Abstract
Low birth weight is associated with insulin resistance and type 2 diabetes in adults. The fetal programming hypothesis has shown that insulin resistance and its associated metabolic disturbances result from a poor gestational environment, for which low birth weight is a surrogate. An at-home questionnaire survey was performed on 660 middle school students (12-15 years) in Seoul, Korea, and 152 cases were randomly selected based on their birth weight. Subjects were divided into three groups according to birth weight. We recorded their birth weight and measured their current anthropometric data, blood pressure, lipid profile, HOMA-IR, and HOMA-β, and compared these parameters among the groups. The relation of birth weight to physiological characteristics in adolescence was examined. Systolic blood pressure, lipid profiles, and fasting plasma glucose, HOMA-β were not significantly different among the groups, but diastolic blood pressure was lower in the third tertile. Insulin, C-peptide, and HOMA-IR were higher in the lower birth weight tertile. After adjustment for confounding factors, birth weight was inversely related to diastolic blood pressure, insulin, C-peptide, and HOMA-IR. We conclude that low birth weight may predict the risk of the insulin resistance and its progression over age, and that adequate gestational nutrition is therefore necessary to prevent low birth weight.
Keywords: Birth weight, insulin resistance, adolescence
INTRODUCTION
Epidemiological studies have demonstrated that adults with a low birth weight are at an increased risk of developing type 2 diabetes mellitus (T2DM), which led to the hypothesis that metabolic syndrome may be closely associated with fetal nutritional state.1 Hales et al. proposed the thrifty phenotype hypothesis as a cause of T2DM, by arguing that malnutrition during the fetal and neonatal periods impairs the development of endocrine function in the pancreas, resulting in an impairment of insulin secretion by β-cells.2,3 In addition, Barker et al.4 reported a higher death rate due to ischemic cardiac disease in a study of 5,654 men with low weight at birth and at 1 year of age. Such results suggest that poor nutrition during the fetal period may cause permanent structural and functional changes in the pancreas that may cause diabetes, hypertension, dyslipidemia, insulin resistance, and other metabolic impairments.1,4
In most studies on birth weight and insulin resistance, low birth weight infants show increased insulin resistance as adolescences or adults.5 However, studies on the relationship between birth weight and insulin resistance are very rare in Korea.6 Accordingly, the purpose of our study is to examine whether birth weight is related to insulin resistance, and to determine whether fetal programming permanently affects insulin resistance in Korean adolescents.
MATERIALS AND METHODS
Subjects
In April 2003, an at-home questionnaire survey was administered to 660 middle school students (12-15 years old) in Seoul, Korea, and 152 cases were selected randomly based on their birth weight. Birth weight data was based on birth records, except in 21 cases (maternal recall) where birth weight was not available from the birth records. Tanner staging was completed during a complete physical examination by a pediatrician.
Subjects were divided into three groups according to birth weight. The subjects born prematurely, earlier than 37 weeks gestation, and those with family history of diabetes (diabetes in either parent) were excluded from the study. The study protocol was approved by the Yonsei University College of Medicine Ethical Committee, and informed consent was obtained from each participant.
Methods
Physical measurements
Body weight and height were measured in the morning, with participants wearing light clothing. Body mass index (BMI) was calculated as body weight divided by height (kg/m2). Waist circumference was determined using a measuring tape placed midway between the lowest rib and the iliac crest, with the participant standing on a flat floor with his or her feet 30 cm apart. Body fat percentage was evaluated by dual-energy X-ray absorptiometry (Hologic QDR 1500; Delphi, Waltham, MA, USA).
Blood pressure (mmHg) was measured twice after the subject had been resting in the supine position for 5 min with the use of an appropriate cuff size in relation to arm size. Diastolic blood pressure was determined as Korotkoff phase V. The mean of the two measurements was used in further analyses.
Blood collection and serological testing
Participants fasted from 10 PM to 9 AM, at which time blood samples were collected. Plasma glucose levels were measured immediately with an autoanalyzer using the hexokinase method (Hitachi 747; Roche, Montclair, NJ, USA). Serum insulin and C-peptide levels were determined using an enzyme chemiluminescence immunoassay (ECIA, DPC, Immulite DPC, Los Angeles, USA). Serum total cholesterol and HDL-cholesterol were measured using a direct enzymatic method (Hitachi 747; Daiichi, Tokyo, Japan). Serum triglyceride levels were measured by an enzymatic colorimetric method (Hitachi 747; Roche, Almere, Japan/Germany), and LDL-cholesterol was calculated using the Friedewald equation.7
Measurement of insulin resistance and insulin secretion
Insulin resistance was estimated after fasting using the homeostasis model assessment method for insulin resistance (HOMA-IR), and the ability to secrete insulin was measured using a homeostasis model assessment of β-cell function (HOMA-β). These models were originally developed by Matthews, subsequently modified,8,9 and have been validated in patients with T2DM and obesity.10,11 The formulas are as follows: HOMA-IR = fasting insulin (µU/mL) × fasting plasma glucose (mmol/L)/22.5; HOMA-β = 20 × fasting insulin (µU/ml)/[fasting plasma glucose (mmol/L)-3.5].
Statistical analysis
Descriptive statistics are presented as mean values ± SD. We used an independent sample t-test or Chi-square test for the comparison of males and females, as appropriate. An ANOVA was performed to compare birth weight, current age, weight, height, BMI, waist circumference, and body fat percentage of each group according to their birth weight. The physiological characteristics (systolic blood pressure, diastolic blood pressure, total cholesterol, triglyceride, HDL-cholesterol, LDL-cholesterol, fasting plasma glucose, insulin, C-peptide, HOMA-IR, and HOMA-β) were adjusted using a general linear model; the covariates used were age, sex, height, weight, waist circumference, body fat percentage, and Tanner stage. The inclusion of covariates in the general linear model removes its contribution to the outcome variables. The relation of birth weight to physiological characteristics in adolescents was examined by partial correlation and adjusted for age, sex, height, weight, waist circumference, body fat percentage, and Tanner stage. Statistical analyses were conducted using SPSS for Windows, version 11.0 (SPSS Inc., Chicago, IL, USA), and p < 0.05 was set as the level of significance.
RESULTS
Physical measurements and the clinical characteristics of the study subjects
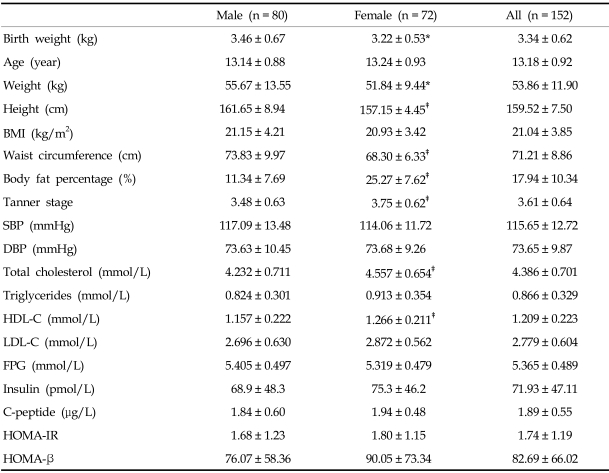
Eighty subjects were male and seventy-two were female, with an average age of 13.18 ± 0.92 years. No statistically significant difference in age between genders was noted. Although height, weight, and waist circumference were greater in males compared to females, there was no difference in BMI. Body fat percentage was higher in females. Blood pressure, fasting plasma glucose, insulin, C-peptide, HOMA-IR, and HOMA-β were not significantly different between males and females. On the other hand, total cholesterol and HDL-cholesterol levels were higher in females (both p < 0.01), but triglyceride and LDL-cholesterol levels were not significantly different (Table 1).
Table 1.
Clinical Characteristics of Subjects
Values are mean ± SD.
*p < 0.05 vs. male, †p < 0.01 vs. male, ‡p < 0.001 vs. male.
BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, HDL-cholesterol, LDL-C, LDL-cholesterol; FPG, fasting plasma glucose; HOMA-IR, Homeostasis model assessment for insulin resistance; HOMA-β, Homeostasis model assessment for β-cell function.
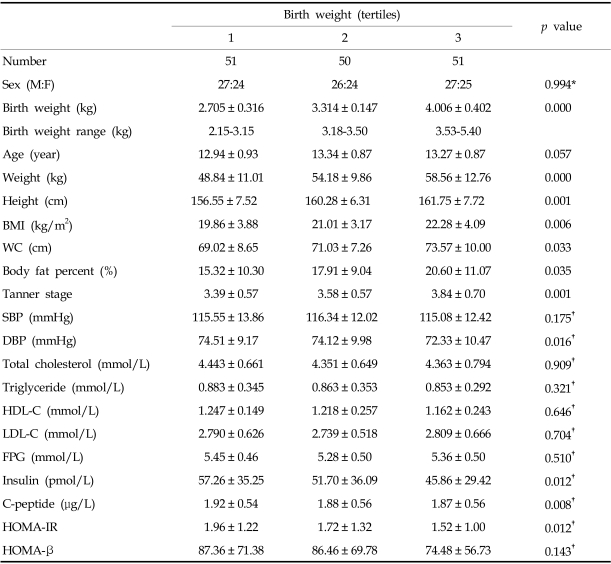
Subjects were grouped into thirds according to birth weight. Analysis of grouped data from males and females showed that birth weight correlated with adolescent body size. Current weight, height, BMI, waist circumference, body fat percentage, and Tanner stage all increased with birth weight tertile. Systolic blood pressure, lipid profiles, fasting plasma glucose, and HOMA-β were not significantly different between groups, but diastolic blood pressure was lower in the third tertile. Lower birth weight was related to insulin resistance in adolescence and insulin, C-peptide, and HOMA-IR were higher in the lower birth weight tertile (all p < 0.05) (Table 2).
Table 2.
Anthropometric and Physiological Characteristics according to Thirds of Birth Weight
Values are mean ± SD.
*Analyzed by chi-square test.
†Adjusted for age, sex, height, weight, waist circumference, body fat percent, and Tanner stage.
BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, HDL-cholesterol, LDL-C, LDL-cholesterol; FPG, fasting plasma glucose; HOMA-IR, homeostasis model assessment for insulin resistance; HOMA-β, homeostasis model assessment for β-cell function.
Association of birth weight with physiological characteristics in adolescence
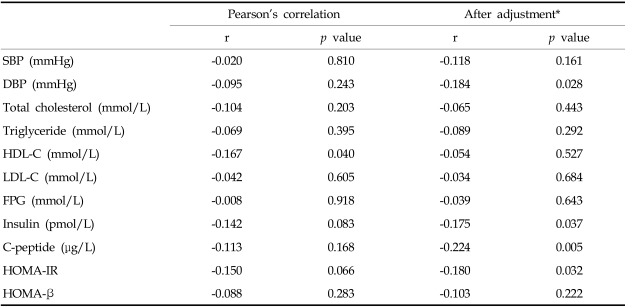
There was no significant relationship between birth weight and physiological characteristics in adolescents, except for HDL-cholesterol (r = -0.167; p = 0.040). However, each physiologic variable in adolescents correlated with current anthropometric parameters. Thus, we performed partial correlation analyses to examine the relationship between each physiological characteristic while controlling for the effects of current anthropometric parameters. After adjustment for gender, current age, height, weight, waist circumference, body fat percentage, and Tanner stage, birth weight was inversely related to diastolic blood pressure (r = -0.184; p = 0.029), insulin (r = -0.194; p = 0.021), C-peptide (r = -0.237; p = 0.004), and HOMA-IR (r = -0.195; p = 0.020). But, the relationship between birth weight and HDL-cholesterol was attenuated (r = -0.071; p = 0.403) (Table 3).
Table 3.
Association of Birth Weight with Physiological Characteristics in Adolescents
*Adjusted for age, sex, height, weight, waist circumference, body fat percent, and Tanner stage. BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, HDL-cholesterol, LDL-C, LDL-cholesterol; FPG, fasting plasma glucose; HOMA-IR, Homeostasis model assessment for insulin resistance; HOMA-β, Homeostasis model assessment for β-cell function.
DISCUSSION
After an epidemiological study showing the increased risk of developing T2DM in adults who were under-weight at birth, the hypothesis was proposed that insulin resistance syndrome and metabolic syndrome are closely related to fetal nutritional state. In a study on residents of the Hertfordshire and Preston areas in England, Hales et al.3 reported that the weight at birth and 1 year correlate inversely to the development of diabetes in adults. The risk of diabetes is decreased as birth weight is increased. Thus, they argued that malnutrition during the fetal and neonatal period impairs the formation of the endocrinal pancreas, resulting in the impairment of insulin secretion by beta cells. Hales et al. proposed the thrifty phenotype hypothesis as a causality of T2DM.2 In addition, they reported that in 5,654 men who were underweight at birth and 2 years after birth, the death rate due to ischemic cardiac diseases was high,4 and in subsequent studies, they observed that blood pressure and plasma fibrinogen were increased in adults who had had low body weight compared to placenta weight.12,13 Such reports argue that maternal malnutrition during important periods for the fetus may cause permanent structural and functional changes in specific organs such as the liver, blood vessels, pancreatic tissues, etc., and that the time of malnutrition and specific characteristics may determine the pattern of metabolic impairment such as diabetes, hypertension, dyslipidemia, etc. They proposed that impairment of the initial formation of beta cells in the pancreas may be an important factor for the development of T2DM. In other words, if the fetus is exposed to nutrition deficiency or malnutrition during the active proliferation of beta cells, the developing fetus undergoes metabolic adaptation.14 The developmental dysgenesis of endocrinal pancreas is induced during such processes and cannot completely recover by the intake of sufficient nutrition. Under such conditions, insulin secretion remains impaired, and in adults requiring increased amounts of insulin, diabetes may develop.
In most studies on birth weight and insulin resistance, low birth weight infants show increased insulin resistance as adolescences or adults.5 Similar results were obtained in a study of Koreans6 that investigated the relationships between birth weight and insulin secretion, resistance, and various anthropometric indices including visceral fat area in healthy young males. They found a positive association between birth weight and insulin sensitivity in adult life. However, there were some limitations in their study. First, the subjects were not randomly selected, and the study did not include both sexes. In addition, although they found a positive relationship between birth weight and insulin sensitivity in adulthood, they omitted the interaction between current insulin sensitivity and current body size.
The hypothesis that an adult disease has fetal origins is plausible, but much supporting evidence is flawed by incomplete and incorrect statistical interpretation.15 Some observational studies show a direct association between low birth weight and current adult health status.12,16-19 However, in other studies this relationship has emerged only after body size at some later period (notably current weight or body mass index) has been adjusted for.20-25 Adjusting for current size has been justified on the grounds that birth weight or size is positively related to later size, and also that current weight or fatness is positively related to the outcome variable of interest (for example, LDL-cholesterol). If not adjusted for current weight or fatness, the relationship between birth weight and the outcome variable could be obscured.26
In our study, each physiologic variable (blood pressure, lipid profiles, HOMA index) in adolescence correlated with current anthropometric parameters (data not shown). Thus, we performed partial correlation analyses to examine the relationship between each physiological characteristic in adolescence while controlling for the effects of current anthropometric parameters. Therefore, low birth weight was related to insulin resistance in adolescence; insulin, C-peptide, and HOMA-IR were higher in the lower birth weight tertile. Meanwhile, birth weight correlated negatively to insulin, C-peptide, and HOMA-IR.
The finding of a non-significant difference in fasting plasma glucose levels among the birth weight tertiles for males and females was somewhat unexpected. However, there were some differences in the patterns of the child-adult relationship; in children, the relationships reported were inconsistent: absent, positive or U-shaped.17,27,28 In the natural progression of T2DM, relative insulin resistance and high insulin secretion are followed by elevated plasma glucose. So in the earlier stages of T2DM, insulin resistance and, as a result, high insulin secretion may be predominant.5
In our study, birth weight was not significantly related to systolic blood pressure. However, we detected a small but significant negative correlation between birth weight and diastolic blood pressure. The majority of studies that have examined the relationship between birth weight and blood pressure in adolescents reported an inverse relationship,29 but it was often smaller than that reported in prepubescent children and adults. In a longitudinal study of Finnish children and adolescents,30 it was found that during puberty, the inverse relationship between birth weight and blood pressure is smaller, but is restored to previous levels after puberty. This change in the association of birth weight and blood pressure may be due to the perturbed tracking of blood pressure during the adolescent growth phase.29 There was no direct association between birth weight and lipid profiles in adolescents in our study, and this result is consistent with previous systematic reviews.31,32
There are a number of possible limitations in our study. Some of the birth weight data were reported by participants' parents. However, validity of parent-reported birth weight has been previously confirmed.20,25,33 This cohort has not been followed-up between birth and adolescence, so we can not show the growth patterns during infancy, which are known to be an important determinant of later insulin resistance.34 In addition, genetic influence cannot be entirely eliminated, although subjects with family history of diabetes (diabetes in either parent) were excluded from the study. Hattersley et al.35 suggested that insulin-regulated fetal growth, and hence birth weight, are determined by fetal insulin secretion and insulin action, which are in turn regulated by fetal genotype. Dunger et al.36 showed an association between common genetic variation at a variable number of tandem repeat (VNTR) loci in the promoter region of the insulin gene and birth size. Moreover, it remains possible that birth weight is not the marker at birth most strongly related to insulin resistance.
In conclusion, low birth weight was related to insulin resistance in Korean adolescents and adequate gestational nutrition is necessary to prevent low birth weight.
Footnotes
This study was supported by a grant from the Korea Health 21 R & D Project, Ministry of & Health Welfare, Republic of Korea (03-PJ1-PG1-CH05-0005).
References
- 1.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–66. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 2.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 3.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, et al. Fetal and infant growth and impaired glucose tolerance at age 64. Br Med J. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJ, Winter PD, Osmond C, Margettes B, Simmonds SJ. Weight in infancy and death from ischemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 5.Newsome CA, Shiell AW, Fal C, Phillips D, Shier R, Law CM. Is birth weight related to later glucose and insulin metabolism? - a systemic review. Diabetic Med. 2003;20:339–348. doi: 10.1046/j.1464-5491.2003.00871.x. [DOI] [PubMed] [Google Scholar]
- 6.Choi CS, Kim C, Lee WJ, Park JY, Hong SK, Lee MG, et al. Association beween birth weight and insulin sensitivity in healthy young men in Korea: role of visceral adipocity. Diabetes Res Clin Pract. 2000;49:53–59. doi: 10.1016/s0168-8227(00)00131-5. [DOI] [PubMed] [Google Scholar]
- 7.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma without use of preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 8.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 9.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program (letter) Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 10.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 11.Hermans MP, Levy JC, Morris RJ, Turner RC. Comparison of tests of beta-cell function across a range of glucose tolerance from normal to diabetes. Diabetes. 1999;48:1779–1786. doi: 10.2337/diabetes.48.9.1779. [DOI] [PubMed] [Google Scholar]
- 12.Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. Br Med J. 1990;301:259–262. doi: 10.1136/bmj.301.6746.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barker DJ, Meade TW, Fall CH, Lee A, Osmond C, Phipps K, et al. Relation of fetal and infant growth to plasma fibrinogen and factor VII concentrations in a adult life. Br Med J. 1992;304:148–152. doi: 10.1136/bmj.304.6820.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001;4:611–624. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- 15.Lucas A, Fewtrell MS, Cole TJ. Fetal origins of adult disease-the hypothesis revisited. Br Med J. 1999;319:245–249. doi: 10.1136/bmj.319.7204.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson S, Walton RJ, Clark PM, Barker DJP, Hales CN, Osmond C. The relation of fetal growth to plasma glucose in young men. Diabetologia. 1992;35:444–446. doi: 10.1007/BF02342441. [DOI] [PubMed] [Google Scholar]
- 17.Law CM, Gordon GS, Shiell AW, Barker DJP, Hales CN. Thinness at birth and glucose tolerance in seven-year-old children. Diabet Med. 1995;12:24–29. doi: 10.1111/j.1464-5491.1995.tb02057.x. [DOI] [PubMed] [Google Scholar]
- 18.Valdez R, Athens MA, Thompson GH, Bradshaw BS, Stern MP. Birth weight and adult health outcomes in a biethnic population in the USA. Diabetologia. 1994;37:624–631. doi: 10.1007/BF00403383. [DOI] [PubMed] [Google Scholar]
- 19.Lithell HO, McKeigue PM, Berglund L, Mohsen R, Lithell U-B, Leon DA. Relation of size at birth to non-insulin dependent diabetes and insulin concentrations in men aged 50-60 years. Br Med J. 1996;312:406–410. doi: 10.1136/bmj.312.7028.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor SJ, Whincup PH, Cook DG, Papacosta O, Walker M. Size at birth and blood pressure: cross sectional study in 8-11 year old children. Br Med J. 1997;314:475–480. doi: 10.1136/bmj.314.7079.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forrester TE, Wilks RJ, Bennett FI, Simeon D, Osmond C, Allen M, et al. Fetal growth and cardiovascular risk factors in Jamaican schoolchildren. Br Med J. 1996;312:156–160. doi: 10.1136/bmj.312.7024.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fall CH, Stein CE, Kumaran K, Cox V, Osmond C, Barker DJ, et al. Size at birth, maternal weight and type 2 diabetes in South India. Diabet Med. 1998;15:220–227. doi: 10.1002/(SICI)1096-9136(199803)15:3<220::AID-DIA544>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 23.Law CM, Barker DJ, Osmond C, Fall CH, Simmonds SJ. Early growth and abdominal fatness in adult life. J Epidemiol Community Health. 1992;46:184–186. doi: 10.1136/jech.46.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fall CH, Pandit AN, Law CM, Yajnik CS, Clark PM, Breier B, et al. Size at birth and plasma insulin-like growth factor-1 concentrations. Arch Dis Child. 1995;73:287–293. doi: 10.1136/adc.73.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whincup PH, Cook DG, Adshead F, Taylor SJC, Walker M, Papacosta O, et al. Childhood size is more strongly related than size at birth to glucose and insulin levels in 10-11-year-old children. Diabetologia. 1997;40:319–326. doi: 10.1007/s001250050681. [DOI] [PubMed] [Google Scholar]
- 26.Leon DA. Fetal growth and adult disease. Eur J Clin Nutr. 1998;52:S72–S82. [PubMed] [Google Scholar]
- 27.Bavdekar A, Yajnik CS, Fall CH, Bapat S, Pandit AN, Deshpande V, et al. Insulin resistance syndrome in 8-year old Indian children: small at birth, big at 8 years, or both. Diabetes. 1999;48:2422–2429. doi: 10.2337/diabetes.48.12.2422. [DOI] [PubMed] [Google Scholar]
- 28.Dabelea D, Pettitt DJ, Hanson RL, Imperatore G, Bennett PH, Knowler WC. Birth weight, type 2 diabetes, and insulin resistance in Pima Indian children and young adults. Diabetes Care. 1999;22:944–950. doi: 10.2337/diacare.22.6.944. [DOI] [PubMed] [Google Scholar]
- 29.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 30.Taittonen L, Nuutinen M, Turtinen J, Uhari M. Prenatal and postnatal factors in predicting later blood pressure among children: cardiovascular risk in young Finns. Pediatr Res. 1996;40:627–632. doi: 10.1203/00006450-199610000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Owen CG, Whincup PH, Odoki K, Gilg JA, Cook DG. Birth weight and blood cholesterol Level: A study in adolescents and systematic review. Pediatrics. 2003;111:1081–1089. doi: 10.1542/peds.111.5.1081. [DOI] [PubMed] [Google Scholar]
- 32.Huxley RR, Owen CG, Whincup PH, Cook DG, Colman S, Collins R. Birth weight and subsequent cholesterol levels: exploration of the "fetal origins" hypothesis. JAMA. 2004;292:2755–2764. doi: 10.1001/jama.292.22.2755. [DOI] [PubMed] [Google Scholar]
- 33.Eaton-Evans J, Dugdale AE. Recall by mothers of the birth weights and feeding of their children. Hum Nutr Appl Nutr. 1986;40:171–175. [PubMed] [Google Scholar]
- 34.Ong KK, Dunger DB. Birth weight, infant growth and insulin resistance. Eur J Endocrinol. 2004;151:U131–U139. doi: 10.1530/eje.0.151u131. [DOI] [PubMed] [Google Scholar]
- 35.Hattersley AT, Beards F, Ballantyne E, Appleton M, Harvey R, Ellard S. Mutations in the glucokinase gene of the fetus result in reduced birth weight. Nat Genet. 1998;19:268–270. doi: 10.1038/953. [DOI] [PubMed] [Google Scholar]
- 36.Dunger DB, Ong KK, Huxtable SJ, Sherriff A, Woods KA, Ahmed ML, et al. Association of the INS VNTR with size at birth. ALSPAC study team. Avon longitudinal study of pregnancy and childhood. Nat Genet. 1998;19:98–100. doi: 10.1038/ng0598-98. [DOI] [PubMed] [Google Scholar]