Abstract
Multiple antibiotic reisistance threatens successful treatment of Acinetobacter baumannii infections worldwide. Increasing interest in the well-known activity of sulbactam against the genus Acinetobacter has been aroused. The purpose of this study was to compare the outcomes for patients with Acinetobacter bacteremia treated with cefoperazone/sulbactam versus imipenem/cilastatin. Forty-seven patients with Acinetobacter baumannii bacteremia were analyzed through a retrospective review of their medical records for antibiotic therapy and clinical outcome. Thirty-five patients were treated with cefoperazone/sulbactam, and twelve patients with imipenem/cilastatin. The percentage of favorable response after 72 hours was not statistically different between cefoperazone/ sulbactam group and imipenem/ cilastatin group. The mortality rate was not statistically different, too. Cefoperazone/sulbactam was found to be as useful as imipenem/cilastatin for treating patients with Acinetobacter bacteremia.
Keywords: Acinetobacter, bacteremia, cefoperazone/sulbactam, imipenem/cilastatin
INTRODUCTION
Acinetobacter baumannii has emerged as an important nosocomial pathogen, and its multiple antibiotic resistance threatens the successful treatment of A. baumannii infections worldwide.1 Nowadays, most nosocomial isolates are resistant to a wide variety of antibiotics, leaving carbapenems as one of the only recognized therapeutic alternative.2,3 In this setting, the overuse of imipenem has been associated with reports of several outbreaks caused by carbapenem-resistant strains, often leaving polymyxin and sulbactam as the only antibiotics with in vitro activity against these organisms.2,4 Moreover, resistance to imipenem is becoming more common.5 Therapy in such cases is a serious challenge, and consequently, the well-known activity of sulbactam against the genus Acinetobacter is receiving renewed attention.6
In one study, the authors reported that sulbactam might prove effective in non-life-threatening A. baumannii infections.2 In another study, the authors found ampicillin-sulbactam to be effective in the treatment of a small number of patients with Acinetobacter ventilator-associated pneumonia.7 However, to our knowledge no reports have been issued about the efficacy of cefoperazone/sulbactam for the treatment of Acinetobacter bacteremia.
The purpose of this study was to compare the outcomes of patients with Acinetobacter bacteremia who were treated with cefoperazone/ sulbactam or imipenem-cilastatin.
MATERIALS AND METHODS
Study population
We reviewed the records of the clinical microbiology laboratory and identified patients with significant bacteremia caused by A. baumannii, who registered between 1998 and 2002 at the Severance Hospital, Yonsei University College of Medicine, retrospectively. Demographic, clinical, and microbiological data were extracted from the patients' medical records.
Identification and antimicrobial susceptibility testing
CLSIThe isolates were identified using conventional techniques and/or ATB 32 GN system (bioMerieux, Marcy-l'Etoile, France).8 The antimicrobial susceptibilities of Acinetobacter isolates were determined by microbiology laboratory staff using a disk-diffusion method. Results were interpreted using the guidelines established by the Clinical and Laboratory Standards Institute (CLSI), formerly the National Committee for Clinical Laboratory Standards (NCCLS).9 The cefoperazone/ sulbactam susceptibility of Acinetobacter was determined using a disk containing 75 µg of cefoperazone and 30 µg of sulbactam. The zone diameter used for cefoperazone in the CLSI guideline was used for cefoperazone/sulbactam. Intermediate susceptibility to the antibiotics was considered as resistance.
Definitions
'Significant bacteremia' was defined as the isolation of bacterial species from one or more blood cultures, and by the presence of signs responsible for sepsis. Standard Center for Disease Control nosocomial infection definitions were used to define the sites of infection.10 Bacteremia was considered 'nosocomial' if (a) a positive blood culture was obtained after 72 h of admission and there was no evidence of infection at the time of admission; or if (b) infections were acquired at other hospitals before transfer to the study hospital; or if (c) infections were acquired during a previous admission within 2 weeks of presentation. Otherwise, the bacteremia was considered to be community-acquired. The initial empirical antimicrobial therapy was considered appropriate if the initial antibiotics, which were administered within 24 h after acquisition of a blood culture samples, included at least one antibiotic that was active in vitro against the causative microorganisms and when the dosage and route of administration conformed with current medical standards.11 Inappropriate initial antimicrobial therapy referred to the administration of antimicrobial agents to which the causative microorganisms were resistant in vitro or to the lack of an antimicrobial therapy for a known causative pathogen. If the antimicrobial agent was not administered within 24 h of bacteremia onset, antimicrobial use was considered inappropriate. Disease severity was scored using the APACHE II system.
Antibiotic therapy and outcome
Empiric antibiotic therapy was initiated immediately after a blood culture had been performed. In most patients, an antipseudomonal β-lactam antimicrobial agent (piperacillin/tazobactam, ceftazidime, cefoperazone/sulbactam, imipenem/cilastatin, or meropenem) was administered as an empiric therapy. Empiric antibiotic therapy was changed to definitive therapy, as needed, on the basis of the results of culture and sensitivity testing. The choice of definitive therapy was made at the discretion of the attending physician, but imipenem/cilastatin generally was used for imipenem-susceptible Acinetobacter isolates. Cefoperazone/sulbactam generally was used for cefoperazone-susceptible isolates. Use of vancomycin or aminoglycoside was done at the discretion of the attending physician but was not routine. The evaluation of efficacy was based on the clinical response to therapy. Clinical outcomes were analyzed using; outcomes after 72 hrs of treatment, 7-day mortality and 30-day mortality. Clinical outcomes after 72 hrs of treatment were categorized as complete response, partial response, failure, or death. 'Complete response' was defined as the eradication of all presenting signs and symptoms of infection, and 'partial response' as the resolution of some but not all of these signs and symptoms. Treatment was considered a 'failure' if these signs and symptoms did not improve appreciably. 'Death' was defined as a death within 72 hrs of initiating treatment. Complete response and partial response were considered favorable response, and failure and death were considered unfavorable response.
Statistical analysis
Discrete variables were compared between groups using Fisher's exact test or χ2 statistics, as appropriate. Continuous variables were compared using the Student's t-test. A p-value of < 0.05 was considered statistically significant. The SPSS (version 11.0) software package was used for all analyses.
RESULTS
Demographic and clinical characteristics
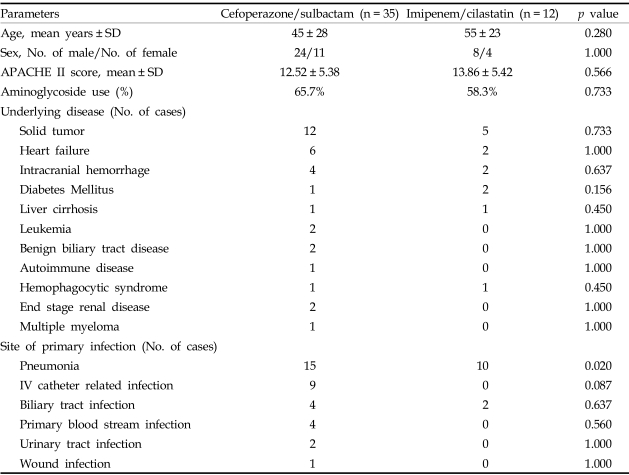
A total of 72 patients with significant Acinetobacter bacteremia were identified, and of these, 47 patients were treated with cefoperazone/sulbactam or imipenem/cilastatin as a definitive treatment regimen. Thirty-five patients with Acinetobacter bacteremia were treated with cefoperazone/sulbactam, and 12 patients were treated with imipenem/cilastatin. The clinical characteristics of patients treated with cefoperazone/sulbactam or imipenem/cilastatin are summarized in Table 1. The baseline characteristics of the patients in the treatment groups were statistically similar. Most isolates (97.9%) were nosocomial pathogen. The common sites of primary infection were the lungs (pneumonia), intravascular catheter related infection, and biliary tract infection. There was a higher incidence of pneumonia among patients in the imipenem/cilastatin group.
Table 1.
Demographic Characteristics of Patients with Acinetobacter Bacteremia Treated with Cefoperazone/sulbactam or Imipenem/cilastatin
Antimicrobial susceptibility patterns and antimicrobial therapy
Regarding antimicrobial susceptibility, all episodes of bacteremia in the imipenem/cilastatin group were caused by isolates that were fully susceptible to imipenem/cilastatin. Two isolates in imipenem/cilastatin group were resistant to cefoperazone/sulbactam. Of the 35 patients that were treated with cefoperazone/sulbactam, 2 were fully resistant to imipenem/cilastatin, 2 were intermediately resistant, and 31 were susceptible. All isolates in cefoperazone/sulbactam group were susceptible to cefoperazone/sulbactam. All definite antimicrobial therapies were'appropriate' antimicrobial therapy. Empiric antimicrobial therapies were 'appropriate' in 29 patients and 'inappropriate' in 18 patients. Appropriateness of empiric antimicrobial therapy was not associated with clinical outcomes such as outcomes after 72 hrs of treatment, 7-day mortality and 30-day mortality.
Clinical responses at 72 hours according to antibiotic treatment
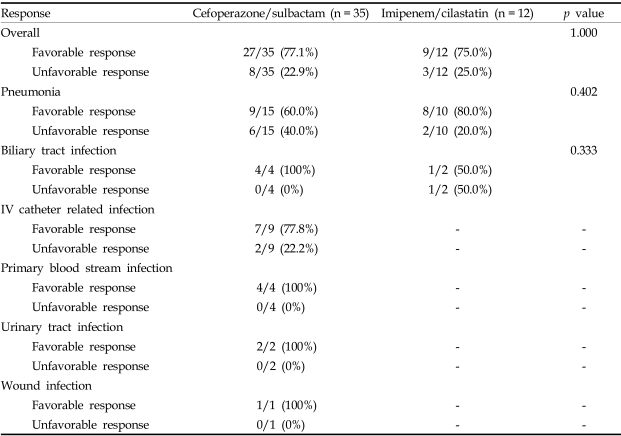
Clinical responses at 72 hours after antibiotic treatment are listed versus the definite antibiotic treatment regimens in Table 2.
Table 2.
Clinical Response at 72 Hours According to Definite Antibiotic Treatment Regimens
The percentage of complete and partial response was not statistically different (77% for the cefoperazone/sulbactam group vs. 75% for the imipenem/cilastatin group; p = 1.000).
In the subgroup of pneumonia patients, the percentage of complete and partial response was not statistically different between cefoperazone/sulbactam group and imipenem/cilastatin group (60% for the cefoperazone/sulbactam group vs. 80% for the imipenem/cilastatin group; p=0.402).
Mortality according to antibiotic treatment
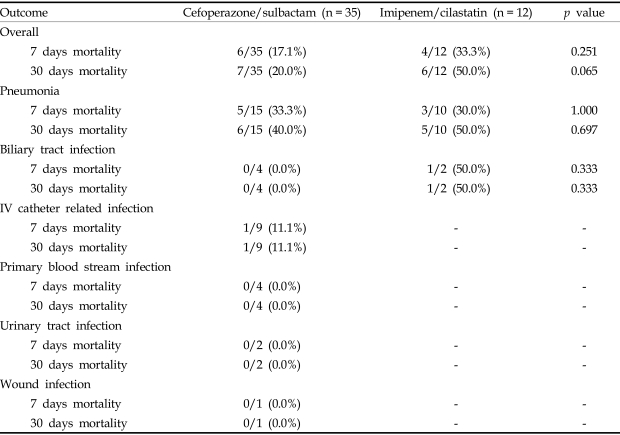
The mortalities of the patients with Acinetobacter bacteremia are listed with their respective antibiotic treatment regimens in Table 3. The 7-day mortality rate was lower in the cefoperazone/sulbactam group than in imipenem/cilastatin group, but this was not statistically significant (17.1% for cefoperazone/sulbactam group vs. 33.3% for imipenem/cilastatin group, p = 0.251). Thirty-day mortality rate was also lower in the cefoperazone/sulbactam group than in the imipenem/cilastatin group, but again this was not significant (20.0% for the cefoperazone/sulbactam group vs. 50.0% for the imipenem/cilastatin group, p = 0.065).
Table 3.
Treatment Outcomes According to the Sites of Primary Infection and Antibiotic Treatment Regimens
DISCUSSION
Over the last 20 years, A. baumannii has emerged as an important nosocomial pathogen. However, the treatment of choice for A. baumannii bacteremia has not been established. There have been no comparative therapeutic trials, and clinical experience is lacking. The usual treatment is an active β-lactam alone or in association with an aminoglycoside, which is similar to the treatment of bacteremia caused by other Gram-negative bacilli.3,12 A general trend towards decreased susceptibility to antibiotics has been observed worldwide in the majority of nosocomial strains. Multiple antibiotic resistance threatens the successful treatment of A. baumannii infections worldwide. Nowadays, most nosocomial isolates are resistant to the variety of antibiotics tested routinely, leaving carbapenems, mainly imipenem, as almost the only recognized therapeutic alternative. Imipenem treatment resulted in a cure for bacteremia in 83% of the cases in one study.4 However, the overuse of imipenem has been associated with several outbreaks of carbapenem-resistant strains, often leaving polymyxin and sulbactam as the only antibiotics with in-vitro activity against these organisms.4
Sulbactam is an inhibitor of β-lactamase, which shows in vitro bactericidal activity against Acinetobacter sp.13,14 Rodriquez-Hernandez et al.15 showed that the efficacy of sulbactam in experimental infections caused by susceptible A. baumannii strains is similar to that of imipenem. Corbella et al.2 treated 42 patents with non-life-threatening multiresistant A. baumannii infections, including seven bacteremias, with sulbactam alone and in combination with ampicillin; 39 improved or were cured with no major adverse affect. Ampicillin-sulbactam was found to be at least as effective as imipenem and a cost-effective alternative for the treatment of non-life-threatening multiresistant A. baumannii infections.16 Wood et al.7 reported that ampicillin-sulbactam was effective at treating a small number of patients with Acinetobacter ventilator-associated pneumonia.
Studies in North America, South America, Europe and Asia have investigated the in vitro activity of cefoperazone-sulbactam, and have shown it to be superior to that of cefoperazone alone against clinical isolates of many Gram-negative bacilli, but particularly against Acinetobacter species, in which activity is due to sulbactam alone.6,17-21 However, the efficacy of cefoperazone/sulbactam against Acinetobacter has not been studied in a clinical setting.
The results of the present study show that cefoperazone/sulbactam appears to be useful for the treatment of Acinetobacter bacteremia. These results suggest that sulbactam could be used for the treatment of life threatening infections by A. baumanni, and suggest that not only ampicillin/sulbactam but also cefoperazone/sulbactam could be used for the treatment of infections by A. baumannii. These results are encouraging because of the potential for high mortality in cases of Acinetobacter infection given increasing imipenem resistance among Acinetobacter isolates and the lack of treatment options.17,22
One of the most important problems associated with previous studies upon the in vitro activity of cefoperazone/sulbactam against Acinetobacter concerns the different criteria used to define susceptibility. In the case of the cefoperazone/sulbactam combination, there is no CLSI standard sulbactam concentration for the agar dilution or disk diffusion tests, and interpretations usually take into account the MICs of cefoperazone. In vitro studies have shown that cefoperazone/sulbactam is more active than a variety of individual β-lactam agents against Acinetobacter species, and only imipenem has demonstrated in vitro activity superior to that of cefoperazone-sulbactam.23,24 A standard method for the evaluation of the sensitivity of Acinetobacter to cefoperazone/sulbactam is needed.
Unfortunately, resistance to sulbactam has been noted in imipenem-resistant strains of A. baumannii, leaving the polymyxin as the only treatment alternative.12,25 Levin et al.26 reported upon the outcomes of 60 nosocomial infections, including bacteremia, caused by A. baumannii and Pseudomonas aeruginosa, which were resistant to all commercially available antimicrobial agents. These infections were treated with colistin, but colistin causes some critical adverse effects, such as nephrotoxicity or neurotoxicity.
The several potential limitations of the present study include its retrospective design, the small number of patients, and potential differences between the groups, which may have favored the use of cefoperazone/sulbactam or imipenem/cilastatin.
In summary, cefoperazone/sulbactam was found to be as useful as imipenem/cilastatin for the treatment of Acinetobacter bacteremia in a small number of patients. Prospective study should be undertaken upon the efficacy of cefoperazone/sulbactam for the treatment of patients with Acinetobacter bacteremia.
Footnotes
This study was supported by the Brain Korea 21 Project for Medical Science, Yonsei University.
References
- 1.Urban C, Segal-Maurer S, Rahal JJ. Considerations in control and treatment of nosocomial infections due to multidrug-resistant Acinetobacter baumannii. Clin Infect Dis. 2003;36:1268–1274. doi: 10.1086/374847. [DOI] [PubMed] [Google Scholar]
- 2.Corbella X, Ariza J, Ardanuy C, Vuelta M, Tubau F, Sora M, et al. Efficacy of sulbactam alone and in combination with ampicillin in nosocomial infections caused by multiresistant Acinetobacter baumannii. J Antimicrob Chemother. 1998;42:793–802. doi: 10.1093/jac/42.6.793. [DOI] [PubMed] [Google Scholar]
- 3.Bergogne-Berezin E, Towner KJ. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9:148–165. doi: 10.1128/cmr.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cisneros JM, Reyes MJ, Pachon J, Becerri B, Caballero FJ, Garcia-Garmendia JL, et al. Bacteremia due to Acibetobacter baumannii: epidemiology, clinical findings, and prognostic features. Clin Infect Dis. 1996;22:1026–1032. doi: 10.1093/clinids/22.6.1026. [DOI] [PubMed] [Google Scholar]
- 5.Lee K, Kim YA, Park YJ, Lee HS, Kim MY, Kim EC, et al. Increasing prevalence of vancomycin-resistant Enterococci, and cefoxitin-, imipenem- and fluoroquinolone-resistant Gram-negative bacilli: a KONSAR study in 2002. Yonsei Med J. 2004;45:598–608. doi: 10.3349/ymj.2004.45.4.598. [DOI] [PubMed] [Google Scholar]
- 6.Levin AS. Multiresistant Acinetobacter infections: a role for sulbactam combinations in overcoming an emerging worldwide problem. Clin Microbiol Infect. 2002;8:144–153. doi: 10.1046/j.1469-0691.2002.00415.x. [DOI] [PubMed] [Google Scholar]
- 7.Wood GC, Hanes SD, Croce MA, Fabian TC, Boucher BA. Comparison of ampicillin-sulbactam and imipenem-cilastatin for the treatment of Acinetobacter ventilator-associated pneumonia. Clin Infect Dis. 2002;34:1425–1430. doi: 10.1086/340055. [DOI] [PubMed] [Google Scholar]
- 8.Farmer JJ., III . Enterobacteriaceae: introduction and identification. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of clinical microbiology. 7th ed. Washington DC: American Society for Microbiology; 1999. pp. 442–458. [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility testing-twelfth informational supplement. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2002. NCCLS document M100-S12. [Google Scholar]
- 10.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 11.Harbarth S, Garbino J, Pugin J, Romand JA, Lew D, Pittet D. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med. 2003;115:529–535. doi: 10.1016/j.amjmed.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Cisneros JM, Rodriquez-Banño J. Nosocomial bacteremia due to Acinetobacter baumannii: epidemiology, clinical features and treatment. Clin Microbiol Infect. 2002;8:687–693. doi: 10.1046/j.1469-0691.2002.00487.x. [DOI] [PubMed] [Google Scholar]
- 13.Obana Y, Nishino T. In-vitro and in-vivo activities of sulbactam and YTR830H against Acinetobacter calcoaceticus. J Antimicrob Chemother. 1990;26:677–682. doi: 10.1093/jac/26.5.677. [DOI] [PubMed] [Google Scholar]
- 14.Vila J, Marcos A, Marco F, Abdalla S, Vergara Y, Reig R, et al. In vitro antimicrobial production of beta-lactamases, aminoglycoside-modifying enzymes, and chloramphenicol acetyltransferae by and susceptibility of clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother. 1993;37:138–141. doi: 10.1128/aac.37.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriquez-Hernandez MJ, Cuberos L, Pichardo C, Caballero FJ, Moreno I, Jimenez-Mejias ME, et al. Sulbactam efficacy in experimental models caused by susceptible and intermediate Acinetobacter baumannii strains. J Antimicrob Chemother. 2001;47:479–482. doi: 10.1093/jac/47.4.479. [DOI] [PubMed] [Google Scholar]
- 16.Jellison TK, McKinnon PS, Rybak MJ. Epidemiology, resistance, and outcomes of Acinetobacter baumannii bacteremia treated with imipenem-cilastin or ampicillin-sulbactam. Pharmacotherapy. 2001;21:142–148. doi: 10.1592/phco.21.2.142.34114. [DOI] [PubMed] [Google Scholar]
- 17.Urban C, Go E, Mariano N, Berger BJ, Avraham I, Rubin D, et al. Effect of sulbactam on infections caused by imipenem-resistant Acinetobacter calcoaceticus biotype anitratus. J Infect Dis. 1993;167:448–451. doi: 10.1093/infdis/167.2.448. [DOI] [PubMed] [Google Scholar]
- 18.Lim VK, Cheong YM. In vitro activity of cefoperazone-sulbactam combination against cefoperazone resistant clinical isolates in a Malaysian general hospital. Malays J Pathol. 1995;17:73–76. [PubMed] [Google Scholar]
- 19.Jones RN, Wilson HW, Thornsberry C, Barry AL. In vitro antimicrobial activity of cefoperazone-sulbactam combinations against 554 clinical isolates including a review and beta-lactamase studies. Diagn Microbiol Infect Dis. 1985;3:489–499. doi: 10.1016/s0732-8893(85)80005-5. [DOI] [PubMed] [Google Scholar]
- 20.Eliopoulos GM, Klimm K, Ferraro MJ, Moellering RCJ. In vitro activity of cefoperazone-sulbactam combinations against cefoperazone-resistant clinical bacterial isolates. Eur J Clin Microbiol Infect Dis. 1989;8:624–626. doi: 10.1007/BF01968142. [DOI] [PubMed] [Google Scholar]
- 21.Knapp CC, Sierra-Madero J, Washington JA. Comparative in vitro activity of cefoperazone and various combinations of cefoperazone/sulbactam. Diagn Microbiol Infect Dis. 1989;13:45–49. doi: 10.1016/0732-8893(90)90053-x. [DOI] [PubMed] [Google Scholar]
- 22.Gales AC, Jones RN, Forward KR, Linares J, Sader HS, Verhoef J. Emerging importance of multidrug-resistant Acibetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: geographic patterns, epidemiological features, and trend in the SENTRY antimicrobial surveillance program (1997-1999) Clin Infect Dis. 2001;32(Suppl 2):S104–S113. doi: 10.1086/320183. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi K, Mathai D, Biedenbach DJ, Lewis MT, Gales AC, Jones RN. Evaluation of the in vitro activity of six broad-spectrum beta-lactam antimicrobial agents tested against over 2,000 clinical isolates from 22 medical centers in Japan. Diagn Microbiol Infect Dis. 1999;34:123–134. doi: 10.1016/s0732-8893(99)00019-x. [DOI] [PubMed] [Google Scholar]
- 24.Jones RN, Salazar JC, Pfaller MA, Doern GV. Multicenter evaluation of antimicrobial resistance to six broad-spectrum beta-lactams in Colombia using the Etest method. Diagn Microbiol Infect Dis. 1997;29:265–272. doi: 10.1016/s0732-8893(97)00157-0. [DOI] [PubMed] [Google Scholar]
- 25.Wood CA, Reboli AC. Infections caused by imipenem-resistant Acinetobacter calcoaceticus biotype anitratus. J Infect Dis. 1993;167:448–451. doi: 10.1093/infdis/168.6.1602. [DOI] [PubMed] [Google Scholar]
- 26.Levin AS, Barone AA, Penco J, Santos MV, Marinho IS, Arruda EA, et al. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin Infect Dis. 1999;28:1008–1011. doi: 10.1086/514732. [DOI] [PubMed] [Google Scholar]