Abstract
Although cultured myoblast transplantation has been extensively studied as a gene complementation approach to muscular dystrophy treatment, clinical success has still been limited. The inability to adequately isolate and purify myoblasts presents a major limitation to the production of sufficient myoblasts for engrafting purposes. This study attempted to purify myoblasts from primary culture by magnetic-activated cell sorting (MACS), complement-mediated cytotoxicity, and a preplating technique. As a result of positive myoblasts selection by MACS, the average percentage of myoblasts in mixed culture was increased from 30.0% to 41.7%. We observed both myoblast lysis and fibroblast lysis after complement-mediated cytotoxicity. Enrichment of myoblasts in mixed culture was found to increase to 83.1% by using the preplating technique. In addition, higher purification (92.8%) was achieved by following the preplating technique with MACS. Thus, preplating in combination with magnetic-activated cell sorting allows for a rapid and effective isolation of myoblasts from human muscle tissue.
Keywords: Muscular dystrophy, myoblast, magnetic-activated cell sorting, complement-mediated cytotoxicity, preplating
INTRODUCTION
Duchenne muscular dystrophy (DMD) is a severe X-linked neuromuscular disease that affects approximately 1/3500 live male births, regardless of ethnicity. This condition is known to be caused by a mutation in the gene that encodes the muscle protein dystrophin.1-7 There is currently no cure for DMD, although intensive research on experimental treatments is underway. One experimental treatment is based on myoblast transfer. This approach has a number of limitations including a low rate of spread, poor survival of injected myoblasts and insufficient donor myoblasts. Furthermore, the production of myoblasts for engrafting purposes is limited by problems in myoblast isolation and purification and in the maintenance of a satellite cell state (i.e., undifferentiated cells) before transplantation.5,7-10 Human myoblasts can be isolated from muscle biopsy, but these are easily contaminated by fibroblasts or other non-myogenic cells in vitro.
Several myoblast purification procedures have been reported. Percoll density centrifugation, selective adhesion and chemical use of fibroblast growth inhibitors have all been used for myogenic cell separation, although the yield of myoblasts from primary culture is limited.11-13 To date, the methods used to improve yields include the incorporation of muscle-specific antibody in fluorescence-activated cell sorting and size separation-based sorting by flow cytometry.14-16 These methods, however, require special equipment and techniques.
This study attempted to purify myoblasts from primary culture by magnetic-activated cell sorting (MACS), complement-mediated cytotoxicity, preplating and combined preplating and MACS. In addition, we compared the results with untreated cells and report upon the efficiency of each of these methods in purifying primary myoblasts. Finally, we observed the differentiation of the purified myoblasts into myotubes in order to verify their functionality.
MATERIALS AND METHODS
Materials
Human skeletal muscle samples were obtained from volunteers with informed consent and following the regulations of the Ethics Committee of Yongdong Severance Hospital, Yonsei University College of Medicine. All patients received a physical examination, blood screening for possible neuromuscular disorders and provided a medical history.
Preparation of primary culture
Primary muscle cells were removed from the vastus lateralis muscles as previously described.17,18 The muscle mass was rinsed in phosphate buffered saline (PBS; GIBCO BRL, Grand Island, NY, USA) and minced into a coarse slurry using blades. Cells were enzymatically dissociated by adding 0.2% collagenase type XI (Sigma, St. Louis, MO, USA) for 1 h at 37℃ and 5% CO2. The isolated cells were then suspended in Dulbecco's modified Eagle's medium with 20% fetal bovine serum (FBS-DMEM; containing 1% gentamycin; GIBCO BRL). The cells were then plated in gelatin-coated flasks at 1 × 106 cells/mL and incubated at 37℃ and 5% CO2 until 50% confluent. The growth medium was replaced twice a week. When cells reached 50% confluence, the medium was discharged and 0.05% trypsin-0.53 mM EDTA (Life Technologies, Rockville, MD, USA) was added. The cells were harvested by centrifugation at 1100 rpm for 10 min, and either subcultured or used to evaluate the isolation methods.
Magnetic-activated cell sorting (MACS)
Primary culture cells were rinsed with PBS after detaching and centrifuged at 1100 rpm for 10 min. Pelleted cells were resuspended in PBS with a 1:100 dilution of the myoblast-specific monoclonal antibody, 5.1H11 (Developmental Studies Hybridoma Bank, Iowa University, Iowa City, IA, USA). The cell-antibody complex was incubated at room temperature for 30 min and rinsed twice with PBS. This step was followed by a 15 min incubation at 6-12℃ with a 1:5 dilution of goat antimouse IgG microbeads (Miltenyl Biotec GmbH, Bergisch Gladbach, Germany). The mixture was subjected to a magnetic field, and myoblasts adhered to IgG microbeads were separated using a MiniMACS separation system (Miltenyl Biotec GmbH).
Complement-mediated cytotoxicity
The primary culture cells were detached from the flask with 0.05% trypsin-0.53 mM EDTA, as described above. The cells were reacted with 1:800 IgM monoclonal antibody 1B10 (Sigma) at 37℃ and 5% CO2 for 50 min. The cells were then rinsed with PBS and incubated at 37℃ and 5% CO2 in 12.5% young rabbit serum (Sigma) and 20% FBS-DMEM for 45 min.
Preplating
The preplating technique was performed using previously described protocols with slight modification.17,18 Briefly, the primary culture cells were detached and then preplated in a gelatin-coated flask. After 5-10 min of incubation at 37℃ and 5% CO2, the supernatant was withdrawn from the flask, and cells were replated in a fresh gelatin-coated flask. Because of different adherence rates, mainly fibroblasts had adhered to the new flask at 50% total adherence.17-19 Serial replatings of the fibroblast-depleted supernatant were then performed in gelatin-coated flasks. After 5 serial platings, the culture was enriched with small, round myogenic cells (pp5). Preplate 1 (pp1) was the population of myoblasts that adhered in the first 10 min, and were incubated for 3-4 days until 70% confluent.
Immunohistochemistry for desmin
Myoblasts were determined by desmin staining. Cultured cells mounted on saline-coated slides were air-dried at room temperature for 10 minutes. Endogenous peroxidase activity was blocked by incubating the cells on slides in 3% H2O2 for 10 minutes and rinsing with tris-buffered saline (TBS; DAKO Co., Carpinteria, CA, USA). The cells were then incubated for one hour at room temperature with monoclonal mouse primary antibodies against desmin (DAKO). After rinsing with TBS, the cells were incubated with biotin-labeled secondary antibodies using a labeled streptavidin-biotin (LSAB) kit (DAKO) at room temperature for 15 min. After rinsing with TBS, the cells were incubated with streptavidin peroxidase at room temperature for 15 minutes, and rinsed with TBS. The cells were developed using 3-amino-9-ethyl carbazole (AEC), rinsed with distilled water and counterstained with hematoxylin. Cell density was determined by counting the number of desmin positive cells per microscope field (×100) in 10 random fields.
Differentiation
Myoblasts treated with MACS and/or by preplating were transferred to 1% FBS-DMEM after the treatment(s) had been completed. The myoblasts were incubated at 37℃ and 5% CO2. Differentiation into myotubes was observed under a phase-contrast microscope.
Statistical analysis
Each experiment was repeated three times to reduce bias, and results expressed are the medians. The Mann-Whitney test for equal proportions was used in SPSS for Windows 10.0 (SPSS Inc. Chicago, IL, USA) to evaluate statistically significant differences (p < 0.05) between "before" and "after" treatments.
RESULTS
Magnetic-activated cell sorting (MACS)
Beads were coated with goat anti-mouse IgG which recognized 5.1H11, a human myoblast-specific antibody. Bridges of antibodies allowed target cells to attach to the magnetic beads, which were isolated using a magnetic collector device, MiniMACS. As a result, positive selection of myoblasts by MACS increased the percentage of myoblasts in mixed culture from 30.0% to 41.7% (Fig. 1).
Fig. 1.
Percentage increase of myoblasts prepared by MACS, complement-mediated cytotoxicity, preplating and combined preplating and MACS. Myoblast percentages were determined by immunochemical staining for desmin. All but complement-mediated cytotoxicity showed an increase in the myoblast fraction after treatment. Asterisks indicate statistical significance (p < 0.05).
Complement-mediated cytotoxicity
Complement-mediated cytotoxicity did not significantly increase the number of myoblasts in mixed culture (Fig. 1). Furthermore, myoblasts were lysed along with fibroblasts. It would appear that 1B10, a monoclonal anti-fibroblast antibody, cross-reacted with myoblasts.
Preplating
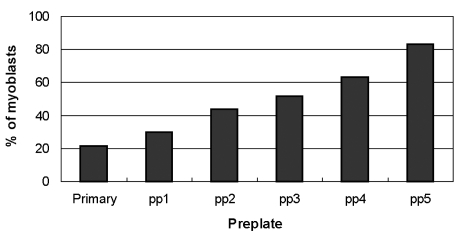
The preplate technique is based on the differential adherence characteristics of primary culture cells to a gelatin-coated surface, and it significantly enriches myoblasts. As shown in Fig. 2, the fraction of desmin positive cells increased from primary culture to pp5, in which 83.1% of cells expressed desmin, as compared to 21.3% in the primary culture. In order to obtain yet higher yields of myoblasts, preplated cells (pp5) were subjected to MACS, whereupon myoblasts increased from 76.4% to 92.8% (Fig. 1).
Fig. 2.
Characterization of the percentage of myoblasts obtained by preplating (pp1 to pp5). The myoblast populations displayed different desmin immunoreactivities ranging from the primary culture (21.3%) to the fifth preplate (pp5; 83.1%). The first preplate (pp1) contained only 29.9% desmin-positive cells, whereas the sequential preplates contained incrementally higher levels of desmin-positive cells.
Differentiation
Myoblasts treated with MACS and/or preplating fused into myotubes in five to seven days in 1% FBS-DMEM.
DISCUSSION
The present study compared three procedures for purifying myoblasts directly from freshly dissociated human skeletal muscles. Immunomagnetic cell sorting, MACS for example, is widely used for blood cells but rarely applied to tissue cell sorting. Lequerica et al.20 reported that positive selection with MACS could be used to increase the percentage of myoblasts in mixed culture from 8.4% to more than 90% without affecting viability. Therefore, we attempted to isolate myoblasts by MACS. MACS only increased the number of myoblasts in mixed culture from 30.0% to 41.7%, an 11.7% increment. This large difference in results may be due to different equipment and methods.
Preplating causes enrichment by differentiating phenotypically distinct myoblasts and fibroblasts based on desmin expression. Results from previous studies using various preplating techniques have shown myoblast enrichments ranging from 78% to more than 95% of the total cell count.17,18,21 In the present study, myoblasts, or desmin-positive cells, reached 83.1% at pp5. This was improved to 92.8% with the addition of MACS. The results demonstrate that anti-mouse immunoglobulin (Ig)-coated beads recognize 5.1H11 on the surface of myoblasts, and allow the cells to be separated when subjected to a magnetic field produced by a simple magnetic device.
Fibroblasts have a proliferate advantage over most other cell types and tend to outgrow them, even when the initial level of fibroblast contamination is low. A variety of methods to reduce fibroblast contamination have been reported. One involves complement-mediated cytotoxicity using an anti-fibroblast antibody. For example, the monoclonal antibodies 6-19 and 1B10 fixed complement have been used to remove fibroblasts from epithelial cell culture.22,23 This study used antibody 1B10 to reduce or eliminate fibroblasts in human skeletal muscle primary culture. 1B10 is specific to fibroblasts and smooth muscle differentiated fibroblasts within the context of vascular smooth muscle cells.24 Our findings reveal that the removal of fibroblasts using 1B10 and its complement was inefficient because myoblasts were also destroyed. This suggests that both cell types express the antigen to which 1B10 binds, and thus, myoblasts and fibroblasts may be of a similar lineage. These results also indicate that the anti-fibroblast antibody 1B10 is not useful for determining fibroblast levels in muscle tissue.
Myoblasts treated with MACS, preplating or both methods differentiated into myotubes, demonstrating that these isolation methods do not affect myoblast differentiation.
There are some limitations to our experiment. Because purifying procedures were not processed from the same primary cell cultures, the myoblast fractions before treatment were not equal (Fig. 1). This is because we were not able to grow enough primary culture cells for all the treatments from one sample.
In conclusion, preplating in combination with immunomagnetic purification is easy, efficient and does not require special equipment. Neither immunomagnetic purification nor preplating altered myoblast function in terms of differentiation into myotubes. We hope that the information obtained from these experiments will be useful for designing strategies to maximize myoblast purification.
ACKNOWLEDGEMENTS
We thank Stuart Hodgett, Ph.D. (University of Western Australia) for assistance with preplating. The monoclonal antibody 5.H11, developed by Blau HM and Walsh FS, was obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD (National Institute of Child Health & Human Development) and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.
Footnotes
This work was supported by a Korea Research Foundation Grant (KRF-2002-003-E00129).
References
- 1.Arahata K, Ishiura S, Ishiguro T, Tsukahara T, Suhara Y, Eguchi C, et al. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature. 1988;333:861–863. doi: 10.1038/333861a0. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman EP, Brown J, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 3.Huard J, Labrecque C, Dansereau G, Robitaille L, Tremblay JP, Labrecque C, et al. Dystrophin expression in myotubes formed by the fusion of normal and dystrophic myoblasts. Muscle Nerve. 1991;14:178–182. doi: 10.1002/mus.880140213. [DOI] [PubMed] [Google Scholar]
- 4.Monaco AP, Neve RL, Colletti-Feener C, Bertlson CJ, Kurnit DM, Kunkel LM. Isolation of candidate cDNAs for portion of the Duchenne muscular dystrophy gene. Nature. 1986;323:646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- 5.Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibers from dystrophin negative to positive by injection of normal myoblasts. Nature. 1989;337:176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- 6.Thomas MA, Fast A, Bach JR. Rehabilitation of the patient with disease of the motor unit. In: DeLisa JA, Gans BM, editors. Rehabilitation medicine: principles and practice. Philadelphia: Lippincott Company; 1993. pp. 1564–1568. [Google Scholar]
- 7.Zubrzycka-Gaarn EE, Bulman DE, Karpati G, Burghes AH, Belfall B, Klamut HJ, et al. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988;333:466–469. doi: 10.1038/333466a0. [DOI] [PubMed] [Google Scholar]
- 8.Morgan JE, Hoffman EP, Partridge TA. Normal myogenic cells from newborn mice restore normal histology to degenerating muscle of the mdx mouse. J Cell Biol. 1990;111:2437–2449. doi: 10.1083/jcb.111.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watt DJ, Lambert K, Morgan JE, Partridge TA, Sloper JC. Incorporation of donor muscle precursor cells into an area of muscle regeneration in the host mouse. J Neurol Sci. 1982;57:319–331. doi: 10.1016/0022-510x(82)90038-7. [DOI] [PubMed] [Google Scholar]
- 10.Watt DJ, Morgan JE, Partridge TA. Use of mononuclear precursor cells to insert allogeneic genes into growing mouse muscles. Muscle Nerve. 1984;7:741–750. doi: 10.1002/mus.880070908. [DOI] [PubMed] [Google Scholar]
- 11.Chiu RC, Zibaitis A, Kao RL. Cellular cardiomyoplasty: myocardial regeneration with satellite cell implantation. Ann Thorac Surg. 1995;60:12–18. [PubMed] [Google Scholar]
- 12.Kao WW, Prockop DJ. Proline analogue removes fibroblasts from cultured mixed cell population. Nature. 1977;266:63–64. doi: 10.1038/266063a0. [DOI] [PubMed] [Google Scholar]
- 13.Yablonka-Reuveni Z, Nameroff M. Skeletal muscle cell populations. Separation and partial characterization of fibroblast-like cells from embryonic tissue using density centrifugation. Histochemistry. 1987;87:27–38. doi: 10.1007/BF00518721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baroffio A, Aubry JP, Kaelin A, Krause RM, Hamann M, Bader CR. Purification of human muscle satellite cells by flow cytometry. Muscle Nerve. 1993;16:498–505. doi: 10.1002/mus.880160511. [DOI] [PubMed] [Google Scholar]
- 15.Blanton JR, Jr, Grant AL, McFarland DC, Robinson JP, Bidwell CA. Isolation of two populations of myoblasts from porcine skeletal muscle. Muscle Nerve. 1999;22:43–50. doi: 10.1002/(sici)1097-4598(199901)22:1<43::aid-mus8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 16.Webster C, Pavlath GK, Parks DR, Walsh FS, Blau HM. Isolation of human myoblasts with the fluorescence-activated cell sorter. Exp Cell Res. 1988;174:252–256. doi: 10.1016/0014-4827(88)90159-0. [DOI] [PubMed] [Google Scholar]
- 17.Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998;142:1257–1267. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qu Z, Huard J. Matching host muscle and donor myoblasts for myosin heavy chain improves myoblast transfer therapy. Gene Therapy. 2000;58:428–437. doi: 10.1038/sj.gt.3301103. [DOI] [PubMed] [Google Scholar]
- 20.Lequerica JL, Mirabet V, Montero JA, Hurtado C, Piquer S, Carbonell F. In vitro proliferation, differentiation and immuno-magnetic bead purification of human myoblasts. Ann Transplant. 1999;4:103–196. [PubMed] [Google Scholar]
- 21.Jankowski RJ, Haluszczak C, Trucco M, Huard J. Flow cytometric characterization of myogenic cell populations obtained via the preplate technique: potential for rapid isolation of muscle-derived stem cells. Hum Gene Ther. 2001;12:619–628. doi: 10.1089/104303401300057306. [DOI] [PubMed] [Google Scholar]
- 22.Abboud CN, Duerst RE, Farntz CN, Ryan DH, Liesveld JL, Brennan JK. Lysis of human fibroblast colony-forming cells and endothelial cell by monoclonal antibody (6-9) and complement. Blood. 1986;68:1196–1200. [PubMed] [Google Scholar]
- 23.Singer KH, Scearce RM, Tuck DT, Whichard LP, Denning SM, Haynes BF. Removal of fibroblasts from human epithelial cell cultures with use of a complement fixing monoclonal antibody reactive with human fibroblasts and monocytes/macrophages. J Invest Dermatol. 1989;92:166–170. doi: 10.1111/1523-1747.ep12276685. [DOI] [PubMed] [Google Scholar]
- 24.Simonart T, Degraef C, Heenen M, Hermans P, Van Vooren JP, Noel JC. Expression of the fibroblast/macrophage marker 1B10 by spindle cells in Kaposi's sarcoma lesions and by Kaposi's sarcoma-derived tumor cells. J Cutan Pathol. 2002;29:72–78. doi: 10.1034/j.1600-0560.2002.290202.x. [DOI] [PubMed] [Google Scholar]