Abstract
The upper chest wall does not grow properly in children with spinal muscular atrophy (SMA) with paradoxical breathing. This suggests that long-term inability to take a deep breath in developing children may result in underdevelopment of the upper chest wall. In addition, a rapid and paradoxical breathing pattern is frequently observed in children with severe cerebral palsy (CP), which often corresponds to the underdevelopment of the upper chest wall. The present study is designed to evaluate the ratio of the upper to lower chest wall in children with severe spastic quadriplegic CP, compared with normal children. We compared normal children with children that had spastic quadriplegic CP who did not have kyphosis or scoliosis. Test subjects were matched in terms of age, height, and weight. The diameters of upper chest (Dapex) and of lower chest (Dbase) were measured on the anteroposterior (AP) view of a chest X-ray and the Dapex to Dbase ratio was calculated. In selected cases the forced vital capacity (FVC) was measured using a Wright Respirometer. The Dapex to Dbase ratio was significantly lower in the CP group than in the control group (p<0.001). The ratio increased linearly with age (p<0.001) in both CP (R = 0.372) and control groups (R = 0.477). The FVC/preFVC showed significant correlation with the Dapex to Dbase ratio (R = 0.542, p<0.01). The results of this study suggest a deviation of optimal chest wall structure in children with spastic quadriplegic CP.
Keywords: Chest wall, cerebral palsy, quadriplegia
INTRODUCTION
Cerebral palsy (CP) patients suffer from a high incidence of respiratory dysfunction such as recurrent pneumonia, atelectasis, bronchiectasis, sleep apnea, chronic obstructive lung disease, and restrictive lung disease.1,2 Respiratory dysfunction is known to be a leading cause of death among individuals with CP.1 However, respiratory dysfunction in children with severe CP has not been well studied, possibly due to the difficulty of testing these uncooperative young children. Clinical symptoms such as poor coughing and airway clearance, respiratory muscle weakness and kyphoscoliosis are thought to be related to respiratory dysfunction in these individuals.26
People of all ages take deep breaths or sigh regularly, and, of course, infants cry. These actions stretch the respiratory structures. Children with CP, particularly quadriplegic CP, breathe in a poorly coordinated fashion, relying on the abdominal muscles instead of the chest muscles.7,8 Eventually, chest movement is restricted and the chest muscles weaken. As a result, the ability to take a large breath is impaired.3,9,10 The shallow and low breathing volume in severe CP leads to the development of widespread microatelectasis and a decrease in lung distensibility.11 In particular, CP can lead to underdevelopment of the lung tissues and an alteration in chest wall development in children who are not fully grown.12-14 The development of pectus excavatum in children with spinal muscular atrophy (SMA) who did not receive early intervention,14 demonstrates the impact of long term respiratory insufficiency on chest wall development.
An intercostal muscle weakness in patients with spinal muscular atrophy results in a paradoxical ventilatory pattern.14-16 Kinematic analysis of breathing revealed a chronic failure to expand the upper chest wall and lungs in these children.16 It is possible that the shallow respiration observed in severe CP leads to an inability to expand the upper chest wall which may ultimately affect upper chest development. This can result in a lower ratio of upper chest to lower chest diameter in children with severe CP compared with normally developed children. Although there are several methods for assessing upper chest volume or expansion, evaluation of these children can be difficult and would ideally involve a simple test that does not require the cooperation of the child. The ratio of the transverse diameters of the upper chest and lower chest measured on a chest radiograph is a simple method for the assessment of chronic hypoventilation of the upper chest compartment in these children. The present study compares the ratio of the transverse diameter of the upper chest to the lower chest in children with severe spastic quadriplegic CP and normally developed children.
MATERIALS AND METHODS
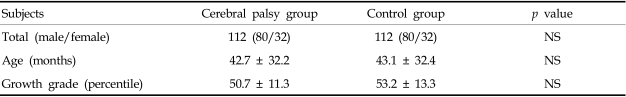
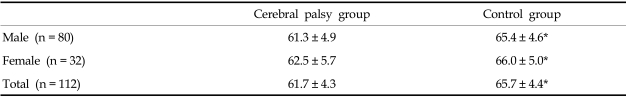
Spastic quadriplegic children of Gross Motor Function Classification System level 4 or 5 and normally developed children were recruited. Supine anteroposterior (AP) chest radiographs were taken and children with musculoskeletal problems such as scoliosis or kyphosis were excluded from the study. A simple chest radiograph was used to exclude those cases with a rotation of the facet joint or spinous process. Each group consisted of 112 children: 80 boys and 32 girls. The mean age was 42.7 ± 32.2 months in the CP group and 43.1 ± 32.4 months in the control group (Table 1). Using the AP view of a chest radiograph horizontal lines were drawn from the inner margin of the rib on one side to the other side, perpendicular to the line connecting the spinous processes. The longest line of the 2nd rib (Dapex) and 9th rib (Dbase) were measured (Fig. 1) and the percentage ratio of the upper to lower chest wall was calculated as Dapex / Dbase × 100(%). In addition, 10 children over age 7 who were able to cooperate with the pulmonary function test were selected from each group. The mean age and height of children in the two groups were similar (p > 0.05; Table 2). The forced vital capacity (FVC) was measured in a sitting position using a Wright Respirometer (Ferraris Development and Engineering Co Ltd., Edmonton, London, UK). Each patient was told to inhale as deeply as possible and to blow the entire volume through the spirometer. This process was repeated at least three times and the highest value was selected as the FVC. This study was approved by the local institutional Ethics Subcommittee for research involving human subjects.
Table 1.
General Characteristics of Subjects
Values are mean ± standard deviation.
NS, not significant.
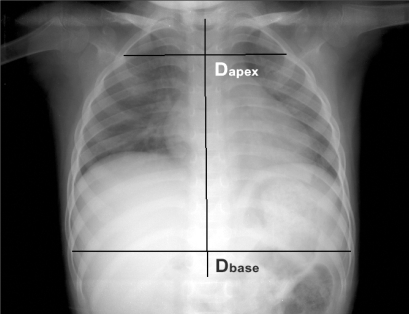
Fig. 1.
Measurement of the diameter of the chest apex (Dapex) and base (Dbase). Horizontal lines were drawn from the inner margin of the rib on one side to the other side perpendicular to the line connecting the spinous processes. The longest line of the 2nd rib (Dapex) and the 9th rib (Dbase) were measured.
Table 2.
Subjects Who Underwent the Pulmonary Function Test
M, male; F, female; SD, standard deviation.
An independent t-test was used to compare the ratio of upper to lower chest wall between the CP group and the control group. The relationship between the percentage ratio of chest wall and age or FVC was analyzed using coefficients of correlation. All data were analyzed with SPSS 11.0 for Windows.
RESULTS
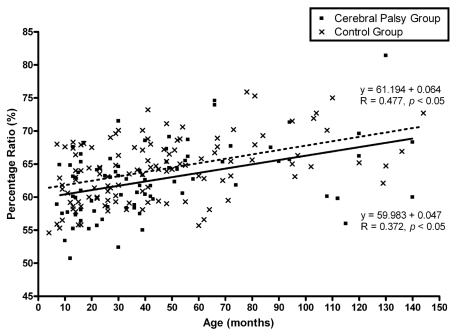
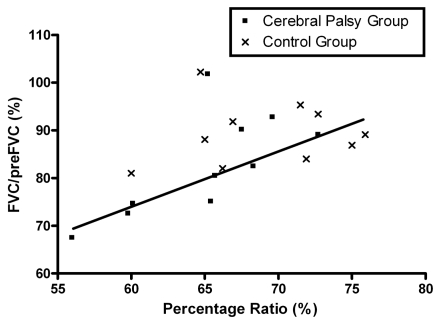
The ratio of the upper to lower chest wall of the CP group was significantly lower than that of the control group (p<0.05; Table 3). In addition, the ratio increased significantly with age in both the CP (R = 0.372, p<0.05) and control group (R = 0.477, p<0.05; Fig. 2). There was no gender difference between the CP and control groups. Table 4 shows the results of the pulmonary function test for the 20 children who underwent the test. The percentage ratio of FVC to the predicted FVC (preFVC) was calculated. There was a significant correlation between the upper to lower chest wall ratio and the percentage ratio of FVC/preFVC (R= 0.542, p<0.05; Fig. 3).
Table 3.
Results of the Percentage Ratio of the Upper to Lower Chest Diameter
Values are mean ± standard deviation.
*p<0.001.
Fig. 2.
Changes in the percentage ratio of the chest wall with age. Percentage ratio was calculated as the Dapex/Dbase diameter × 100. Filled squares and the solid line represent the cerebral palsy group, whereas the cross marks and dotted line represent the control group.
Table 4.
Results of the Pulmonary Function Test
Values are mean ± standard deviation.
FVC, forced vital capacity; preFCV, predicted value of forced vital capacity.
Fig. 3.
Relationship between the ratio of upper to lower chest wall diameter and the forced vital capacity. Percentage ratio was represented as Dapex/Dbase diameter × 100. The squares represent the cerebral palsy group, and the cross marks represent the control group.
DISCUSSION
The chest wall plays a dual role as a scaffold and a pump for the respiratory system. Normal respiratory function requires a scaffolding structure that allows adequate mobility and girth for lung expansion. For optimal pump function, the scaffolding needs to be rigid enough to withstand the distorting force of the inward lung recoil but not so rigid as to impede ventilation. Deviation from the optimal chest wall structure can result in respiratory dysfunction17; however, there have been few studies on the clinical implications of developmental abnormalities of the chest wall.
If left untreated, pectus excavatum can develop in patients with SMA type 1 or SMA type 2 with paradoxical breathing.14 Kinematic analysis of the breathing pattern in these children reveals paradoxical decreases in the upper thoracic volume during autonomous inspiration.16 In the case of CP, the adverse effects of a spinal deformity such as scoliosis, which commonly develops in non-ambulatory CP cases, on pulmonary function are well documented. However, there are very few reports on the effect of deviation from the optimal chest configuration on respiratory function in children with CP. Many studies have described the rapid and paradoxical breathing pattern of subjects with severe CP.3,9-10 In developing children the long-term inability to take a deep breath may result in development of a bell shaped chest, similar to cases of SMA with paradoxical breathing. The present study shows that children with severe CP have a low upper to lower chest diameter ratio compared with normally developed children, suggesting chronic hypoventilation of the upper thoracic compartment in children with CP.
The chest wall compliance of an infant is three times the normal compliance, resulting in paradoxical rib movement during inspiration. The highly compliant chest wall of infants predisposes them to deformation, necessitating that a portion of the respiratory muscle energy be expended in stabilizing the rib cage rather than in effecting ventilation.17 With increasing age the chest wall stiffens17 and developmental changes in chest wall compliance increase the respiratory efficiency. Therefore, the tendency for respiratory muscle fatigue, atelectasis, and respiratory failure decreases with age.18-21 In the present study the ratio of the upper to lower thoracic diameter increased with age in both CP and control groups. This can be explained in part by normal developmental changes in chest wall compliance.
A deviation from the optimal chest wall structure can result in mechanical insufficiency, respiratory muscle fatigue and failure, abnormal ventilation and abnormal resting lung volume.17 The correlation observed in the present study between the upper to lower chest wall ratio and the forced vital capacity demonstrates the relationship between chest wall structure and respiratory efficiency. Assessment of pulmonary mechanics in children with severe CP is challenging due to the difficulty of testing subjects who are unable to cooperate. Therefore, a simple index representing lung function might be a useful screening test for respiratory risk. It is important to note that, although the ratio of the upper to lower chest wall was significantly related to the percentage ratio of FVC/preFVC in this study, this index may not directly represent lung function. In the present study forced vital capacity was determined in a limited number of children who could cooperate with the testing procedure. Moreover, the ratio of the upper to lower chest wall appears to be related to a restrictive lung dysfunction rather than an obstructive lung dysfunction. Various causes of respiratory dysfunction in CP have been reported including: a restrictive pattern,5 abnormal compliance of the chest wall and lung, mismatched ventilation and perfusion,3,4 upper airway obstruction,2 and obstructive sleep apnea.22 Therefore, the low ratio of the upper to lower thoracic compartment can be considered to be one of several factors affecting lung function in these children.
The pectus excavatum of SMA type 1 can resolve, and the lungs and chest walls can grow more normally, if there is intervention with a high-span positive inspiratory pressure plus a positive end-expiratory pressure.14 Therefore, it is possible that the deviation from the optimal chest wall observed in children with CP can resolved if there is proper intervention in the early stages of development.
In conclusion, this study revealed a deviation from optimal chest wall structure in children with spastic quadriplegic CP. The correlation between the ratio of the upper to lower chest wall diameter and the forced vital capacity indicates the importance of the chest wall in respiration, and may provide a simple index for the assessment of respiratory function in children with CP.
References
- 1.Strauss D, Cable W, Shavelle R. Causes of excess mortality in cerebral palsy. Dev Med Child Neurol. 1999;41:580–585. doi: 10.1017/s001216229900122x. [DOI] [PubMed] [Google Scholar]
- 2.Seddon PC, Khan Y. Respiratory problems in children with neurological impairment. Arch Dis Child. 2003;88:75–78. doi: 10.1136/adc.88.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumberg ML. Respiration and speech in the cerebral palsied child. AMA Am J Dis Child. 1955;89:48–53. doi: 10.1001/archpedi.1955.02050110064009. [DOI] [PubMed] [Google Scholar]
- 4.Hardy JC. Lung function of athetoid and spastic quadriplegic children. Dev Med Child Neurol. 1964;89:378–388. doi: 10.1111/j.1469-8749.1964.tb08139.x. [DOI] [PubMed] [Google Scholar]
- 5.Bjure J, Berg K. Dynamic and static lung volumes of school children with cerebral palsy. Acta Paediatr Scand Suppl. 1970;204:35–41. doi: 10.1111/j.1651-2227.1970.tb06140.x. [DOI] [PubMed] [Google Scholar]
- 6.Keesee PD. Abnormal postural reflex activity and voice usage deviations in cerebral palsy. Phys Ther. 1976;56:1358–1360. doi: 10.1093/ptj/56.12.1358. [DOI] [PubMed] [Google Scholar]
- 7.Benditt JO. Management of pulmonary complications in neuromuscular disease. Phys Med Rehabil Clin North Am. 1998;9:167–185. [PubMed] [Google Scholar]
- 8.Redstone F. The effects of seating position on the respiratory patterns of preschoolers with cerebral palsy. Int J Rehabil Res. 2004;27:283–288. doi: 10.1097/00004356-200412000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Muller H. Speech. In: Finnie NR, editor. Handling the young child with cerebral palsy at home. 3rd ed. Oxford: Butterworth-Heinemann; 1997. pp. 112–117. [Google Scholar]
- 10.Stamer MH. Posture and movement of the child with cerebral palsy. San Antonio, Texas: Therapy Skill Builders; 2000. p. 79. [Google Scholar]
- 11.Leopando MT, Moussavi Z, Holbrow J, Chernick V, Pasterkamp H, Rempel G. Effect of a soft Boston orthosis on pulmonary mechanics in severe cerebral palsy. Pediatr Pulmonol. 1999;28:53–58. doi: 10.1002/(sici)1099-0496(199907)28:1<53::aid-ppul9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.De Troyer A, Deisser P. The effects of intermittent positive pressure breathing on patients with respiratory muscle weakness. Am Rev Respir Dis. 1981;124:132–137. doi: 10.1164/arrd.1981.124.2.132. [DOI] [PubMed] [Google Scholar]
- 13.Estenne M, De Troyer A. The effects of tetraplegia on chest wall statics. Am Rev Respir dis. 1986;134:121–124. doi: 10.1164/arrd.1986.134.1.121. [DOI] [PubMed] [Google Scholar]
- 14.Bach JR, Bianchi C. Prevention of pectus excavatum for children with spinal muscular atrophy type 1. Am J Phys Med Rehabil. 2003;82:815–819. doi: 10.1097/01.PHM.0000083669.22483.04. [DOI] [PubMed] [Google Scholar]
- 15.Lissoni A, Aliverti A, Molteni F, Bach JR. Spinal muscular atrophy: kinematic breathing analysis. Am J Phys Med Rehabil. 1996;75:332–339. doi: 10.1097/00002060-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Lissoni A, Aliverti A, Tzeng A, Bach JR. Kinematic analysis of patients with spinal muscular atrophy during spontaneous breathing and mechanical ventilation. Am J Phys Med Rehabil. 1998;77:188–192. doi: 10.1097/00002060-199805000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Papastamelos C, Panitch HB, England SE, Allen JL. Developmental changes in chest wall compliance in infancy and early childhood. J Appl Physiol. 1995;78:179–184. doi: 10.1152/jappl.1995.78.1.179. [DOI] [PubMed] [Google Scholar]
- 18.Goldman MD, Grimby G, Mead J. Mechanical work of breathing derived from rib cage and abdominal V-P partitioning. J Appl Physiol. 1976;41:752–763. doi: 10.1152/jappl.1976.41.5.752. [DOI] [PubMed] [Google Scholar]
- 19.Mortola JP, Saetta M, Fox G, Smith B, Weeks S. Mechanical aspects of chest wall distortion. J Appl Physiol. 1985;59:295–304. doi: 10.1152/jappl.1985.59.2.295. [DOI] [PubMed] [Google Scholar]
- 20.Guslits BG, Gaston SE, Bryan MH, England SJ, Bryan AC. Diaphragmatic work of breathing in premature human infants. J Appl Physiol. 1987;62:1410–1415. doi: 10.1152/jappl.1987.62.4.1410. [DOI] [PubMed] [Google Scholar]
- 21.Heldt GP, Mcllroy MB. Distortion of chest wall and work of diaphragm in preterm infants. J Appl Physiol. 1987;62:164–169. doi: 10.1152/jappl.1987.62.1.164. [DOI] [PubMed] [Google Scholar]
- 22.Kotagal S, Gibbons VP, Stith JA. Sleep abnormalities in patients with severe cerebral palsy. Dev Med Child Neurol. 1994;36:304–311. doi: 10.1111/j.1469-8749.1994.tb11850.x. [DOI] [PubMed] [Google Scholar]