Abstract
Synaptotagmin is a Ca2+ sensing protein, which triggers a fusion of synaptic vesicles in neuronal transmission. Little is known regarding the expression of Ca2+-dependent synaptotagmin isoforms and their contribution to the release of secretory vesicles in mouse and rat parotid acinar cells. We investigated a type of Ca2+-dependent synaptotagmin and Ca2+ signaling in both rat and mouse parotid acinar cells using RT-PCR, microfluorometry, and amylase assay. Mouse parotid acinar cells exhibited much more sensitive amylase release in response to muscarinic stimulation than did rat parotid acinar cells. However, transient [Ca2+]i increases and Ca2+ influx in response to muscarinic stimulation in both cells were identical, suggesting that the expression or activity of the Ca2+ sensing proteins is different. Seven Ca2+-dependent synaptotagmins, from 1 to 7, were expressed in the mouse parotid acinar cells. However, in the rat parotid acinar cells, only synaptotagmins 1, 3, 4 and 7 were expressed. These results indicate that the expression of Ca2+-dependent synaptotagmins may contribute to the release of secretory vesicles in parotid acinar cells.
Keywords: Synaptotagmin, calcium signaling, exocytosis, parotid acinar cells
INTRODUCTION
Mammalian salivary protein secretion is evoked largely by β-adrenergic stimulation, which generates an intracellular second messenger, cAMP, followed by the activation of cAMP-dependent protein kinase (PKA).1,2 Salivary fluid secretion is regulated by increases in the intracellular concentration of Ca2+ ([Ca2+]i), via cholinergic and α-adrenergic stimulation, which opens Ca2+-activated Cl- channels at the luminal membrane and evokes fluid secretion.3 In the parotid acinar cells most exocytosis is regulated by [cAMP]i increases without affecting [Ca2+]i and PKA activation is essential for cAMP-dependent exocytotic secretion.1,2,4 However, [Ca2+]i increases with muscarinic stimulation also evoke the release of secretory vesicles without affecting [cAMP]i, although the amount of protein secretion by Ca2+ is lower than by cAMP.5,6 However, the exact mechanism underlying the Ca2+-dependent exocytotic pathway in salivary protein secretion has not yet been clearly elucidated.
The mechanism of Ca2+-dependent exocytotic pathway in neuronal cells has been known well and is known to be mediated by SNAREs (soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptors). In neuronal cells, the assembly of a core complex composed of three proteins mediates exocytosis: Vesicle-associated membrane protein 2 (VAMP2) is a vesicle-associated SNARE, and syntaxin1 and SNAP-25 are target membrane SNAREs (t-SNARE).7,8 These proteins spontaneously assemble into a functional complex, and are sufficient to mediate exocytosis.9,10 In neuronal cells, the family of Ca2+ binding proteins, the synaptotagmins, are believed to comprise, or at least be a part of the block.11 The synaptotagmin family consists of at least 15 members, which can be divided into 2 groups: Ca2+-dependent or independent types.12 Genetic and biochemical evidence demonstrate that synaptotagmin 1 functions as a low-affinity Ca2+ sensor.11 Synaptotagmin 3 may have other Ca2+-dependent functions in neuronal cells,13 but in neuroendocrine cells, such as pancreatic islet β-cells, it appears to serve as a high-affinity Ca2+ sensor for exocytosis.14-16 The role played in cell function by other synaptotagmins remains unknown at present.
In non-neuronal cells, syntaxin 4 and SNAP-23 are the most plausible candidates for t-SNAREs. Both have been detected in rat parotid acinar cells, but they were not found to be coimmunoprecipitated with VAMP-2, which suggests that their interactions with VAMP2 may be weak.17 In addition, the presence of synaptotagmins and their role in exocytosis in the parotid acinar cells are not clearly understood. Parotid acinar cells appear to secrete proteins in a Ca2+-dependent manner like neuronal cells, because protein secretion occurs in parotid acinar cells by increase in [Ca2+]i. Stimulation with muscarinic, substance P, peptidergic or α-adrenergic receptors also elicits significant amylase release from the rat parotid. The stimulation of these receptors activates phosphatidyl-inositide metabolism and induces an increase in [Ca2+]i without affecting intracellular cAMP levels.5,6 The elevated [Ca2+]i affects factors related to the protein secretion in the parotid acinar cells. Accordingly, synaptotagmin is the most plausible candidate with regard to Ca2+-dependent protein secretion in the parotid acinar cells.
According to previous reports, the Ca2+-dependent synaptotagmin 2 and 3 are expressed in rat parotid acinar cells.18 However, the expression of Ca2+-dependent synaptotagmins has not been elucidated in mouse parotid acinar cells. In the present work, we investigated the expression of Ca2+-dependent synaptotagmins and Ca2+ signaling in rat and mouse parotid acinar cells, because the expression of Ca2+-dependent synaptotagmins and their role in the release of secretory vesicles are not known in mouse and rat parotid acinar cells.
MATERIALS AND METHODS
Preparation of parotid acinar cells from mice and rats
Male Sprague-Dawley rats (150-250 g) and ICR strain mice (23-28 g) were sacrificed by cervical dislocation. The cells were prepared from the parotids of SD rats and ICR mice by limited collagenase digestion as previously described.19 In order to achieve pure isolation of acinar cells, density gradient centrifugation was performed with Accudenz, and pure acinar cells were confirmed via light microscope.20 After isolation, the acinar cells were resuspended in an extracellular physiologic salt solution (PSS), the composition of which was as follows: NaCl, 140 mM; KCl, 5 mM; MgCl2, 1 mM; CaCl2, 1 mM; HEPES, 10 mM; and glucose, 10 mM titrated to pH 7.4 with NaOH. The osmolality of the extracellular solution (measured with a FISKE 110 osmometer), was 310 mOsm.
[Ca2+]i measurement
Cells were incubated for 40 min in PSS containing 5 µM fura 2-acetoxymethyl ester (Teflabs Inc., Austin, TX, USA) with Pluronic F-127 to enhance dye loading. Changes in [Ca2+]i were measured by means of fura 2 fluorescence, with excitation wavelengths of 340 nm and 380 nm, and an emission wavelength of 510 nm at room temperature. Background fluorescence was subtracted from the raw signals at each excitation wavelength before calculating the fluorescence ratio as follows: Ratio=F340/F380. The emitted fluorescence was monitored with a CCD camera (Photon Technology International Inc., Lawrenceville, NJ) attached to an inverted microscope. Fluorescence images were obtained at 2 s interval.21 Each cell was then stimulated by carbachol in a dose-dependent manner.
Amylase assay
Animals were allowed water but starved for 24 hr prior to the experiment. Each acinar cell was stimulated with equal concentrations of the carbachol used in the [Ca2+]i measurement study. Acinar cells were incubated with carbachol for 20 min in a shaking incubator at 37℃ and 60 rpm. The carbachol concentrations were followed in a dose-dependent manner. Acinar cells were lysed by sonication. The lysates were clarified by centrifugation at 13,000 rpm for 10 min. The total amylase content, or content of amylase released into the medium, was determined by the method described by Bernfeld et al.22 Aliquots of the incubation medium and the supernatants of the homogenized glands were incubated at 37℃ with a 0.5% starch suspension for 10 min. Absorbance was measured at a wavelength of 540 nm. Amylase activity in the medium was expressed as a percentage of the total activity.
Western blotting
Protein extracts were prepared from parotid acinar cells as follows. Pure acinar cells were washed with ice-cold PBS and then lysed by the addition of Tris-Hcl, NaCl, and EDTA buffer (1% NP-40, 10 mM of Tris HCl [pH 7.8], 150 mM NaCl, 1 mM of EDTA, 2 mM of Na3VO4, 10 mM of NaF, 10 µg/mL of aprotinin, 10 µg/mL leupeptine, 10 µg/mL of PMSF). The lysates were clarified by centrifugation at 13,000 rpm for 10 min. Samples were separated by 12% SDS-PAGE. Proteins in the gel were transferred onto nitrocellulose membrane (Schleicher and Schuell Bioscience, Dassel, Germany) for 1 h at a current of 200 mA. The nitrocellulose membrane was blocked by incubation in 6% skim milk in TTBS buffer [1X TBS solution + 0.1% Tween 20]. The membrane was then probed overnight at 4℃ with each primary antibody, anti-synaptotagmin 1 (Alomone Labs, Jerusalem, Israel) polyclonal antibody (1 : 500). After the membrane was washed three times with buffer TTBS, it was incubated with horseradish peroxidase conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) in TTBS buffer containing 3% skim milk for 1 h at room temperature, and again washed with TTBS buffer. Detection was performed using an ECL detection system (Amersham Biosciences, Uppsala, Sweden) and immunoreactive bands were visualized using Medical X-Ray film (AGFA).
RT-PCR
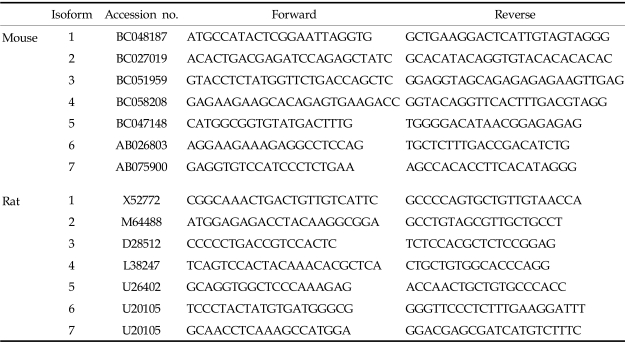
Total RNA was isolated from brain and acinar cells. Brains were homogenized using homogenizer with Liquid Nitrogen. Total RNA was isolated using Trizol reagent (Sigma, Saint Louise, Missouri, USA), chloroform, and isopropanol. Relative RT-PCR was performed to measure the gene expression of the synaptotagmin isoforms. The specific primers for each synaptotagmin isoform are described in Table 1. Polymerase Chain Reactions were performed on a T gradient 96 PCR machine (Biometra Co., Gottingen, Germany) using 1~2 nM of cDNA, 5 pmoles of each oligonucleotide primer, 200 µM of each dNTP, 1 unit of Taq polymerase (Applied Biosystems, Foster City, California, USA) and 10× Taq polymerase buffer in a 50 µL volume. The PCR program started with 94℃, denaturation for 30 sec, followed by 40 cycles of 94℃/30 sec, Ta/1 min, 72℃/1 min (Ta, annealing temperature; 56℃ to 61℃).
Table 1.
The PCR-Primers Encording Calcium Dependent Synaptotagmins
Data analysis and statistics
Results are expressed as the mean ± S.E.M. The statistical significances of differences between groups were determined using Student's T-tests. In statistical tests, p values of less than 0.05 were considered to be significant.
RESULTS
Ca2+-dependent exocytosis is much more sensitive to carbachol in mouse parotid acinar cells
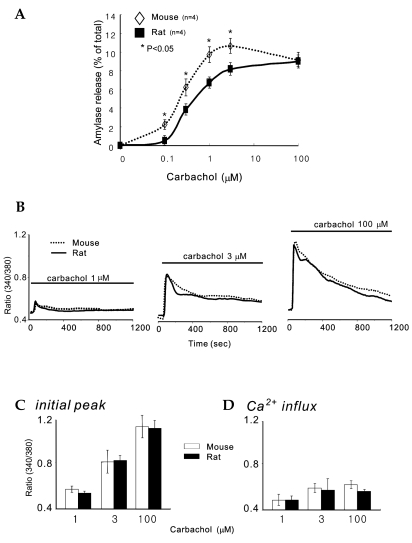
In order to compare amylase release between rat parotid acinar cells and mouse parotid acinar cells, we performed amylase release assays. Fig. 1A shows a typical amylase secretion curve with stimulation of carbachol, a muscarinic agonist, in a range of 0.1 µM to 100 µM, in parotid acinar cells. In mice, amylase release increased in a dose-dependent manner with up to 3 µM carbachol, but at 100 µM, amylase release decreased slightly: 2.33 ± 0.48% at 0.1 µM, 6.22 ± 0.93% at 0.3 µM, 9.71 ± .85% at 1 µM, 10.7 ± 0.81% at 3 µM, and 9.15 ± 0.86% at 100 µM. In rats, increases in agonist concentration evoked dose-dependent increases in amylase release: 0.55 ± 0.3% at 0.1 µM, 3.83 ± 0.56% at 0.3 µM, 6.68 ± 0.48% at 1 µM, 8.2 ± 0.66% at 3 µM, and 9.01 ± 0.41% at 100 µM. Ca2+-triggered exocytosis from the mouse acinar cells occurred with an apparent affinity of ~ 0.26 ± 0.03 µM. Therefore, there was a significant difference of amylase release rates between mouse and rat parotid acinar cells (p<0.05, n = 4). In contrast, Ca2+-triggered exocytosis from rat acinar cells occurred with an apparent affinity of ~ 0.48 ± 0.02 µM (n = 4). Thus, Ca2+-dependent exocytosis in mouse cells was about twice as sensitive to agonist than it was in rat cells. It is of note that the extent of exocytosis at the optimal concentration of 100 µM carbachol was the same in both cell types.
Fig. 1.
Concentration-response curve of muscarinic agonist, carbachol, on amylase release and carbachol-induced [Ca2+]i increases in parotid gland acinar cells from rats and mice. A, Amylase releases were measured with stimulation of the muscarinic agonist, carbachol, in a concentration range of 0.1 µM to 100 µM in parotid acinar cells from rats and mice. Results are expressed as the mean ± S.E.M. of four experiments in each group. B, [Ca2+]i in parotid acinar cells was measured with the relative ratio of fura2 fluorescence in response to each concentration of carbachol for 20 mins. The traces are representatives of 7 different experiments. C, analysis of carbachol-induced [Ca2+]i initial peaks. D, analysis of carbachol-induced Ca2+ influx. Results are expressed as the mean ± S.E.M. of seven experiments in each group. *Significant difference between rat and mice parotid acinar cells (p<0.05).
Ca2+ release and Ca2+ influx by carbachol stimulation in rat and mouse parotid acinar cells are identical
Amylase release depends on [cAMP]i, and [Ca2+]i increases in the parotid acinar cells.3 In both cells, amylase release was different with the same concentration of muscarinic stimulation. It was possible that the mice were more capable a maintaining Ca2+ levels during the 20 minutes long agonist stimulation than the rat. Therefore, we attempted to ascertain whether or not the same concentration of muscarinic stimulation evoked the same transient [Ca2+]i increases and Ca2+ influx in both cells. The fluorescence of fura 2-loaded cells was measured at the relative ratio of [Ca2+]i in response to the above-mentioned concentrations of carbachol. Although we did not detect changes in [Ca2+]i at 0.1 and 0.3 µM of carbachol, 1 µM, 3 µM, and 100 µM carbachol evoked [Ca2+]i increases in a dose-dependent manner (Fig. 1B and C), in which transient [Ca2+]i increases were followed by a sustained plateau for 20 min. The initial peak amplitudes of these transient [Ca2+]i increases in rats were 0.58 ± 0.03 at 1 µM, 0.83 ± 0.1 at 3 µM, and 1.15 ± 0.1 at 100 µM (n = 7); in mice 0.54 ± 0.02 at 1 µM, and 0.84 ± 0.05 at 3 µM, and 1.14 ± 0.07 at 100 µM (n = 7). The amplitudes of Ca2+ influx in rats were 0.49 ± 0.05 at 1 µM, 0.58 ± 0.1 at 3 µM, and 0.57 ± 0.01 at 100 µM (n = 7); in mice, they were 0.49 ± 0.04 at 1 µM, and 0.6 ± 0.04 at 3 µM, and 0.63 ± 0.03 at 100 µM (n = 7). Interestingly, rat and mouse parotid acinar cells generated identical transient [Ca2+]i increases and Ca2+ influx with the same concentrations of agonist, suggesting that both cells have similar mechanism for the increasing and decreasing of [Ca2+]i levels.
Different expression of Ca2+-dependent synaptotagmins
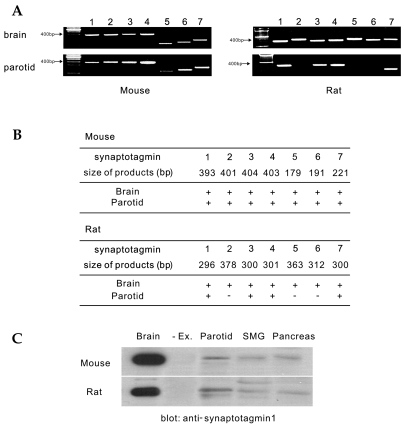
Since there were no differences in Ca2+ response between rat and mouse parotid acinar cells, we hypothesized that the expression of Ca2+-sensing protein differed between the cell types. It is generally accepted that one of the protein closely related to Ca2+-triggering exocytosis is synaptotagmin, which has 15 different isoforms and 7 Ca2+-dependent types from 1 to 7.8,23-25 RT-PCR for the specific 7 Ca2+-dependent types was performed in both the rat and mouse brains as a positive control, as the brains of both animals are known to harbor all varieties of synaptotagmin. Mice and rats brain expressed 7 Ca2+-dependent synaptotagmins in Fig. 2A. In parotid acinar cells, mice express 7 Ca2+-dependent synaptotagmins, whereas rats express only synaptotagmin types 1, 3, 4, and 7.
Fig. 2.
RT-PCR products encoding Ca2+-dependent synaptotagmins in parotid acinar cells and Western blotting of synaptotagmin 1 in exocrine glands. A, RT-PCR results of the Ca2+-dependent synaptotagmin family in brain microsome and parotid acinar cells from mice and rats. Lane numbers indicate synaptotagmin isoforms. B, size of PCR products encoding synaptotagmin isoforms and summary of results. Note that rat parotid acinar cells express synaptotagmin isoforms 1, 3, 4, and 7. Each result is the representative of five to eight different experiments. C, Western blotting of synaptotagmin 1 in brain, parotid, submandibular, and pancreatic acinar cells from mice and rats. The brain contains 40 µg and the parotid, submandibular, and pancreatic acinar cells contain 100 µg of proteins in each lane. Each result is the representative of four different experiments. -Ex; no extract.
Synaptotagmin 1 is expressed in exocrine gland cells
Since the expression of synaptotagmin 1 in rat parotid gland acinar cells remains unclear,18,26 we performed Western blotting on brain, parotid, submandibular, and pancreatic acinar cells from both rats and mice using pAb against synaptotagmin 1 (Fig. 2C). Western blot data show that synaptotagmin 1 was expressed in exocrine gland cells including rat parotid acinar cells (n=4). The expression levels of synaptotagmin I compared to the brain microsome in mice, were 0.77 ± 0.11% in the parotid acini, 0.2 ± 0.07% in the submandibular acini, and 0.55 ± 0.08% in pancreatic acini; in rats, 0.69 ± 0.16% in the parotid acini, 0.25 ± 0.04% in the submandibular acini, and 0.46 ± 0.04% in pancreatic acini. Interestingly, there are no differences in expression levels between rat and mouse brain microsome.
DISCUSSION
In the present work, we studied the expression of Ca2+-dependent synaptotagmins in rat and mouse parotid acinar cells, the expression of which has been mainly reported in brain tissues.27 Synaptotagmin functions as a Ca2+ sensor in neuronal cells, in which the cytoplasmic domain contains two C2 repeats capable of binding phospholipids in a Ca2+-dependent manner.28 Among the synaptotagmins, synaptotagmin 1 mainly found in the nervous system has been intensively studied and found especially in synaptic vesicles and dense-core vesicles and serves as a major Ca2+ regulator of neurotransmitter release in the fast release of synaptic vesicles.27 In this study we have shown that synaptotagmin 1 is also present in parotid acini with RT-PCR and western blot. Moreover, we found that submandibular and pancreatic acinar cells also express synaptotagmin 1 with the same Ab, suggesting that most of the exocrine gland acini have synaptotagmin 1, and that the protein may be generally involved in regulating exocytosis of secretory vesicles. However, still in non-neuronal cells including pancreas and parotid, the functional role of synaptotagmins is not investigated, because method for identifying its functional role is not available. Previously we published a low level expression of synaptotagmins in pancreatic acinar cells from sarco/endoplasmic reticulum ATPase 2 heterozygote mice and its wild type.29 In Fig. 2C, the expression level of synaptotagmin 1 in the parotid, submandibular, and pancreatic acinar cells was lower than that in the brain, indicating that the exocrine gland cells utilize a different Ca2+-sensing and regulatory mechanism to secrete secretory vesicles than do neuronal cells.
The most notable finding in this study is that rat and mouse parotid acinar cells have different Ca2+-dependent synaptotagmins and that this could be related to their ability with regard to exocytosis of salivary proteins, such as amylase. RT-PCR results in Fig. 2 show that mouse parotid acinar cells express 7 Ca2+-dependent synaptotagmins, and rat parotid acinar cells express types 1, 3, 4, and 7, but not 2, 5, and 6. In the previous reports, recombinant synaptotagmin 2 exhibited Ca2+-dependent binding to syntaxin 1, a component of the synaptic vesicle fusion complex, with half maximal binding occurring ~200 µM Ca2+ in agreement with the high, local Ca2+ concentrations estimated to be required for transmitter release at many synapses.23 Although the exact functions and subcellular localization of synaptotagmins 5 and 6 remain unclear, synaptotagmin 5 is a dense core vesicle-specific synaptotagmin isoform that controls a specific type of calcium-regulated secretion in pancreatic α-cells,24 and synaptotagmin 6 is a key component of the secretory machinery involved in acrosomal Ca2+-dependent exocytosis.25 Based on these reports, we suggest that the expression of Ca2+-dependent synaptotagmins evoked the high sensitivity of amylase release in respond to muscarinic stimulation in mouse parotid acinar cells in Fig. 1A.
Ca2+ is a key regulator of many cellular processes.30 In Figs. 1B and C, muscarinic stimulation induced concentration-dependent [Ca2+]i increases and Ca2+ influx. First, rapid [Ca2+]i increases within a milisecond were observed, followed by a long-term sustained plateau. Rapid [Ca2+]i increases are generated by the activation of G-protein coupled receptors, Gαq, and phospholipase C β to generate 1,4,5-tris-inositolphosphate (IP3) in the cytosol, resulting in the release Ca2+ from the ER. A sustained plateau level of [Ca2+]i depends on the relative ratio between the activity of store-operated Ca2+ channels, and the plasma membrane Ca2+ pumps.31 In parotid acinar cells, muscarinic stimulation directly generates transient [Ca2+]i increases and Ca2+ influx. The difference in amylase release profiles in Fig. 1A promptly led us to investigate [Ca2+]i. Interestingly, both cells exhibited dose-dependent patterns, but identical transient [Ca2+]i increases and Ca2+ influx, indicating that both cells have similar mechanism for increasing and decreasing [Ca2+]i levels. In addition, our work demonstrates that increasing concentrations of muscarinic stimulation increase the amplitude of [Ca2+]i in Fig. 1B. This behavior can be best explained by the quantal properties of Ca2+ release, with increasing concentrations of IP3 reducing the threshold for initiation of Ca2+ release.32
In the present study, different expressions of Ca2+-dependent synaptotagmin were found in rat and mouse parotid acinar cells, and may be related to different amylase release. Although our observation did not directly demonstrate the functions of Ca2+-dependent synaptotagmins, these proteins may represent the specific targets of intracellular Ca2+ signaling for salivary protein secretion. Further study is required, to elucidate the exact role of Ca2+-dependent synaptotagmin.
Footnotes
This work was supported by the Research Fund from Yonsei University College of Dentistry for 2003 to Dong Min Shin.
References
- 1.Quissell DO. Stimulus-exocytosis coupling mechanism in salivary gland cells. In: Dobrosielski-Vergona K, editor. Biology of the Salivary Glands. Florida: CRC Press; 1993. pp. 181–200. [Google Scholar]
- 2.Takuma T, Ichida T. Catalytic subunit of protein kinase A induces amylase release from streptolysin O-permeabilized parotid acini. J Biol Chem. 1994;269:22124–22128. [PubMed] [Google Scholar]
- 3.Turner RJ, Sugiya H. Understanding salivary fluid and protein secretion. Oral Dis. 2002;8:3–11. doi: 10.1034/j.1601-0825.2002.10815.x. [DOI] [PubMed] [Google Scholar]
- 4.Takuma T, Ichida T. Does cyclic AMP mobilize calcium for amylase secretion from rat parotid cells? Biochim Biophys Acta. 1986;887:113–117. doi: 10.1016/0167-4889(86)90130-8. [DOI] [PubMed] [Google Scholar]
- 5.Butcher FR, Putney JW. Regulation of parotid gland function by cyclic nucleotides and calcium. Adv Cyclic Nucleotide Res. 1980;13:215–249. [PubMed] [Google Scholar]
- 6.Sugiya H, Furuyama S. The activation of Ca2+ mobilizing receptors in salivary gland. Biomed Res. 1989;10:111–121. [Google Scholar]
- 7.Hay JC, Scheller RH. SNAREs and NSF in targeted membrane fusion. Curr Opin Cell Biol. 1997;9:505–512. doi: 10.1016/s0955-0674(97)80026-9. [DOI] [PubMed] [Google Scholar]
- 8.Jahn R, Sudhof TC. Membrane fusion and exocytosis. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- 9.Weber T. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 10.Mcnew JA. Compartmental specificity of cellular mem brane fusion encoded in SNARE protein. Nature. 2000;407:153–159. doi: 10.1038/35025000. [DOI] [PubMed] [Google Scholar]
- 11.Geppert M, Sudhof TC. RAB3 and synaptotagmin: the yin and yang of synaptic membrane fusion. Annu Rev Neurosci. 1998;21:75–95. doi: 10.1146/annurev.neuro.21.1.75. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda M. Molecular cloning and characterization of human, rat and mouse synaptotagmin XV. Biochem Biophys Res Commun. 2003;306:64–71. doi: 10.1016/s0006-291x(03)00911-2. [DOI] [PubMed] [Google Scholar]
- 13.Butz S, Fernandez CR, Schmitz F, Jahn R, Sudohf TC. The subcellular localization of atypical synaptotagmin III and VI. Synaptotagmin III enriched in synapses and synaptic plasma membrane but not in synaptic vesicles. J Biol Chem. 1999;274:18290–18296. doi: 10.1074/jbc.274.26.18290. [DOI] [PubMed] [Google Scholar]
- 14.Brown H, Meister B, Deeney J, Corkey BE, Yang SN, Larsson O, et al. Synaptotagmin III isoform is compartmentalized in pancreatic-cells and has a functional role in exocytosis. Diabetes. 2000;49:383–391. doi: 10.2337/diabetes.49.3.383. [DOI] [PubMed] [Google Scholar]
- 15.Gao Z, Reavey CJ, Young RA, Jegier P, Wolf BA. Synaptotagmin III/VII isoforms mediate Ca2+ induced insulin secretion in pancreatic islet β cells. J Biol Chem. 2000;275:36079–36085. doi: 10.1074/jbc.M004284200. [DOI] [PubMed] [Google Scholar]
- 16.Mitzuda M. Localization and functional role of synaptotagmin in insulin secretory vesicles in pancreatic-cells. Diabetes. 1997;46:2002–2006. doi: 10.2337/diab.46.12.2002. [DOI] [PubMed] [Google Scholar]
- 17.Takuma T, Arakawa T, Tajima Y. Interaction of SNARE proteins in rat parotid acinar cells. Arch Oral Biol. 2000;45:369–375. doi: 10.1016/s0003-9969(00)00004-2. [DOI] [PubMed] [Google Scholar]
- 18.Imai A, Nashida T, Shimomura H. mRNA expression of membrane-fusion-related proteins in rat parotid gland. Arch Oral Biol. 2001;46:955–962. doi: 10.1016/s0003-9969(01)00048-6. [DOI] [PubMed] [Google Scholar]
- 19.Zeng W, Lee MG, Yan M, Diaz J, Benzamin I, Marino CR, et al. Immuno and functional characterization of CFTR in submandibular and pancreatic acinar and duct cells. Am J Physiol. 1997;273:C442–C455. doi: 10.1152/ajpcell.1997.273.2.C442. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Diaz J, Zhao H, Muallem S. Chacterization, localization and axial distribution of calcium signaling receptors in the rat submandibular salivary glands ducts. J Physiol (Lond) 1996;491:647–662. doi: 10.1113/jphysiol.1996.sp021246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong JH, Lee SI, Kim KE, Yong TS, Seo JT, Sohn MH, et al. German cockroach extract activates protease-activated receptor 2 in human airway epithelial cells. J Allergy Clin Immunol. 2004;113:315–319. doi: 10.1016/j.jaci.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Bernfeld P. Enzymes of starch degradation and synthesis. Adv Enzymol Relat Subj Biochem. 1951;12:379–428. doi: 10.1002/9780470122570.ch7. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Ullrich B, Zhang JZ, Anderson RG, Brose N, Sudhof TC, et al. Calcium dependent and independent activities of neural and non-neural synaptotagmin. Nature. 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- 24.Saegusa C, Fukuda M, Mikoshiba K. Synaptotagmin V is targeted to dense-core vesicles that undergo calcium dependent exocytosis in PC12 cells. J Biol Chem. 2002;277:24499–24505. doi: 10.1074/jbc.M202767200. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Fukuda M, Bocktaele EV, Pascual O, Haydon PG. Synaptotagmin IV regulates glial glutamate release. Proc Natl Acad Sci USA. 2004;101:9441–9446. doi: 10.1073/pnas.0401960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levious O, Feinstein N, Linial M. Expression and localization of synaptotagmin 1 in rat parotid gland. Eur J Cell Biol. 1997;73:81–92. [PubMed] [Google Scholar]
- 27.Sudhof TC. Synaptotagmins: Why so many? J Biol Chem. 2002;277:7629–7632. doi: 10.1074/jbc.R100052200. [DOI] [PubMed] [Google Scholar]
- 28.Chapman ER. Synaptotagmin a calcium sensor that triggers exocytosis? Nat Rev Mol Cell Biol. 2002;3:498–508. doi: 10.1038/nrm855. [DOI] [PubMed] [Google Scholar]
- 29.Zhao XS, Shin DM, Liu LH, Shull GE, Muallem S. Plasticity and adaptation of Ca2+ signaling and Ca2+-dependeny exocytosis in SERCA2+/- mice. EMBO J. 2001;20:2680–2689. doi: 10.1093/emboj/20.11.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 31.Kiselyov K, Shin DM, Muallem S. Signaling specificity in GPCR-dependent calcium signaling. Cell Signal. 2003;15:243–253. doi: 10.1016/s0898-6568(02)00074-8. [DOI] [PubMed] [Google Scholar]
- 32.Shin DM, Luo X, Wilkie TM, Miller LJ, Peck AB, Humphreys-Beher MG. Polarized expression of G protein-coupled receptors and an all-or-none discharge of Ca2+ pools at initiation sites of [Ca2+]i waves in polarized exocrine cells. J Biol Chem. 2001;276:44146–44156. doi: 10.1074/jbc.M105203200. [DOI] [PubMed] [Google Scholar]