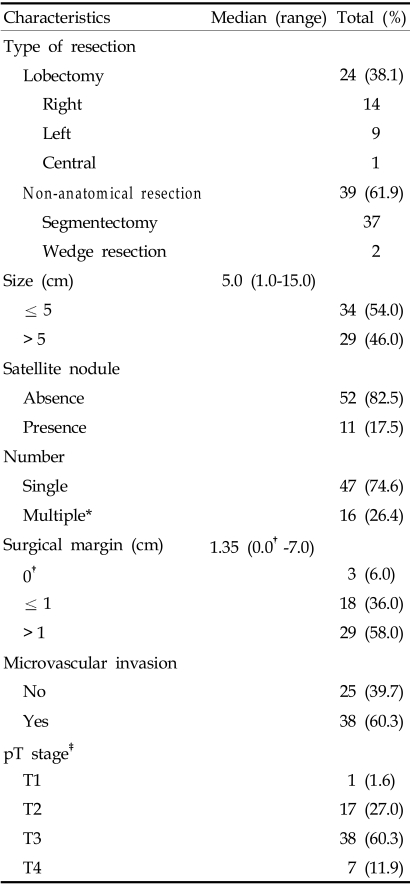
Twenty-four patients had received a lobectomy, and thirty-nine had received a segmentectomy or wedge resection.
The median tumor size was 5 cm and ranged from 1.0 to 15.0 cm. Thirty-four patients had tumors less than 5 cm. Multiple nodules were noted in the gross findings of five patients, and in 11 patients, satellite nodules were found by microscopy. Thirty-eight patients had microvascular invasion. The median distance from the tumor to the resection margin was 1.35 cm (ranging from 0 to 7.0 cm).
The number of cases in each pathologic T stage (pT stage) was as follows: stage 1, 1 case, stage 2, 17 cases, stage 3, 38 cases, and stage 4, 7 cases (Table 3).
Table 3.
Tumor Characteristics
*Multiple, the number of tumors including microscopic satellite nodules.
†0.0 cm, abutting the tumor at resection margin.
‡pT stage, postoperative pathologic tumor stage.
Survival rate and factors affecting survival
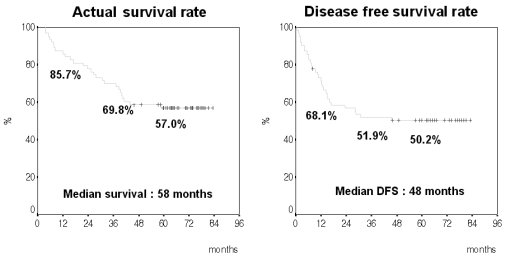
The actual 1-, 3-, and 5-year survival rates were 85.7%, 69.8%, and 57.0%, respectively The median survival time was 58 months. The actual 1-, 3-, and 5-year disease-free survival (DFS) rates were 68.1%, 51.9%, and 50.2%, respectively. The median DFS time was 46 months (Fig. 1). A total of 31 patients (49.2%) had recurrences.
Fig. 1.
The actual survival rate compared to the disease-free survival rate.
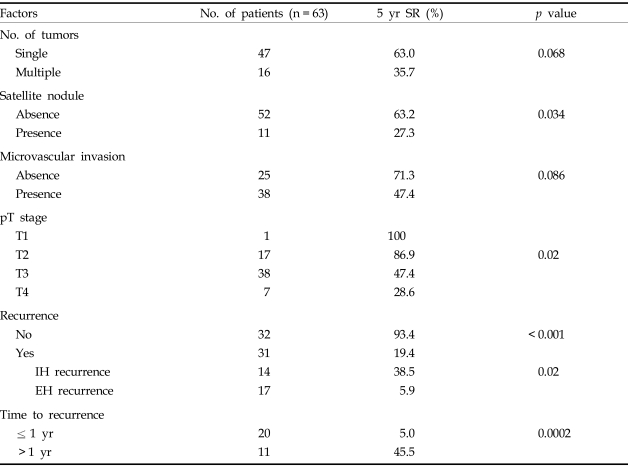
A univariate analysis showed the prognostic factors for survival were the presence of satellite nodules (p = 0.03), pT stage (p = 0.02), recurrence (p < 0.01), time to recurrence (p < 0.01), and extrahepatic metastasis (p = 0.02). In contrast, tumor number and the presence of microvascular invasion were not statistically significant factors (Table 4). Patient age, ICG R15 values, AFP levels, preoperative treatments, extent of hepatic resection, intraoperative transfusions, tumor size and surgical margins were also not significant factors.
Table 4.
Factors Affecting Survival Rate (Univariate Analysis)
5 yr SR, 5-year survival rate; IH, intrahepatic; EH, extrahepatic and/or intrahepatic.
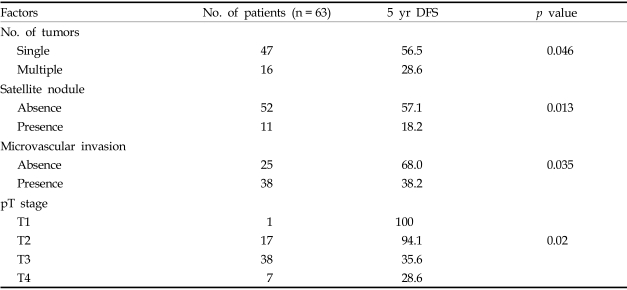
Univariate analysis revealed that the following factors affected DFS rates: tumor number (p = 0.046), the presence of satellite nodules (p = 0.013), microvascular invasion (p = 0.035), and pT stage (p = 0.02) (Table 5).
Table 5.
Factors Affecting Disease Free Survival (Univariate Analysis)
DFS, disease free survival.
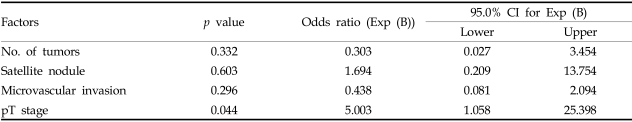
Multivariate analysis showed that pT stage was the only factor affecting survival rate, and that no factors affected DFS (Table 6).
Table 6.
Multivariative Analysis for Factors Affecting 5-Year Survival Rate
CI, confidence interval.
DISCUSSION
HCC is the fifth most common malignancy worldwide. According to the Korean National Statistical Office, in 2002, HCC was the third most common malignancy in Korea. Curative resection is the most effective therapy for HCC. However, many HCC patients are not able to have resections and show poor prognosis due to many factors. These factors include difficulty in achieving an early diagnosis, multiplicity, vascular invasion, and advanced liver cirrhosis. Recently, the incidence of hepatic resection has been increased by early diagnosis through screening high risk groups, improving imaging study modalities such as CT-scans or MRI, having an increased understanding of liver anatomy, developing surgical techniques and instruments, improving perioperative management, and decreasing the high rate of postoperative complications and mortalities. Despite these advances, long-term survival rates remain low because of high recurrence rates.
Until now, the 5-year cumulative survival rate was reported as 30 to 60%,4-11 and the actual 5-year survival rate was reported as 30 to 50%.12-14 Subjects of this study are patients with HCC who underwent hepatic resection during a two year period from January 1998 until December 1999. According to this study, the actual 5-year survival rate is 57%, which is higher than previous studies, and is similar to or better than the cumulative survival rate reported recently in other studies. The actual 5-year DFS rate in this study is 50%, which is similar to or better than the 15 to 50% 5-year DFS rate reported in other studies.
All previous studies have reported different prognostic factors affecting the long term survival after HCC resection. The factors found in these studies include: HBsAg, HBeAg, AFP, Child-Pugh classification, ICG R15, perioperative transfusion, intrahepatic tumor characteristics, tumor size and number, satellite nodules, vascular invasion, resection margin distance, and stage.4-19
In this study, we classified pT stages according to the modified UICC stages defined by the Korean liver cancer study group. Multiplicity was defined as the gross presence of multiple nodules or the presence of satellite nodules that were observed by microscopy. Vascular invasion was defined as either the gross or microscopic presence of vascular invasion. Large differences between pT stage and preoperative T stage were present because the preoperative T stage did not include microscopic findings such as microscopic satellite nodules and microvascular invasion.
In this study, there were differences in the 5-year survival rate of patients with microvascular invasions and with an increased tumor number. However, these factors were not statistically significant (0.05 < p ≤ 0.1). The five-year survival rate of patients who did not have satellite nodules was 63.2%. Interestingly, this rate is much higher than that of patients who had satellite nodules (p = 0.034). The 5-year survival rate was lowered significantly in patients with a high pT stage. In a multivariate analysis, pT stage was the only prognostic factor. Thus, three factors, such as tumor size, tumor number, and vascular invasion, affect tumor stage cumulatively rather than independently.
Tumor recurrence is the most important prognostic factor in HCC. Recurrence is classified as either early or late. Early recurrence is defined as recurrence within 12 months or 3 years and consists of a residual tumor that has spread from the primary tumor remaining in the residual liver. In contrast, late recurrence results from multicentric tumor development.7 Prognosis is worse in cases of early recurrence or concurrent extrahepatic metastasis.7 In this study, we defined early recurrence as a tumor recurring within one year after curative resection. Thirty-one out of 63 patients (49%) in our study had recurrences, and the 5-year survival rate of patients with recurrences (19.4%) was much lower than those of patients who did not have recurrences (93.4%). Twenty patients (65%) had recurrences within 1 year. The 5-year survival rate of these patients with early recurrences was significantly lower than the five year survival rate of patients who had recurrences after 1 year (5% versus 45.5%, respectively).
Nineteen of 31 patients who had recurrences within 1 year had a high pT stage (pT3, 16 cases, pT4, 3 cases). Fourteen patients exhibited microvascular invasion, while 10 patients had tumors greater than 10 cm in diameter.
The five-year survival rate of patients with extrahepatic metastasis was 5.9%. This rate was lower than that of patients without extrahepatic metastasis (38.5%). Seventeen patients with extrahepatic metastasis showed pT3 (15 cases) and pT4 (2 cases), while 12 of these patients exhibited microvascular invasion.
Some studies have found that the factors affecting tumor recurrence are: AFP, tumor number and size, satellite nodules, portal vein invasion, and microvascular invasion.6,9,10 In contrast, other studies did not find any significant factors.12 In this study, tumor number, satellite nodules, and microvascular invasion significantly affected the 5- year DFS. These are the same factors that were found in other studies. However, in our study no significant factors were found using multivariate analysis.
Factors that were found to affect survival and recurrence rates in previous studies were not statistically significant in this study. These factors included: age, ICG R15, preoperative treatment, AFP, intraoperative transfusion, type of hepatic resection, tumor size, and resection margin distance.
In this study, the 1-, 3-, and 5-year survival rates of HCC patients after hepatic resection were 85.7%, 69.8%, and 57.0%, respectively, and the 1-, 3-, and 5-year DFS rates were 68.1%, 51.9%, and 50.2%, respectively. Recurrences, especially then they occurred within 1 year, were the most adverse prognostic factor in long-term survival rates. The pathologic characteristics of HCC (satellite or multiple nodule and microvascular invasion) were found to be significantly adverse prognostic factors. Moreover, tumor recurrence was affected by the pT stage. Early recurrence and extrahepatic metastasis were more frequent in patients with higher pT stages. Hence, reducing early recurrences requires an aggressive treatment strategy that includes postoperative adjuvant therapy. Furthermore, in patients with high pT stages, prospective study is needed for adequate adjuvant therapy.
In conclusion, the rate of HCC recurrences after resection is high, and recurrences are the most adverse prognostic factor of HCC. However until now, postoperative adjuvant therapy was not established for prevention of recurrence. Therefore, the prospective study of postoperative adjuvant therapy is necessary for the prevention of recurrences after hepatic resection. In high-risk groups such as patients with advanced pT stages, effort should be put forth to look for early recurrences while an active strategy for recurrence prevention should be implemented in follow-up treatment regimens.
References
- 1.Lai EC, Fan ST, Lo CM, Chu KM, Liu CL, Wong J. Hepatic resection for hepatocellular carcinoma. An audit of 343 patients. Ann Surg. 1995;221:291–298. doi: 10.1097/00000658-199503000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cance WG, Stewart AK, Menck HR. The national cancer database report on treatment patterns for hepatocellular carcinoma: improved survival of surgically resected patients, 1985-1996. Cancer. 2000;88:912–920. doi: 10.1002/(sici)1097-0142(20000215)88:4<912::aid-cncr23>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 3.Takano S, Oishi H, Kono S, Kawakami S, Nakamura M, Kubota N, et al. Retrospective analysis of type of hepatic resection for hepatocellular carcinoma. Br J Surg. 2000;87:65–70. doi: 10.1046/j.1365-2168.2000.01308.x. [DOI] [PubMed] [Google Scholar]
- 4.Bae TS, Kim SB, Park SH, Choi DW. Outcome of hepatic resection for hepatocellular carcinoma patients. J Korean Surg Soc. 2003;64:480–486. [Google Scholar]
- 5.Choi YM, Kang KC, Ahn SI, Lee KY, Hong KC, Choi SK, et al. Clinical analysis of prognostic factors in hepatocellular carcinoma. J Korean Surg Soc. 2003;65:42–48. [Google Scholar]
- 6.Park CK, Jang WY, Lee JI, Song SY, Choi MS, Cho JW, et al. Prognostic factors after hepatic resection of hepatocellular carcinoma: Univariate and multivariate analysis. Korean J Gastroenterol. 2002;39:33–39. [Google Scholar]
- 7.Song TJ, IP EW, Fong Y. Hepatocellular carcinoma: Current surgical management. Gastroenterology. 2004;127:248–260. doi: 10.1053/j.gastro.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 8.Yu AS, Keeffe EB. Management of hepatocellular carcinoma. Rev Gastroenterol Disord. 2003;3:8–24. [PubMed] [Google Scholar]
- 9.Marin-Hargreaves G, Azoulay D, Bismuth H. Hepatocellular carcinoma: surgical indications and results. Crit Rev Oncol Hematol. 2003;47:13–27. doi: 10.1016/s1040-8428(02)00213-5. [DOI] [PubMed] [Google Scholar]
- 10.Yeh CN, Lee WC, Chen MF, Tsay PK. Predictors of long-term disease-free survival after resection of hepatocellular carcinoma: two decades of experience at Chang Gung Memorial Hospital. Ann Surg Oncol. 2003;10:916–921. doi: 10.1245/aso.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101:796–802. doi: 10.1002/cncr.20426. [DOI] [PubMed] [Google Scholar]
- 12.Hwang IS, Hong SW, Nah YW, Jang YG, Kim KH, Lee HS. Analysis of 38 long-term survivors after liver resections for hepatocellular carcinomas. Korean J HBP Surg. 2000;4:67–76. [Google Scholar]
- 13.Chang CH, Chau GY, Lui WY, Tsay SH, King KL, Wu CW. Long-term results of hepatic resection for hepatocellular carcinoma originating from the noncirrhotic liver. Arch Surg. 2004;139:320–325. doi: 10.1001/archsurg.139.3.320. [DOI] [PubMed] [Google Scholar]
- 14.Shimozawa N, Hanazaki K. Longterm prognosis after hepatic resection for small hepatocellular carcinoma. J Am Coll Surg. 2004;198:356–365. doi: 10.1016/j.jamcollsurg.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Ramacciato G, Mercantinie P, Cautero N, Corigliano N, di Benedetto F, Quintini C, et al. Prognostic evaluation of the new American joint committee on cancer/international union against cancer staging system for hepatocellular carcinoma: analysis of 112 cirrhotic patients resected for hepatocellular carcinoma. Ann Surg Oncol. 2005;14 doi: 10.1245/ASO.2005.03.098. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Qin LX, Tang ZY. The prognostic significance of clinical and pathological features in hepatocellular carcinoma. World J Gastroenterol. 2002;8:193–199. doi: 10.3748/wjg.v8.i2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau H, Fan ST, Ng IOL, Wong J. Long term prognosis after hepatectomy for hepatocellular carcinoma: A survival analysis of 204 consecutive patients. Cancer. 1998;83:2302–2311. [PubMed] [Google Scholar]
- 18.Kim GS, Roh JH, Cho CK, Kim HJ. Long-term survival rates and prognostic factors for a hepatocellular carcinoma after a curative hepatic resection. J Korean Surg Soc. 1999;57:715–727. [Google Scholar]
- 19.Han SH, Lee WJ, Noh SH, Kim MW, Kim BR, Lee KS. Univariate and multivariate analysis of prognostic factors in survival after resection of primary hepatoma. J Korean Surg Soc. 1994;47:393–400. [Google Scholar]
- 20.Park JW. Practice guideline for diagnosis and treatment of hepatocellular carcinoma. Korean J Hepatol. 2004;10:88–98. [PubMed] [Google Scholar]
- 21.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]