Abstract
We characterized and compared the characteristics of Ca2+ movements through the sarcoplasmic reticulum of inferior oblique muscles in the various conditions including primary inferior oblique overaction (IOOA), secondary IOOA, and controls, so as to further understand the pathogenesis of primary IOOA. Of 15 specimens obtained through inferior oblique myectomy, six were from primary IOOA, 6 from secondary IOOA, and the remaining 3 were controls from enucleated eyes. Ryanodine binding assays were performed, and Ca2+ uptake rates, calsequestrins and SERCA levels were determined. Ryanodine bindings and sarcoplasmic reticulum Ca2+ uptake rates were significantly decreased in primary IOOA (p<0.05). Western blot analysis conducted to quantify calsequestrins and SERCA, found no significant difference between primary IOOA, secondary IOOA, and the controls. Increased intracellular Ca2+ concentration due to reduced sarcoplasmic reticulum Ca2+ uptake may play a role in primary IOOA.
Keywords: Calcium, calsequestrin, inferior oblique overaction, ryanodine receptor, sarcoplasmic reticulum, sarcoplasmic reticulum Ca2+-ATPase 1
INTRODUCTION
Inferior oblique overaction (IOOA), defined as an elevation of an eye as it moves toward adduction, is a common oculomotor disease.1 From the clinical point of view, it has been customary to distinguish between primary and secondary overactions of this muscle. Secondary overactions differ in as much that the vertical deviation is large in the primary position and is accompanied by a torsional deviation and the Bielschowsky head tilt test is positive. Although the clinical differentiation of primary and secondary overaction is not difficult, the explanations given for apparent primary overaction in the older literature are vague. Secondary overaction is caused by paresis or paralysis of the ipsilateral superior oblique muscle or by paresis or paralysis of the contralateral superior rectus muscle. However, primary overaction, in which there is no evidence for past or present ipsilateral superior oblique muscle paralysis or paresis, is difficult to explain. A number of studies have been undertaken to elucidate the pathogenesis of primary IOOA.1-5 However, none of the resulting explanations are convincing, and it remains doubtful as to whether a true primary overaction exists at all from an innervational perspective.
Ca2+ ions trigger muscle contraction and the release of Ca2+ from the sarcoplasmic reticulum (the intracellular Ca2+ storage site) plays an important role in determining the intramuscular Ca2+ concentration. Ca2+ binds with the protein participating in muscle contraction to induce muscle contraction and is then reabsorbed into the sarcoplasmic reticulum via Ca2+ ATPase. In addition, muscle relaxation is possible when Ca2+ is reabsorbed by the sarcoplasmic reticulum and during this process Ca2+-ATPase (the Ca2+ pump) plays a key role. This process of Ca2+ uptake by the sarcoplasmic reticulum via Ca2+-ATPase reduces intramuscular Ca2+ concentrations and results in muscle relaxation.6,7
Several regulatory proteins present in the sarcoplasmic reticulum plays fundamental roles in regulating the intramuscular concentration of Ca2+. These include ryanodine receptor, a Ca2+ release channel which participates in Ca2+ release from sarcoplasmic reticulum into muscle cells, Ca2+-ATPase, which drives Ca2+ uptake, and calsequestrin that binds with Ca2+.8-11 Thus, since Ca2+ uptake by the sarcoplasmic reticulum and Ca2+ release from the sarcoplasmic reticulum play key roles in the regulation of Ca2+ concentrations in muscle cells, the above proteins are important elements in studies on the mechanisms of muscle contraction and relaxation. Furthermore, changes in these proteins may be of key importance in many muscular diseases. Reported studies on diseases associated with the dysfunction of the ryanodine receptor mainly concern with heart disease, although studies concerning its involvement with skeletal muscle in malignant hyperthermia, myasthenia gravis, and muscular dystrophy have been conducted. However, little is known about its relation with extraocular muscles.12-21
In the present study, Ca2+ release, uptake, and storage, were examined in inferior oblique muscles with primary or secondary overaction, and compared with those of the normal inferior oblique muscles, particularly with respect to Ca2+ movements through the sarcoplasmic reticulum so as to further understand the pathogenesis of primary IOOA.
MATERIALS AND METHODS
The inferior oblique muscles of patients diagnosed as having primary or secondary IOOA were obtained through a weakening procedure (myectomy) under general anesthesia. IOOA was graded according to the amount of eye over-elevation in adduction versus the position of the fellow eye: 1+ represented an over-elevation of 1mm, 2+ 2 mm, 3+ 3 mm, and 4+ 4 mm. Patients with an IOOA 3+ or 4+ underwent inferior oblique weakening surgery for functional or cosmetic reasons. Those who had a history of other ocular disease or who had undergone previous ocular surgery were excluded. Three patients (6 eyes) had primary IOOA and 9 (10 eyes) secondary IOOA. Additional inferior oblique muscles (normal controls) were obtained from 4 patients (4 eyes) who underwent enucleation due to phthisis bulbi. Patients' ages ranged from 3 to 71 years and there were 10 males and 6 females. All surgical procedures were performed by one of the authors (JBL), and each muscle specimen was obtained at the same location from the muscle insertion with minimal manipulation. Although procedures were performed in all 16 patients, muscle homogenation was possible in 12 patients. Muscles were frozen in liquid nitrogen immediately after the procedure, and muscle tissues were homogenized in a cold room at -4℃.
Preparation of whole homogenates
After removing blood and connective tissues from the inferior oblique muscles in 0.9% NaCl solution, the muscle specimens were dried with filter paper and weighed. About 50 mg of inferior oblique muscle tissue per ml of homogenate buffer was placed in solution (20 mM morpholinopropanesulphonic acid, 1 M KCl, leupeptin 1 µM, pepstatin 1 µM, aprotinine 1 µM, PMSF 0.1 mM, trypsin inhibitor 10 µM/ml, sucrose 0.3 M), and the mixture was homogenated for 3 × 30 sec using a polytron PT probe (Brinkmann Instruments Co, Westburg, NY, USA). Total protein was assayed by the Bradford method.22
Characteristics of ryanodine receptor in primary and secondary IOOA
Whole homogenate (2.5 mg) was placed in buffer solution [1 M KCl, 20 mM morpholinopropanesulphonic acid (pH 7.4), 20 nM [3H]ryanodine (54.7 Ci/mmol, New England Nuclear Co, Billerica, MA, USA), 1 mM EGTA] at 37℃ for 2 hours to determine ryanodine binding value (total binding value). After blocking ryanodine receptor in the sarcoplasmic reticulum, the same amounts of whole homogenate and non-labeled ryanodine were placed into the buffer solution to obtain the 'non-specific binding value'. The following reactions were performed upon the two ryanodine containing homogenates mentioned above. 100 µl of PEG solution [30% PEG, 1 mM EDTA Tris (pH 7.4)] was added to homogenate and left at room temperature for 5 min. The mixture was then centrifuged for 15 min at 14,000 rpm to precipitate sarcoplasmic reticulum, and the supernatant was washed with 0.4 ml of ryanodine binding buffer solution. Soluen 350 (100 µl) was then added to the precipitate to dissolve the lipid layer from the sarcoplasmic reticulum. The mixture was left at 70℃ for 15 min and moved to a scintillation vial to measure radioactivity after adding 4 ml of picofluor.
Uptake of oxalate-supported Ca2+ by sarcoplasmic reticulum
In order to load Ca2+ into the sarcoplasmic reticulum, the reaction mixture [KCl 100 mM, morpholinopropanesulphonic acid (pH 6.8) 20 mM, NaN3 10 mM,] was added to 300 µg of whole homogenate and reacted for 4 min at 37℃. 5 mM of MgATP, 10 mM potassium oxalate, and 0.2 mM 45CaCl2 0.2 mM (104 cpm/nmol; Amersham, Arlington Height, IL, USA) were then added and the whole was filtered through a Minipore filter (0.45 µm pore size) for 2, 4, or 7 min. The filtrants were placed into scintillation vials to measure radioactivity after adding 4 ml of picofluor and left for 15 min.
Western blot analysis of calsequestrin and sarcoendoplasmic reticulum Ca2+-ATPase 1 (SERCA1)
Calsequestrin is a 65 KDa protein which stores Ca2+ in the sarcoplasmic reticulum. SERCA 1 is a 120 KDa enzyme which is related with fast muscle. After quantifying the amount of protein by Bradford method, 50 µg of protein was mixed with sample buffer and boiled at 95℃ for 5 min. Proteins were separated by electrophoresis, and blotted onto nitrocellulose paper using buffer solution. The paper was then placed in a blocking solution containing phosphate buffered saline (PBS) containing 5% bovine serum albumin and 0.1% Tween 20 for an hour at room temperature. Primary antibody (mouse monoclonal anti-human antibody; Department of Bioscience, Kwangju Science and Research Institute, Kwangju, Korea) for calsequestrin and primary antibody (mouse monoclonal anti-human antibody; Transduction Laboratories, Lexington, KY, USA) for SERCA1 were reacted for 3 hours at room temperature. The proteins were washed with PBS containing 0.1% Tween 20 3 times, and reacted for 90 minutes at room temperature with secondary antibody (alkaline phosphatase-conjugated goat anti-mouse IgG; Santa Cruz, CA, USA). Protein bands were detected by enhanced chemiluminescence (Amersham Life Science, Little Chalfont, England, UK).
Statistical analysis
The above procedure was repeated eight times. All measured values are expressed as means ± standard deviations. Comparisons between the three groups were performed using the Kruscal-Wallis test and comparison between two groups using the Wilcoxon rank sum test; p values of <0.05 were considered statistically significant.
RESULTS
Ryanodine binding measurement
Ryanodine receptor binding was measured in the whole homogenate of each inferior oblique muscle tissue. The amount of ryanodine receptor binding per unit of protein was the greatest for the control group, followed by the secondary IOOA group and then the primary IOOA group. Specific binding between ryanodine receptor and ryanodine in the primary IOOA group was significantly lower than that in the control group, suggesting that the activity of the ryanodine receptor in the primary IOOA group was lower than that in the control group (Table 1). However, no significant difference in receptor binding properties was observed between the other two group combinations (p>0.05).
Table 1.
Concentration of the Ryanodine Receptor in the Sarcoplasmic Reticulum of Inferior Oblique Muscles
Values are means ± standard deviations.
IOOA, inferior oblique overaction.
*Statistically significant difference when compared with the control (p<0.05).
Uptake of Ca2+ by the sarcoplasmic reticulum
The rate of Ca2+-uptake by the sarcoplasmic reticulum was the fastest for the control group, followed by the secondary IOOA group, and then the primary IOOA group. The difference between all three groups was statistically significant (p<0.05). More specifically, Ca2+ reabsorbed into sarcoplasmic reticulum was fastest for the control group and slowest for the primary IOOA group (Table 2), and all intergroup differences were significant.
Table 2.
The Rate of 45Ca2+ Uptake by the Sarcoplasmic Reticulum of Inferior Oblique Muscles
Values are means ± standard deviations.
IOOA, inferior oblique overaction.
*Ca2+ uptake rates (nmole/mg protein/min) were significantly different in the three groups (p<0.05).
Western blot analysis for calsequestrin and SERCA1
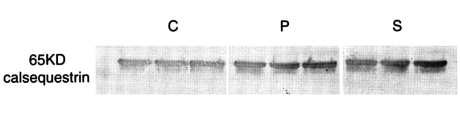
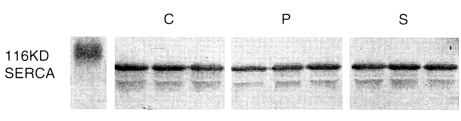
Differences between the Ca2+ storage capacities of the sarcoplasmic reticulum in the oblique muscles of each of the three groups were estimated by determining the amount of calsequetrin. No significant inter-group differences were observed in terms of the amount of calsequestrin (Fig. 1). In order to determine whether a lower Ca2+ uptake was due to the down-regulation of SERCA1 (Ca2+ pump), western blot analysis was performed. However, no significant difference was observed between the three groups with respect to SERCA1 expression (Fig. 2).
Fig. 1.
The expression of calsequestrin in inferior oblique muscles. C, P, and S represent inferior oblique muscles from the controls, and primary and secondary inferior oblique overaction cases, respectively. These three groups were similar with respect to calsequestrin expression.
Fig. 2.
The expressions of sarco-endoplasmic reticulum Ca2+-ATPase 1 in inferior oblique muscles. C, P, and S represent inferior oblique muscles from the controls, and primary and secondary inferior oblique overaction cases, respectively. No significant difference was found between these three groups in terms of SERCA1 expression.
DISCUSSION
A number of hypotheses have been proposed concerning the pathogenesis of primary IOOA. Bielschowsky, for instance, suggested that eyes are elevated in adduction due to an abnormal fascial structure of the inferior oblique muscle.1 Guibor suggested that IOOA could be caused by a synkinesis of inferior oblique muscle with the ipsilateral medial rectus muscle owing to an impulse spread within the central nervous system.4 Spencer and McNeer reported that an electron microscopic structure of the inferior oblique muscle showed no difference between primary and secondary IOOA, and concluded primary IOOA might be due to incomplete palsy of the ipsilateral superior oblique muscle.5 The discovery of muscle pulleys has directed our attention to other etiologic possibilities for this apparent overaction.3 The distribution of the isoforms of lactate dehydrogenase in children with IOOA was different from that in the control group.2
In the present study, we evaluated the functional affinity of the ryanodine receptor by attaching the [3H] to ryanodine. Moreover, because muscle contractility in primary IOOA is presumed to be larger than that of normal controls, intramuscular Ca2+ concentrations in primary IOOA are expected to be higher than normal, which suggests Ca2+ release by the sarcoplasmic reticulum is enhenced. However, unexpectedly, ryanodine receptor binding affinity, which resulted in Ca2+-release, was significantly low in the primary IOOA compared with that in the controls. Thus, we postulated that, in primary IOOA, the Ca2+ storage within the sarcoplasmic reticulum is reduced due to Ca2+-ATPase inactivation, and that consequently Ca2+-release capacity is reduced. As a result, the amount or the function of ryanodine receptor was supposed to be reduced. However, other investigators have reported that the inactivation of Ca2+-ATPase is closely related with ischemic heart disease, heart failure, and hypertrophy of cardiac muscle; thus, as the intracellular Ca2+ concentration increases, the concentration of ryanodine receptor decreases, and in some cases, down regulation has been found to be associated with the decreased expression of its mRNA.23,24
By measuring Ca2+ uptake using oxalate, we were able to determine the amount of Ca2+ sequestration by the sarcoplasmic reticulum and to estimate the function of the ryanodine receptor indirectly. In the present study, the amount and rate of Ca2+ uptake was determined when the ryanodine receptor was completely blocked. Moreover, uptake rate differences between the three groups were significant. Ca2+ was reabsorbed most promptly in control group, followed by the secondary IOOA group, and the primary IOOA group. This result is consistent with our assumption that if Ca2+ is not sequestered rapidly into the sarcoplasmic reticulum, but remains in the cytoplasm, that the intracellular Ca2+ concentration would be highest in primary IOOA muscle cells. In secondary IOOA, the rate of Ca2+ uptake was slower than that of the control group, which suggests that this is a transitional phenomenon, which results from the relative overaction of the inferior oblique muscle due to paralysis of its antagonist, the superior oblique muscle.
Ca2+ pump activity is known to be reduced in muscular dystrophy and in Brody disease.22,25 However, little information is available on the role of Ca2+ pump in ocular disease, especially in oculomotor disease, although it was reported that Ca2+ pump activity changes in lens epithelial cells after treated with an oxidant or heat.26 In the present study, Ca2+ pump levels, which is one of the factors that impacts the Ca2+ uptake rate, were assayed by western blotting for SERCA 1, a fast twitch isoform of SERCA. However, no significant difference was found between the three groups. Thus we infer that a reduction in Ca2+ uptake in IOOA is not due to reduced Ca2+ pump levels, but rather is probably due to a functional abnormality in SERCA 1, or in phospholamban, a SERCA regulator, or alternatively to a reduced level of ATP phosphorylation. Further studies are needed to resolve this issue.
Calsequestrin is a Ca2+ storage protein that binds Ca2+ within the lumen of the sarcoplasmic reticulum. The protein is usually fixed in the junctional sarcoplasmic reticulum and located near the ryanodine receptor. Calsequestrin is negatively charged and interacts with the positively charged triadin and ryanodine receptors. Furthermore, calsequestrin has been reported to be related to Ca2+-ATPase present in the sarcoplasmic reticulum.27,28 In the present study, no significant difference was found between the three groups with respect to calsequestrin expression, which suggests that Ca2+ concentrations in the sarcoplasmic reticulum might not be different between groups. However, this does not exclude the possibility that Ca2+ storage capacity in the sarcoplasmic reticulum by calsequestrin is impaired.
In the present study, we attempted to unravel the mechanism of IOOA from a molecular perspective rather than from the anatomical or histological standpoints. Thus, we evaluated ryanodine receptor binding, Ca2+ uptake, the relation between SERCA and uptake, and calsequestrin (Ca2+ storage), in normal, primary IOOA, and secondary IOOA. Initially we hypothesized that the pathogenesis of IOOA (especially in primary IOOA) is associated with changes in intracellular Ca2+ concentration and Ca2+ movement. The observed delay in Ca2+ uptake by the sarcoplasmic reticulum in primary IOOA seems remarkable. However, this study has some limitations. First, age-matched controls are required to exclude the possibility of age-related changes in Ca2+ signaling or, may be, in tissue stability. Second, the number of sample is small. Last, there are many other proteins involved in the Ca2+ signaling and contraction in muscles that would deserve a closer analysis. Further study is needed to determine whether delayed Ca2+ uptake per se has a causal relationship on increased intracellular Ca2+ concentration. Moreover, a significant reduction in ryanodine receptor binding was also observed in primary IOOA. Thus, the simultaneous measurements of intracellular Ca2+ concentration and muscle contraction, and functional analysis of regulating protein abnormalities require further investigation.
References
- 1.Von Noorden GK, Campos EC. Binocular vision and ocular motility. 6th ed. revised. St. Louis London Philadelphia Sydney Toronto: Mosby; 2002. [Google Scholar]
- 2.Aihara T, Miyata M, Ishikawa S. The lactate dehydrogenase isoenzyme pattern in the overacting inferior oblique muscle. J Pediatr Ophthalmol Strabismus. 1978;15:43–47. doi: 10.3928/0191-3913-19780101-14. [DOI] [PubMed] [Google Scholar]
- 3.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotropic muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2:17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]
- 4.Guibor GP. Synkinetic overaction of the inferior oblique muscle. Am J Ophthalmol. 1949;32:221–229. doi: 10.1016/0002-9394(49)90137-3. [DOI] [PubMed] [Google Scholar]
- 5.Spencer RF, McNeer KW. Structural alterations in overacting inferior oblique muscles. Arch Ophthalmol. 1980;98:128–133. doi: 10.1001/archopht.1980.01020030130015. [DOI] [PubMed] [Google Scholar]
- 6.Block BA, Imagawa T, Campbell KP, Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988;107(6 Pt 2):2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dulhunty AF, Gage PW. Effect of extracellular calcium concentration and dihydropyridines on contraction in mammalian skeletal muscle. J Physiol. 1988;399:63–80. doi: 10.1113/jphysiol.1988.sp017068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986;261:6300–6306. [PubMed] [Google Scholar]
- 9.Pessah IN, Warterhouse AL, Casida JE. The calciumryanodine receptor complex of skeletal and cardiac muscle. Biochem Biophys Res Commun. 1985;128:449–456. doi: 10.1016/0006-291x(85)91699-7. [DOI] [PubMed] [Google Scholar]
- 10.Mintz E, Guillian F. Ca2+ transport by the sarcoplasmic reticulum ATPase. Biochim Biophys Acta. 1997;1318:52–70. doi: 10.1016/s0005-2728(96)00132-6. [DOI] [PubMed] [Google Scholar]
- 11.Damiani E, Margreth A. Specific protein-protein interaction of calsequestrin with junctional sarcoplasmic reticulum of skeletal muscle. Biochem Biophys Res Commun. 1990;172:1253–1259. doi: 10.1016/0006-291x(90)91584-f. [DOI] [PubMed] [Google Scholar]
- 12.Cheung WY. Calmodulin. Sci Am. 1982;246:62–70. doi: 10.1038/scientificamerican0682-62. [DOI] [PubMed] [Google Scholar]
- 13.Coronado R, Morrissette J, Sukhareva M, Vaughn DM. Structure and function of ryanodine receptors. Am J Physiol. 1994;266(6 Pt 1):C1485–C1504. doi: 10.1152/ajpcell.1994.266.6.C1485. [DOI] [PubMed] [Google Scholar]
- 14.Jenden DJ, Fairhurst AS. The pharmacology of ryanodine. Pharmacol Rev. 1969;21:1–25. [PubMed] [Google Scholar]
- 15.Jones LR, Zhang L, Sanborn K, Jorgensen AO, Kelley J. Purification, primary structure, and immunological characterization of the 26-kDa calsequestrin binding protein (junctin) from cardiac junctional sarcoplasmic reticulum. J Biol Chem. 1995;270:30787–30796. doi: 10.1074/jbc.270.51.30787. [DOI] [PubMed] [Google Scholar]
- 16.Knudson CM, Stang KK, Moomaw CR, Slaughter CA, Campbell KP. Primary structure and topological analysis of a skeletal muscle-specific junctional sarcoplasmic reticulum glycoprotein (triadin) J Biol Chem. 1993;268:12646–12654. [PubMed] [Google Scholar]
- 17.Brillantes AM, Allen P, Takahashi T, Izumo S, Marks AR. Differences in cardiac calcium release channel (ryanodine receptor) expression in myocardium from patients with end-stage heart failure caused by ischemic versus dilated cardiomyopathy. Circ Res. 1992;71:18–26. doi: 10.1161/01.res.71.1.18. [DOI] [PubMed] [Google Scholar]
- 18.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 19.Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, et al. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987;61:70–76. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- 20.Go LO, Moschella MC, Watras J, Handa KK, Fyfe BS, Marks AR. Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. J Clin Invest. 1995;95:888–894. doi: 10.1172/JCI117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schillinger W, Meyer M, Kuwajima G, Mikoshiba K, Just H, Hasenfuss G. Unaltered ryanodine receptor protein levels in ischemic cardiomyopathy. Mol Cell Biochem. 1996;160-161:297–302. doi: 10.1007/BF00240062. [DOI] [PubMed] [Google Scholar]
- 22.Daniel MB, Michael DR, Stuart JE. Protein methods. 2nd ed. New York: Wiley-Liss publisher; 1996. [Google Scholar]
- 23.Airey JA, Deerinck TJ, Ellisman MH, Houenou LJ, Ivanenko A, Kenyon JL, et al. Crooked neck dwarf (cn) mutant chicken skeletal muscle cells in low density primary cultures fail to express normal alpha ryanodine receptor and exhibit a partial mutant phenotype. Dev Dyn. 1993;197:189–202. doi: 10.1002/aja.1001970304. [DOI] [PubMed] [Google Scholar]
- 24.Riccardo Z, Simonetta RT. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: Modulation by endogenous factors, drugs and disease states. Pharmacol Rev. 1997;49:1–43. [PubMed] [Google Scholar]
- 25.Martonosi AN, Beeler TJ. Skeletal muscle. In: Peachey LD, Adrian RH, Geiger SR, editors. Handbook of physiology. 3rd ed, revised. Bethesda: American physiological society Press; 1983. pp. 417–485. [Google Scholar]
- 26.Orlova EV, Serysheva II, van Heel M, Hamilton SL, Chiu W. Two structural configurations of the skeletal muscle calcium release channel. Nat Struct Biol. 1996;3:547–552. doi: 10.1038/nsb0696-547. [DOI] [PubMed] [Google Scholar]
- 27.Anderson K, Lai FA, Liu QY, Rousseau E, Erikson HP, Meissner G. Structural and functional characterization of the purified cardiac ryanodine receptor-Ca2+ release channel complex. J Biol Chem. 1989;264:1329–1335. [PubMed] [Google Scholar]
- 28.Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res. 1993;72:463–469. doi: 10.1161/01.res.72.2.463. [DOI] [PubMed] [Google Scholar]