Abstract
Neurofibromatosis type 1 (von Recklinghausen's disease, NF-1) is an autosomal-dominant neurocutaneous disorder characterized by abnormal skin pigmentation (café au lait spots and axillary freckling), cutaneous and plexiform neurofibromas, skeletal dysplasias, and Lisch nodules (pigmented iris hamartomas). Gastrointestinal stromal tumors (GISTs) are the most common tumors of mesenchymal origin in the gastrointestinal tract, mesentery, omentum, and retroperitoneum. Here, we report a case of GIST in the ileum of a 76-year-old woman previously diagnosed as NF-1. She was admitted due to sudden onset of abdominal pain. Contrast enhanced CT scan revealed a moderately defined, peripherally enhanced soft tissue mass of about 8.8×7.3cm, originating from the small bowel in the left of the abdomen. Surgical excision was performed and the tumor was found to be composed of tumor cells that were positive for c-kit protein. The patient started imatinib treatment a month later, but stopped medication due to dyspepsia after a few months and eventually progressed after 18 months
Keywords: Gastrointestinal Stromal Tumors, Neurofibromatosis
INTRODUCTION
Neurofibromatosis type 1 (von Recklinghausen's disease, NF-1) is a relatively common autosomal-dominant neurocutaneous disorder characterized by abnormal skin pigmentation (café au lait spots and axillary freckling), cutaneous and plexiform neurofibromas, skeletal dysplasias, and Lisch nodules (pigmented iris hamartomas). The genetic abnormality in neurofibromatosis has been localized to the neurofibromatosis 1 gene on chromosome 17, which is a tumor suppressor gene in some cell types1). Abnormal tumor suppression increases the incidence of benign and malignant neoplasia in children and adults with NF-1. Optic pathway gliomas and neurofibromas are the most common neoplasms occurring in patients with neurofibromatosis1).
Gastrointestinal abnormalities in patients with NF-1 are reported to occur in up to 10~25% of patients and consist of four groups of lesions: mesenchymal neoplasms; hyperplasias of intestinal neural tissue; neuroendocrine tumors of the duodenum; and rarely, other gastrointestinal neoplasms such as adenocarcinomas2). Although neurofibromas are the most commonly encountered mesenchymal neoplasm of the gastrointestinal tract in patients with NF-1, Gastrointestinal stromal tumors (GISTs), leiomyomas, and leiomyosarcomas also occur3).
GISTs are the most common tumors of mesenchymal origin in the gastrointestinal tract, mesentery, omentum, and retroperitoneum. The most significant risk factor for development of these tumors is the presence of NF-15). Other risk factors include urticaria pigmentosa and the very rare familial GIST syndrome.
Although the increased incidence of GIST in patients with neurofibromatosis is well documented in pathology literature in English, this association has rarely been documented in Korea. Here we report a case of GIST in the ileum of a 76-year-old woman diagnosed as NF-1.
CASE REPORT
A previously healthy 76-year-old woman who was diagnosed with NF-1 40 years ago presented with sudden onset of left lower quadrant pain. When she visited the emergency room, vital signs were blood pressure 100/60 mmHg, pulse rate 96/minute, respiratory rate 20/min, and body temperature 37℃. The physical examination revealed a palpable nonpulsatile mass in the left flank area, which showed tenderness and rebound tenderness. There were numerous café-au-lait patches and multiple cutaneous neurofibromas on the upper extremities and trunk.
When admitted, CBC showed leukocytes 4,880/mm3 (segmented neutrophils 85.9%), hemoglobin 5.6 g/dL, and platelets 218,000/mm3. Blood chemistry results were protein 5.7 g/dL, albumin 3.1 g/dL, total bilirubin 0.5 mg/dL, alkaline phosphatase 144 IU/L, AST/ALT 26/14 IU/L, and BUN/creatinine 26/1.2 mg/dL. There were no significant findings for electrolytes or urinalysis.
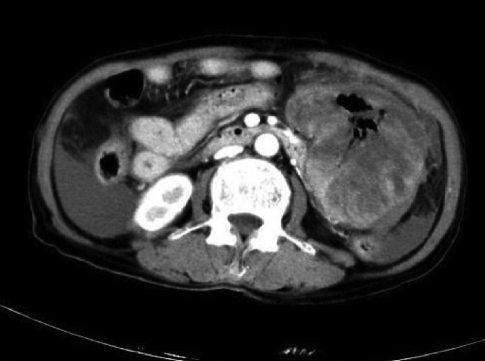
A contrast enhanced CT scan was performed and it revealed a soft tissue mass in the small bowel suggesting a diagnosis of GIST (Figure 1).
Figure 1.
Small-bowel GIST with a diffusely thickened bowel wall. Central portion of the tumor has an area of necrosis, with air present within the necrotic cavity that communicates with the lumen of the small bowel.
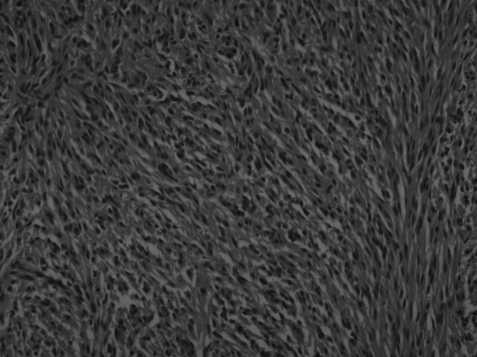
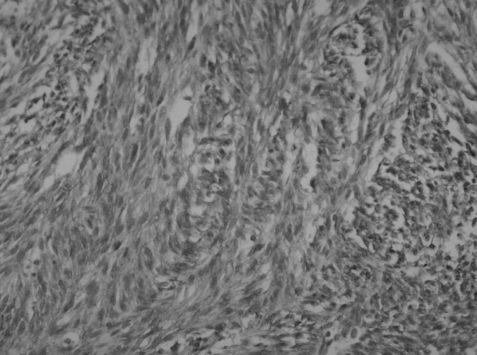
On the basis of the radiological finding, the ileal tumor was surgically excised. A gelatine-like, ruptured mass in very fragile state originating from the bowel wall was located just below the Treiz ligament. There were multiple fibrotic nodules though the whole small bowel. The fragmented mass measured 13.0×3.0×4.0 cm in total and was a brown tan color with a hemorrhagic appearance. The cut section showed a fishy-flesh appearance. A second mass measuring 4.0×3.0×2.0 cm had an irregular shape and was attached to the omentum. Its cut section was also a brown tan color with a fish-fleshy appearance. The tumors were composed of tumor cells that were positive for c-kit protein and the number of mitosis was 13/50 high power fields (HPF) (Figure 2 and 3).
Figure 2.
Histologically, the tumors were composed of multiple spindle cells with eosinophilic cytoplasm and ovoid to elongated nuclei. The number of mitosis was 13/50HPF (hematoxyline-eosin, ×200).
Figure 3.
Immunohistochemistry showing positive for the surface tyrosine kinase receptor c-Kit (CD-117), the defining characteristics of GIST (×200).
As the tumor was found by biopsy to be a GIST, the patient started receiving imatinib a month later. After a few months however, she stopped medication due to dyspepsia and eventually progressed after 18 months.
DISCUSSION
Neurofibromatosis type-1 (von Recklinghausen disease) is an autosomal dominant hereditary disease that may affect the gastrointestinal tract in up to 25% of patients2, 6, 7). Stromal tumors have been reported to be the most common lesion of the gastrointestinal tract, but the symptomatic cases account for less than 5% of patients2, 8). These tumors may cause obstruction, volvulus, intussusception, ulcertation, bleeding or perforation. In our patient, bleeding of ruptured tumors caused hemoperitoneum.
GISTs in patients with NF-1 usually occur in their middle-age after the appearance of cutaneous manifestations2). GISTs in patients with NF-1 are histologically and immunophenotypically identical to those in patients without NF-1. Although most GISTs arise in the stomach, a search in the pathology literature shows that GISTs in patients with NF-1 tend to be multiple and to be located predominantly within the small intestine4).
Clinical symptoms are related to the size and location of the GISTs. Most pathologists use a combination of tumor size and mitotic rate to assess the malignant potential of these tumors. In general, malignant GISTs are larger, more cellular, and more mitotically active. GISTs that are smaller than 5 cm with five mitoses per 50 consecutive high-power fields or less are considered to be benign with a low risk for metastasis. Tumors larger than 10 cm with more than five mitoses per 50 high-power fields are considered to be malignant. All tumors falling between these two extremes are considered to be of uncertain malignant potential with intermediate risk for metastasis. Tumors with more than 50 mitoses per 50 high-power fields are considered to be highly malignant with an aggressive clinical behavior. The liver and the peritoneum are the most common sites of spread in malignant GISTs5).
This case illustrates the increased prevalence and association of GISTs in patients with NF-1. A medical history of NF-1 in a patient who has an intra-abdominal mass with nonspecific CT features and central liquefaction may help in diagnosing GIST by virtue of the well-known association of these two entities.
References
- 1.Gutmann DH, Aylsworth A, Carey JC, Korf B, Marks J, Pyeritz RE, Rubenstein A, Viskochil D. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA. 1997;278:51–57. [PubMed] [Google Scholar]
- 2.Fuller CE, Williams GT. Gastrointestinal manifestations of type 1 neurofibromatosis (von Recklinghausen's disease) Histopathology. 1991;19:1–11. doi: 10.1111/j.1365-2559.1991.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 3.Riddle RH, Petras RE, Williams GT, Sobin LH. Tumors of the intestines. Washington: Armed Forces Institute of Pathology; 2003. [Google Scholar]
- 4.Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol. 2003;27:625–641. doi: 10.1097/00000478-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 5.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumours: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis GB, Berk RN. Intestinal neurofibromas in von Recklinghausen's disease. Am J Gastroenterol. 1973;60:410–414. [PubMed] [Google Scholar]
- 7.Hochberg FH, Dasilva AB, Galdabini J, Richardson EP., Jr Gastrointestinal involvement in von Recklinghausen's neurofibromatosis. Neurology. 1974;24:1144–1151. doi: 10.1212/wnl.24.12.1144. [DOI] [PubMed] [Google Scholar]
- 8.Riccardi VM. Neurofibromatosis: phenotype, natural history, and pathogenesis. 2nd ed. New York: Johns Hopkins; 1992. [Google Scholar]