Abstract
Background/Aims
To date, an effective salvage chemotherapy regimen for the treatment of refractory or relapsing non-Hodgkin's lymphoma (NHL) has not been discovered. This study was conducted to evaluate the efficacy and safety of gemcitabine, etoposide, cisplatin, and dexamethasone in relapsed or refractory NHL patients.
Methods
All patients had histologically proven relapsed or refractory NHL. Treatments consisted of gemcitabine 700 mg/m2 by continuous i.v. on days 1 and 8; etoposide 40 mg/m2 by i.v. on days 1-4; cisplatin 60 mg/m2 by i.v. on day 1; or dexamethasone 40 mg by i.v. on days 1-4 (GEPD) every 21 days. The primary end point was the patient response rate following two cycles of treatment. After two cycles, stem cells were harvested using mobilizing regimens (ESHAP or GEPD plus filgrastim), and this was followed by autologous stem cell transplantation or four additional cycles of GEPD.
Results
Between January 2005 and January 2006, 20 patients (13 males and 7 females) were enrolled in the study. The median age was 53 (range 16-75) years. The most common histology was diffuse large B-cell lymphoma (n=10). The median follow-up duration was 5.2 (range 1.0-16.0) months. After two cycles, the overall response rate was 50.0% (10/20), including two complete responses and eight partial responses. The dose-limiting toxicity was myelosuppression. Grade IV neutropenia and thrombocytopenia occurred in 13 (65.0%) and 6 patients (30.0%), respectively. The median number of CD34-positive cells collected was 6.0 (range, 2.8-11.6) ×106/kg. Of the 17 patients < 66 years of age, 4 (23.5%) proceeded to autologous stem cell transplantation.
Conclusions
GEPD chemotherapy in patients with refractory or relapsed NHL was effective as a salvage therapy and helpful for stem cell harvest followed by autologous transplantation.
Keywords: Non-Hodgkin's lymphoma, Refractory or relapsed, Gemcitabine
INTRODUCTION
Salvage therapies for relapsed or refractory aggressive non-Hodgkin's lymphomas (NHLs) commonly include platinum and etoposide. Two common drug regimens are DHAP [dexamethasone, cytosine arabinoside (Ara-C), and cisplatin] and ESHAP (etoposide, methylprednisone, Ara-C, and cisplatin). The EPIC regimen (etoposide, prednisolone, ifosfamide, and cisplatin) has been shown to be an effective low-toxicity regimen for relapsing lymphoma [1]. However, these salvage protocols are limited by poor responsiveness and toxicity [2]. Currently, there is no effective salvage regimen for relapsed or refractory NHL. Even though NHLs are commonly chemosensitive, 50-60% of patients experience primary treatment failure or relapse after an initial response.
Gemcitabine has a chemical structure that is similar to cytarabine. However, its pharmacological characteristics and mechanisms of action differ. Gemcitabine has been tested in a number of phase II studies as a single agent for the treatment of NHL. In these studies, moderate activity was noted in heavily pretreated lymphoma patients, while drug-related toxicities with the single-agent gemcitabine were mild [3-8]. In a phase II study of gemcitabine, cisplatin, and methylprednisolone (GEM-P) in poor prognostic primary progressive or multiply relapsed Hodgkin's and non-Hodgkin's lymphoma, the overall objective response rate was 80% in 20 patinets [9].
The aim of this study was to evaluate the overall response, disease-free survival, overall survival, and efficacy of stem cell mobilization on gemcitabine, etoposide, cisplatin, and dexamethasone (GEPD) in relapsed or refractory non-Hodgkin's lymphoma.
METHODS
Eligibility criteria
All patients evaluated for NHL had refractory or relapsed disease after previous chemotherapy. The eligibility criteria for patients in this study included: (1) men or women aged between 16-75 yr; (2) a histologically proven diagnosis of NHL (previous diagnoses were reformulated according to the WHO classification of lymphoid neoplasms)[10]; (3) documentation of refractory or relapsing disease after one or more chemotherapy treatment regimens; (4) an Eastern Cooperative Oncology Group Scale performance status less than 2; (5) at least one site of disease measurable in two dimensions using clinical examination, CT scan, or MRI scans; (6) no previous therapy with gemcitabine, high-dose chemotherapy, or stem cell transplantation; (7) a glomerular filtration rate of >60 mL/min, normal hepatic function, and normal bone marrow function. The patients were graded according to the Ann Arbor classification and international prognostic index (IPI). We excluded patients with acquired immunodeficiency syndrome (AIDS)-related lymphoma and those testing positive for human immunodeficiency virus. The study was conducted in accordance with the Declaration of Helsinki and was consistent with the International Conference in Harmonization Good Clinical Practice (ICH GCP) and applicable regulatory requirements. A recognized ethics committee reviewed and approved the study protocol.
Disease evaluation
The initial evaluation before commencement of chemotherapy included: medical history and physical examination; laboratory analyses including a complete blood count; renal and liver function and serum lactate dehydrogenase (LDH); glomerular filtration rate assessment by EDTA51Cr clearance; CT scans of the chest, abdomen and pelvis; and bone marrow biopsies for all NHL patients. All assessments were performed within 7 days of the first treatment, except the CT scans and bone marrow biopsies, which were performed within 28 days.
Study treatment
The GEPD regimen consisted of gemcitabine (700 mg/m2) and was delivered as a continuous intravenous infusion over 70 min on days 1 and 8. Etoposide (40 mg/m2) was delivered as an intravenous infusion over 30 min on days 1-4. Cisplatin (60 mg/m2) was given over 1 hour on day 1. Pre- and post-chemotherapy hydration was given on the day of cisplatin administration. Patients also received dexamethasone (40 mg) intravenously on days 1-4. The cycle was repeated every 21 days. Patients were given allopurinol 300 mg once a day for the first cycle. Throughout their treatment, cotrimoxazole (480 mg) was given twice a day three times a week to all patients for prophylaxis against pneumocystis carinii pneumonia. The institutional standard antiemetic regimen including metoclopromide, any anti-serotonin anti-emetic agent, and dexamethasone was provided prior to chemotherapy. Chemotherapy was delayed on day 8 until recovery for a maximum of 3 weeks if the neutrophil count was <0.5×109/L and/or the platelet count was <50×109/L or if the patient demonstrated grade 3/4 non-hematological toxicity (except for nausea, vomiting, and alopecia). If, on day 21, the neutrophil count was <1.0×109/L and/or the platelet count was <75×109/L chemotherapy treatment was delayed. The dose of cisplatin was reduced by 50% in the event of grade 2 neurological toxicity or grade 1 renal toxicity. In the event of febrile neutropenia, grade 4 thrombocytopenia, or ≥ grade 3 non-hematological toxicity (except alopecia), treatment with 75% of the dose for the three drugs (except for dexamethasone) was given and was returned to the full dose if the reduced dose was well-tolerated (absence of toxicity).
Response and safety assessment
The response to salvage therapy was assessed after a minimum of two courses of chemotherapy. International Workshop NHL response criteria were used to assess the response to treatment [11]. In addition, the toxicity was evaluated and graded according to the National Cancer Institute Common Toxicity Criteria (NCI CTC) version 3.0 grading system.
Statistical analysis
This study was designed as a prospective, non-randomized, open-labeled, multicenter, phase II study. The primary end points of the study were overall response (CR+CRu+PR) and toxicity. The secondary end points were efficacy of stem cell mobilization, progression-free survival, and overall survival. The patient characteristics, responses, toxicities, and efficacy of stem cell mobilization were evaluated by descriptive methods. The progression-free survival and overall survival were calculated using the Kaplan-Meier method. Statistical analysis was performed using SPSS 13.0 for Windows. All p values<0.05 were considered to be significant. In addition, the current trial used a two-stage optimal design, as proposed by Simon, with an 80% power to accept the hypothesis and 5% significance to reject the hypothesis. The duration of a complete remission was measured from the date of achieving the complete remission to the date of relapse for patients who relapsed, or the date of the last contact for patients who had not relapsed. The disease-free survival was estimated from the start date of chemotherapy to the date of relapse for patients who relapsed, or the date of last contact for patients who had not relapsed. Patients who died in remission were included as treatment failures and were not excluded from the analysis. Overall survival was estimated from the start date of chemotherapy to the date of death for patients who died.
RESULTS
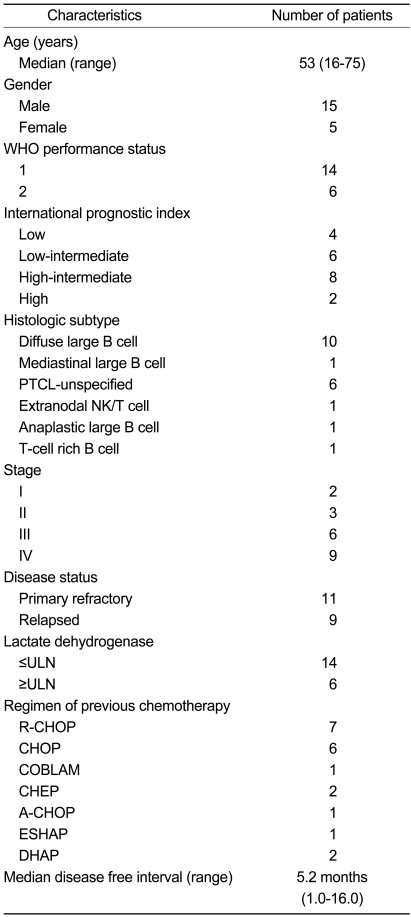
Patients' Characteristics
Twenty patients were enrolled between January 2005 and January 2006 from three medical centers in Korea. The patients' characteristics are summarized in Table 1. Among these patients, WHO performance status I and II were noted in 14 and 6 patients, respectively. The histological type was diffuse large B cell in 10 patients, PTCL (peripheral T cell lymphoma) in 6 patients, and mediastinal large B cell, extranodal NK/T cell, anaplastic large B cell, and T-cell rich B cell in the remaining patients. Before the first GEPD cycle, the disease status was primary refractory in 11 patients, and relapses had occurred in 9 patients. Patients with stage I, II, III, and IV disease included 2, 3, 6, and 9 patients, respectively. Previous treatment regimens were R-CHOP in 7 patients, CHOP in 6 patients, CHEP in 2 patients, DHAP in 2 patients, A-CHOP in 1 patient, COBLAM in 1 patient, and ESHAP in 1 patient. The median disease-free interval was 5.2 months.
Table 1.
Patients' characteristics
PTCL, peripheral T cell lymphoma; ULN, upper limit of normal range
Response to treatment
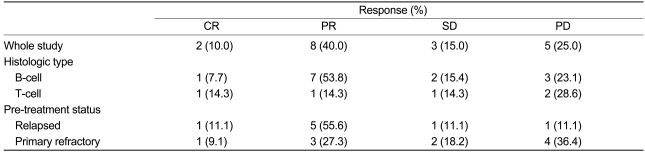
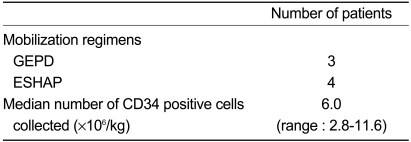
The objective response rate (RR) for all evaluated patients was 50.0% (95% CI:28.1-71.9%). Two CRs (10.0%) and 8 PRs (40.0%) were noted. The response was not evaluated for 2 patients who died early due to progression of disease. However, they are included in the analysis of response rate for intent-to-treat analysis. The responses are summarized in Table 2. Each of the response rates were divided into pretreatment status and histological type. The response rate of B-cell type lymphoma was better than that of T-cell type lymphoma. The response rates of B-cell type and T-cell type lymphomas were 61.5% (95% CI:23.5-76.5%) and 28.6% (95% CI:11.0-68.2%), respectively. The response rates of the relapsed patients and the primary refractory patients were 66.7% (95% CI:34.0-99.4%) and 36.4% (95% CI:6.6-68.2%), respectively. One of the relapsed patients and one of the primary refractory patients were not evaluated for response, due to early death. Autologous SCT as a consolidation therapy was performed in 7 patients. The mobilization regimen was GEPD or ESHAP. The CD34 positive cell yields were adequate (median: 6.03×106/kg, Table 3). No transplant-related mortality occurred. The median follow-up duration was 5.2 months (range, 1.0-16.0 months). The median survival time and the median time to progression were not reached due to short follow-up duration (data not shown).
Table 2.
Response to GEPD chemotherapy with relapsed or refractory Non-Hodgkin's lymphoma
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Table 3.
Mobilization regimens and CD34-positive cell counts
Toxicity
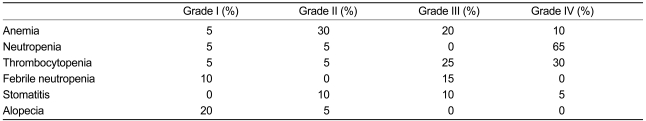
Toxicity was recorded in all 20 patients. The most important toxicity was myelosuppression. NCI-CTC grade IV neutropenia was observed in 13 patients (65%, Table 4).
Table 4.
Adverse reactions of GEPD chemotherapy
DISCUSSION
NHLs are responsive to current chemotherapy treatment regimens; however, the outcome of patients who do not achieve a response to initial treatment is poor. To date, there is no available salvage chemotherapy that is effective for primary refractory or relapsing NHL.
Gemcitabine is a novel nucleoside analogue with proven activity in solid tumors, and was used for NHLs in the late 1990s. It acts as a competitive substrate with deoxycytidine for incorporation into DNA, thus inhibiting DNA replication and repair. Despite its structural similarity to Ara-C, gemcitabine's cellular pharmacology and mechanism of action differs markedly [13]. Laboratory evidence suggests that a prolonged infusion rate of 10 mg/m2 per min may be more effective. In fact, maximal intracellular levels of difluorodeoxycitidine triphosphate, which is the principle active metabolite of gemcitabine, are generated at sustained plasma gemcitabine concentrations of 15-20 µmol/L.
In a phase II study of gemcitabine, cisplatin, and methlyprednisolone (GEM-P) in poor prognostic primary progressive or multiply relapsed Hodgkin's and non-Hodgkin's lymphoma involving 20 patients, the overall objective response rate was 80% (including 25% CR). No case of febrile neutropenia or hemorrhage with thrombocytopenia was encountered [9]. The overall response rate of chemotherapy with ifosfamide, carboplatin, and etoposide (ICE) in primary refractory or relapsed NHLs was 65%. Only 58% of the 163 patients in that study proceeded to transplantation, and the event-free survival for the entire cohort was 25% at 40 months. The response rate to gemcitabine as a single agent in the treatment for relapsed or refractory NHLs was 20% [4]. In a phase II study conducted by the National Cancer Institute of Canada, the clinical trial group that was treated with gemcitabine, dexamethasone, and cisplatin (GDP) had a response rate of 49% [15]. The response rate with GEPD chemotherapy was similar to that of other regimens.
Hematological toxicity of salvage regimens used before SCT is often substantial and may interfere with subsequent attempts at stem cell mobilization. For example, in the initial report on DHAP chemotherapy, 43 of 90 patients (48%) required hospitalization for the management of febrile neutropenia or documented infection, and 10 of 90 patients (11%) died [14]. In our study, 7 patients proceeded to autologous stem cell collection. The mobilization regimens were GEPD chemotherapy (n=3) and ESHAP chemotherapy (n=4). The median number of harvested CD 34-positive cells was 6.03×106/kg. This result was not different from results reported with ICE and DHAP chemotherapy.
The principal toxicity of GEPD was myelosuppression, which was well tolerated. The non-hematological toxicity was mild with no observed grade III-IV toxicity. No transplant-related mortality occurred. The main toxicity of GEM-P and GDP chemotherapy was myelosuppression [9]. Therefore, the toxicity associated with GEPD chemotherapy was the same as GEM-P and GDP chemotherapy and lower than ESHP chemotherapy. The median overall survival and relapse-free survival was 6.9 and 6.3 months, respectively, which is consistent with prior studies [4,6].
In conclusion, GEPD chemotherapy is an effective regimen for patients with primary refractory or relapsed NHL and does not interfere with the ability to harvest autologous stem cells for subsequent transplantation. The associated toxicity was myelosuppression, and this was the principal toxicity and cause of treatment-related mortality. The median overall survival and relapse-free survival were 6.9 months and 6.3 months, respectively. Longer follow-up intervals are needed to determine the overall survival and relapse-free survival in our study. Additional studies are planned to compare GEPD with DHAP or ESHAP in randomized phase III trials as a second-line therapy before ASCT.
Footnotes
This work was supported by the 2003 Inje University research grant.
References
- 1.Hickish T, Roldan A, Cunningham D, et al. EPIC: an effective low toxicity regimen for relapsing lymphoma. Br J Cancer. 1993;68:599–604. doi: 10.1038/bjc.1993.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sweetenham JW, Johnson PW. ESHAP chemotherapy for relapsed/refractory non-Hodgkin's lymphoma. J Clin Oncol. 1994;12:2766a. [PubMed] [Google Scholar]
- 3.Bernell P, Ohm L. Promising activity of gemcitabine in refractory high-grade non-Hodgkin's lymphoma. Br J Haematol. 1998;101:203–204. doi: 10.1046/j.1365-2141.1998.00667.x. [DOI] [PubMed] [Google Scholar]
- 4.Fossa A, Santoro A, Hiddemann W, et al. Gemcitabine as a single agent in the treatment of relapsed or refractory aggressive non-Hodgkin's lymphoma. J Clin Oncol. 1999;17:3786–3792. doi: 10.1200/JCO.1999.17.12.3786. [DOI] [PubMed] [Google Scholar]
- 5.Sallah S, Wan JY, Nguyen NP. Treatment of refractory T-cell malignancies using gemcitabine. Br J Haematol. 2001;113:185–187. doi: 10.1046/j.1365-2141.2001.02743.x. [DOI] [PubMed] [Google Scholar]
- 6.Santoro A, Bredenfeld H, Devizzi L, et al. Gemcitabine in the treatment of refractory Hodgkin's disease: results of a multicenter phase II study. J Clin Oncol. 2000;18:2615–2619. doi: 10.1200/JCO.2000.18.13.2615. [DOI] [PubMed] [Google Scholar]
- 7.Savage DG, Rule SA, Tighe M, et al. Gemcitabine for relapsed or resistant lymphoma. Ann Oncol. 2000;11:595–597. doi: 10.1023/a:1008307528519. [DOI] [PubMed] [Google Scholar]
- 8.Zinzani PL, Bendandi M, Stefoni V, et al. Value of gemcitabine treatment in heavily pretreated Hodgkin's disease patients. Haematologica. 2000;85:926–929. [PubMed] [Google Scholar]
- 9.Chau I, Harries M, Cunningham D, et al. Gemcitabine, cisplatin and methylprednisolone chemotherapy (GEM-P) is an effective regimen in patients with poor prognostic primary progressive or multiply repalsed Hodgkin's and non-Hodgkin's lymphoma. Br J Haematol. 2003;120:970–977. doi: 10.1046/j.1365-2141.2003.04226.x. [DOI] [PubMed] [Google Scholar]
- 10.Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Organization classification of tumors, pathology and genetics of tumors of hematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. [Google Scholar]
- 11.Cheson BD, Horning SJ, Coiffler B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 12.Storniolo AM, Allerhiligen SR, Pearce HL. Preclinical, pharmacologic and phase I studies of gemcitabine. Semin Oncol. 1997;24:S7-2–S7-7. [PubMed] [Google Scholar]
- 13.Grunewald R, Kanraejan H, Keating MJ, Abbruzzese J, Tarassoff P, Plungett W. Pharmacologically directed design of the dose rate and schedule of 2',2'-difluorodeoxycitidine (gemcitabine) administration in- leukemia. Cancer Res. 1990;50:6823–6826. [PubMed] [Google Scholar]
- 14.Velaquez WS, Cabanillas F, Salvador P, et al. Effective salvage therapy for lymphoma with cisplatin in combination with high-dose Ara-C and dexamethasone (DHAP) Blood. 1988;71:117–122. [PubMed] [Google Scholar]
- 15.Crump M, Baetz T, Couban S, et al. Gemcitabine, dexamethasone, and cisplatin in patients with recurrent or refractory aggressive histology B-cell non-Hodgkin lymphoma: a Phase II study by the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) Cancer. 2004;101:1835–1842. doi: 10.1002/cncr.20587. [DOI] [PubMed] [Google Scholar]
- 16.Choi CW, Paek CW, Seo JH, et al. ESHAP salvage therapy for relapsed or refractory non-Hodgkin's lymphoma. J Korean Med Sci. 2002;17:621–624. doi: 10.3346/jkms.2002.17.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumontet C, Morschhauser F, Solal-Celigny P, et al. Gemcitabine as a single agent in the treatment of relapsed or refractory low-grade non-Hodgkin's lymphoma. Br J Haematol. 2001;13:772–778. doi: 10.1046/j.1365-2141.2001.02795.x. [DOI] [PubMed] [Google Scholar]
- 18.Kroep JR, Peters GJ, van Moorsel CJ, et al. Gemcitabine-cisplatin: a schedule finding study. Ann Oncol. 1999;10:1503–1510. doi: 10.1023/a:1008339425708. [DOI] [PubMed] [Google Scholar]
- 19.van Moorsel CJ, Kroep JR, Pinedo HM, et al. Pharmacokinetic schedule finding study of the combination of gemcitabine and cisplatin in patients with solid tumors. Ann Oncol. 1999;10:441–448. doi: 10.1023/a:1008301522349. [DOI] [PubMed] [Google Scholar]
- 20.Nagourney RA, Link JS, Blitzer JB, Forsthoff C, Evans SS. Gemcitabine plus cisplatin repeating doublet therapy in previously treated, relapsed breast cancer patients. J Clin Oncol. 2000;18:2245–2249. doi: 10.1200/JCO.2000.18.11.2245. [DOI] [PubMed] [Google Scholar]
- 21.Peters GJ, Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Braakhuis B. Interaction between cisplatin and gemcitabine in vitro and in vivo. Semin Oncol. 1995;22(4 Suppl 11):72–79. [PubMed] [Google Scholar]
- 22.Zinzani PL, Baliva G, Magagnoli M, et al. Gemcitabine treatment in pretreated cutaneous T-cell lymphoma: experience in 44 patients. J Clin Oncol. 2000;18:2603–2606. doi: 10.1200/JCO.2000.18.13.2603. [DOI] [PubMed] [Google Scholar]