Abstract
The objective of this study was to investigate the correlation between factor XIII (FXIII) activity and disseminated intravascular coagulation (DIC) parameters and also to evaluate the clinical usefulness of DIC diagnosis. Citrated plasma from eighty patients with potential DIC was analyzed for FXIII activity. The primary patient conditions (48 male and 32 female, mean age, 51 years) were malignancy (n = 29), infection (n = 25), inflammation (n = 6), heart disease (n = 3), thrombosis (n = 2), injury (n = 2), and other miscellaneous conditions (n = 13). FXIII testing was performed using the CoaLinkTM FXIII Incorporation Assay Kit (PeopleBio Inc.). Among 80 patients who were suspected to have DIC based on clinical analysis, 46 (57.5%) fulfilled the overt DIC criteria (DIC score > = 5) according to the International Society of Thrombosis and Haemostasis. FXIII levels in the plasma were significantly decreased in overt DIC compared to non-overt DIC patients (mean 75.1% and 199.7% respectively, p < 0.0001). Interestingly, we found a significant inverse correlation between DIC scores and FXIII activity. In addition, FXIII activity significantly correlated with other hemostatic markers that included platelet count, prothrombin time, activated partial thromboplastin time, fibrinogen, and D-dimer. FXIII levels were significantly lower in patients with liver or renal dysfunction. In conclusion, FXIII cross-linking activity measurements may have differential diagnostic value as well as predictive value in patients who are suspected to have DIC.
Keywords: Disseminated intravascular coagulation, factor XIII, hemostasis
INTRODUCTION
Factor XIII (FXIII) is a plasma protein that plays an important role in the final stages of the clotting cascade and the regulation of fibrinolysis.1,2 FXIII deficiency, caused by dyspoiesis or increased consumption, results in bleeding tendencies and wound healing complications.3 Postoperative hemorrhage in patients with congenital and acquired FXIII deficiency has been described in various surgical fields.4 Low activity levels were found in patients with various conditions such as sepsis, multiple fractures, and disseminated intravascular coagulation (DIC).5 However, little data has been generated on the clinical relevance of FXIII activity in the differential diagnosis of non-overt and overt DIC. The objective of this study was to investigate the correlation between FXIII activity and DIC coagulation parameters, and to evaluate the clinical usefulness of DIC diagnosis.
MATERIALS AND METHODS
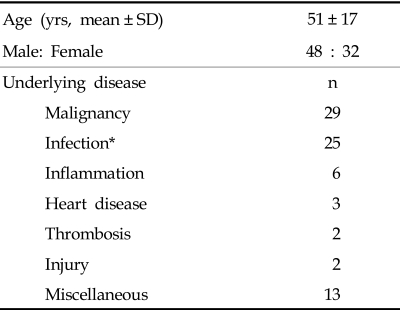
The citrated plasma from 80 patients with potential DIC were analyzed for FXIII activity. The primary patient conditions (48 male and 32 female, mean age, 51 years) were malignancy (n = 29), infection (n = 25), inflammation (n = 6), heart disease (n = 3), thrombosis (n = 2), injury (n = 2), and other miscellaneous conditions (n=13) (Table 1). FXIII testing was performed using the CoaLink™ FXIII Incorporation Assay Kit (PeopleBio Inc., NY, USA) according to the manufacturer's instructions. The assay kit is designed to quantitate the true functional FXIII and FXIIIa activity in plasma by measuring transglutaminase activity. In this assay, the thrombin-activated FXIIIa from the patient plasma binds to a substrate coated plate. Next, a horse radish peroxidase (HRP)-conjugated FXIII is cross-linked to the captured FXIIIa. The cross-linked HRP is then detected with a HRP chromogenic substrate at 450 nm. The data was analyzed to evaluate the correlation between FXIII activity and other DIC markers which included platelet count, fibrinogen activities, prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrin degradation product (D-dimer). PT, APTT, fibrinogen, and D-dimer results were generated with an automated clotting analyzer (STA-R) using reagents from Diagnostica Stago (Asnieres, France). Comparisons of mean group values were performed using a Student's t-test. The Pearson correlation coefficients (r) were calculated to evaluate the relationship of FXIII with other hemostatic markers. A value of p < 0.05 was considered to be statistically significant.
Table 1.
Characteristics of the Study Population
*29 cases, including 4 secondary infections.
SD, standard deviation.
RESULTS
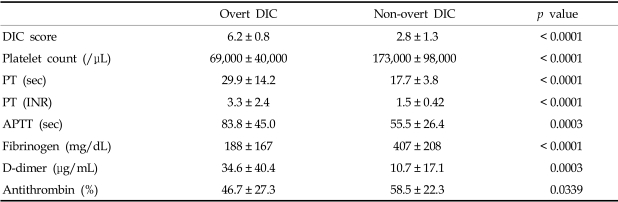
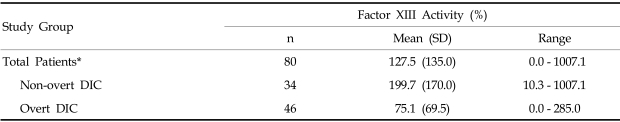
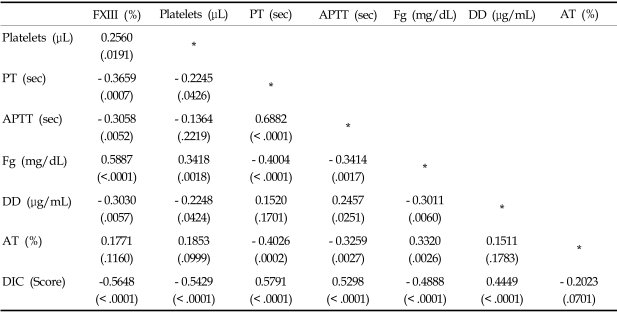
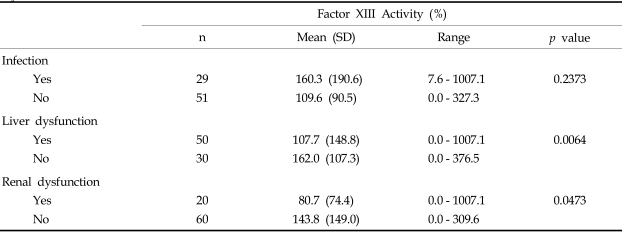
Among the 80 patients who, upon clinical examination, potentially had DIC, 46 (57.5%) fulfilled the overt DIC criteria (DIC score > = 5) according to the ISTH (International Society of Thrombosis and Haemostasis) scoring system.6 Patient characteristics at the time of study entry are shown in Table 2. The plasma levels of FXIII was significantly decreased in overt DIC patients (mean, 75.1%) compared to non-overt DIC patients (mean, 199.7%) (p < 0.0001, Table 3). DIC scores were inversely correlated to FXIII activity (p < 0.0001, Table 4). In addition, FXIII activity was significantly correlated to other hemostatic markers including platelet count (r = 0.2560, p = 0.0191), PT (r = - 0.3659, p = 0.0007), APTT (r = - 0.3058, p = 0.0052), fibrinogen (r = 0.5887, p < 0.0001), and D-dimer (r = - 0.3030, p = 0.0057). In contrast, FXIII activity did not significantly correlate with antithrombin levels. The FXIII levels were significantly lower in patients with liver or renal dysfunction than those without organ dysfunction (p = 0.0064, p = 0.0473, respectively). However, no significant difference in FXIII levels was detected when we compared patients with infections to patients without infections (Table 5).
Table 2.
Hemostatic Markers in Patients with Overt and Non-overt DIC
DIC, disseminated intravascular coagulation; PT, prothrombin time; APTT, activated partial thromboplastin time.
Table 3.
Plasma Factor XIII activity in Patients with Disseminated Intravascular Coagulation (DIC)
Non-overt DIC vs. Overt DIC*, p value < 0.0001.
Table 4.
Correlations (r) between Factor XIII Activity and DIC Coagulation Parameters (p value)
Table 5.
Percentages of Plasma Factor XIII Activity in Patients with Infection, Liver Dysfunction, or Renal Dysfunction
PT, prothrombin time; APTT, activated partial thromboplastin time; Fg, fibrinogen. DD, D-dimer; AT, antithrombin; DIC, disseminated intravascular coagulation.
DISCUSSION
In this study, FXIII plasma activity is compared with other hemostatic markers in a group of patients with a clinical potential for DIC. Of the 80 patients in this study, 46 (57.5%) patients were diagnosed with overt DIC as determined by a DIC score > = 5, according to the ISTH scoring system. FXIII activity was highly variable, but decreased significantly in overt DIC patients compared with non-overt DIC patients. We detected a significant correlation between FXIII activity and global coagulation tests, such as platelet counts, PT, APTT, and fibrinogen. Interestingly, these tests are also important for the diagnosis of DIC. These results suggest that measuring FXIII activity may be helpful in diagnosing overt DIC.
FXIII activity was significantly correlated to DIC score, suggesting that the measurement of FXIII activity could be a clinically useful prognostic marker for DIC. FXIII circulates as an intact zymogen composed of a tetramer of paired A- and B- subunits and becomes enzymatically active after thrombin or factor Xa activation. The peptide responsible for enzymatic activation is located in the A subunit.7 In this study, we used the true functional assay of transglutaminase activity to determine the levels of FXIII incorporation.
Lower FXIII activity in overt-DIC patients compared to non-overt DIC patients in this study may reflect a decreased number of A subunits as the result of a state of consumptive coagulopathy. In DIC cases which improved after treatment, the ratio of A to B subunits has been reported to be between 0.5 and 1.0. These ratios were less than 0.5 in cases of DIC that deteriorated in spite of treatment.8 A functional ELISA-based FXIII incorporation assay may be a good alternative to using two separate ELISA systems to measure FXIII A and B subunit ratios. In addition, this functional assay may have prognostic value as a predictive marker for DIC.
DIC occurs frequently as a complication of severe sepsis and other conditions,9 contributes to the pathogenesis of multiple organ dysfunctions, and is an independent predictor of mortality.10 Recent advances in our understanding of DIC pathogenic mechanisms have resulted in novel therapeutic approaches, such as the administration of activated human protein C in order to restore physiologic pathways.11 In this study, FXIII is associated with renal dysfunction and may have clinical implications in the treatment of patients with both DIC and organ dysfunction. Hypothetically, a combination of overt DIC and low FXIII activity may provide one of the new therapeutic strategies aimed at reducing mortality or the sepsis-related organ failure assessment (SOFA) score. There are several reports documenting the success of using FXIII in conjunction with antithrombin concentrate or antiplasmin drugs to treat patients with life-threatening DIC and decreased FXIII activity.12,13
In this study, FXIII activity was found to be associated with liver dysfunction. Because FXIII is synthesized in the liver, impaired synthesis could lead to acquired FXIII deficiency in acute or chronic liver failure, as previously described.14 The mean FXIII activity (107.7%) in patients with liver dysfunction was only slightly decreased compared to healthy subjects (121.3%, ranging from 40.5% to 196%, SD, 28.9, n = 100). This finding supports that FXIII is synthesized not only in the liver but also in other cells, including platelets and monocytes.15,16 These cell-associated FXIII molecules could also be one of the regulating factors of plasma FXIII activity and the cross-linking of fibrin that is associated with DIC pathogenesis.
In conclusion, we found a significant difference in plasma FXIII activity between patients with overt DIC and non-overt DIC. The measurement of FXIII cross-linking activity may have differential diagnostic value as well as predictive value in patients suspected to have DIC. FXIII concentrate could be used beneficially as an adjunct in therapeutic intervention.
ACKNOWLEDGEMENT
We are grateful to PeopleBio Inc., for their supply of the FXIII assay kit used in our study.
Footnotes
This study was supported in 2004 by the Brain Korea 21 Project for Medical Science, Yonsei University.
References
- 1.Carell NA, Erickson HP, McDonagh J. Electron microscopy and hydrodynamic properties of factor XIII subunits. J Biol Chem. 1989;264:551–556. [PubMed] [Google Scholar]
- 2.Greenberg CS, Birckbichler PJ, Rice RH. Transglutaminases: multifunctional cross-linking enzymes that stabilize tissues. FASEB J. 1991;5:3071–3077. doi: 10.1096/fasebj.5.15.1683845. [DOI] [PubMed] [Google Scholar]
- 3.Heinle K, Adam O, Rauh G. Factor XIII insufficiency in a patient with severe psoriasis vulgaris, arthritis, and infirmity. Clin Rheumatol. 1998;17:346–348. doi: 10.1007/BF01451020. [DOI] [PubMed] [Google Scholar]
- 4.Gerlach R, Raabe A, Zimmermann M, Siegemund A, Seifert V. Factor XIII deficiency and postoperative hemorrhage after neurosurgical procedures. Surg Neurol. 2000;54:260–266. doi: 10.1016/s0090-3019(00)00308-6. [DOI] [PubMed] [Google Scholar]
- 5.Egbring R, Kroniger A, Seitz R. Factor XIII deficiency: pathogenic mechanisms and clinical significance. Semin Thromb Hemost. 1996;22:419–425. doi: 10.1055/s-2007-999041. [DOI] [PubMed] [Google Scholar]
- 6.Taylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–1330. [PubMed] [Google Scholar]
- 7.Ichinose A, Davie EW. Characterization of the gene for a subunit of human factor XIII (plasma transglutaminase), a blood coagulation factor. Proc Natl Acad Sci USA. 1988;85:5829–5833. doi: 10.1073/pnas.85.16.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugimura D, Fukue H, Arai M, Matsumoto K, Fukutake K. Changes of factor XIII a and b subunit in patients with disseminated intravascular coagulation syndrome. Rinsho Byori. 1996;44:355–361. [PubMed] [Google Scholar]
- 9.Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586–592. doi: 10.1056/NEJM199908193410807. [DOI] [PubMed] [Google Scholar]
- 10.Levi M, ten Cate H, van der Poll T, van Deventer SJ. Pathogenesis of disseminated intravascular coagulation in sepsis. JAMA. 1993;270:975–979. [PubMed] [Google Scholar]
- 11.Heslet L. Cinical implications of a validated scoring system for disseminated intravascular coagulation. Crit Care Med. 2004;32:2548–2549. doi: 10.1097/01.ccm.0000148088.91570.07. [DOI] [PubMed] [Google Scholar]
- 12.Delshammar M, Lasson A, Nilsson IM, Ohlsson K, Wallmark A, Vernersson E. Abnormal proteolysis (DIC)-Successful treatment with antithrombin III concentrate and a concentrate containing FXIII and native von Willebrand factor. J Intern Med. 1989;225:21–27. doi: 10.1111/j.1365-2796.1989.tb00031.x. [DOI] [PubMed] [Google Scholar]
- 13.Ryo R, Sugano W, Yoshida A, Adachi M, Yasunaga M, Yoneda N, et al. Antiplasmin drugs and factor XIII concentrates in the treatment of a patient with acute promyelocytic leukemia(M3) Gan No Rinsho. 1989;35:1479–1482. [PubMed] [Google Scholar]
- 14.Biland L, Duckert F, Prisender S, Nyman D. Quantitative estimation of coagulation factors in liver disease. The diagnostic and prognostic value of factor XIII, factor V and plasminogen. Thromb Haemost. 1978;39:646–656. [PubMed] [Google Scholar]
- 15.Ballerini G, Guerra S, Rodeghiero F, Castaman G. A contribution to the pathology of acquired plasma factor XIII deficiency. Semin Thromb Hemost. 1985;11:357–361. doi: 10.1055/s-2007-1004394. [DOI] [PubMed] [Google Scholar]
- 16.Francis CW, Marder VJ. Rapid formation of large molecular weight alpha-polymers in cross-linked fibrin induced by high factor XIII concentrations. Role of platelet factor XIII. J Clin Invest. 1987;80:1459–1465. doi: 10.1172/JCI113226. [DOI] [PMC free article] [PubMed] [Google Scholar]