Abstract
Background/Aims
The optimal time point for initiating renal replacement therapy in patients with end-stage renal disease remains controversial. The primary objective of our study was to determine the effects of residual renal function at the beginning of renal replacement therapy on the mortality of patients with end-stage renal disease.
Methods
We retrospectively studied the clinical outcomes in patients (n=210) with end-stage renal disease who underwent renal replacement therapy at our hospital between 2000 and 2005; all patients were followed for more than 1 year. We used the Modification of Diet in Renal Disease equation to estimate residual renal function.
Results
Of the 210 patients who received renal replacement therapy, 108 were treated with hemodialysis and 102 were treated with peritoneal dialysis. Thirty-three patients died, and the mean survival period was 37.3±17.7 months. The survival rates were compared based on the estimated glomerular filtration rate; no difference in survival rates was observed (p=0.27). Subgroup analysis in the hemodialysis group showed that patients who began chronic dialysis at a lower estimated glomerular filtration rate had higher mortality rates (p<0.05); patients treated with peritoneal dialysis showed no significant difference in mortality rate (p=0.50).
Conclusions
Although there was no difference in the mortality rate based on residual renal function, hemodialysis patients with a lower estimated glomerular filtration rate showed a higher mortality rate than those with a higher estimated glomerular filtration rate.
Keywords: End-stage renal disease, Renal replacement therapy, Prognosis
INTRODUCTION
Before deciding to initiate chronic dialysis in a patient with chronic renal failure, both the physician and patient must consider the subjective and objective parameters. The indications for the initiation of dialysis in chronic kidney disease (CKD) include pericarditis or pleuritis, progressive uremic encephalopathy or neuropathy, clinically significant uremic bleeding diathesis, and malnutrition and fluid overload refractory to diuretics. Persistent metabolic disturbances that are refractory to medical therapies are among the most important indications. However, in cases with no clinical or metabolic disturbances, the time point for initiating dialysis remains controversial.
Some guidelines recommend that dialysis be initiated when the estimated glomerular filtration rate (eGFR) falls below the recommended level in asymptomatic CKD patients. In Canada, clinical practice guidelines recommend starting dialysis when the GFR is <12 mL/min/1.73m2 in the presence of uremia or malnutrition, or when the GFR is <6 mL/min/1.73m2 regardless of whether the case is symptomatic or asymptomatic [1]. Earlier guidelines outlined by the National Kidney Foundation in the Dialysis Outcomes Quality Initiative recommend that renal replacement therapy (RRT) be considered when the GFR falls below 10.5 mL/min/1.73m2 [2]. In their more recent 2006 update, the National Kidney Foundation recommends that nephrologists evaluate the risks and benefits of initiating dialysis when patients reach stage 5 CKD [3].
Despite these guidelines, there exists a wide variation in the time point selected for the initiation of chronic hemodialysis based on levels of residual renal function, and many patients continue to be treated with dialysis when having a very low GFR. Short-term study results on the benefits and risks of both early and late initiation of dialysis in an asymptomatic patient with CKD have been obtained, but long-term results have yet to be reported. Accordingly, we conducted a retrospective study to determine the relationship between mortality and GFR at the initiation of dialysis in patients with CKD.
METHODS
Patients who were admitted to the hospital for the initiation of RRT between 1 January 2000 and 31 June 2005 were enrolled in the study. Of these, patients with acute renal failure and those who had recovered renal function were excluded. Follow-up was continued until 31 June 2007.
The baseline data collected included age, gender, and the cause of renal failure. The most prominent symptom or the indication prompting the initiation of dialysis therapy was also noted.
For all study subjects, the decision to initiate dialysis was made by an attending nephrologist. Pre-dialysis blood urea nitrogen (BUN), serum creatinine, and serum albumin concentrations, as well as estimated GFR calculated by the Modification of Diet in Renal Disease (MDRD) equation, were measured once before the first dialysis. The high and low estimated GFRs were defined as values more than and less than 5 mL/min/1.73m2. Deaths during follow-up were noted, and the cause of mortality was obtained from death certificates.
Survival duration was defined as the period from the initiation of chronic dialysis to death. Hospitalization refers to the admission frequency after the initiation of chronic dialysis, except for hospitalization due to trauma.
Mortality analyses were performed using Kaplan-Meier survival curves, with survival rates during the follow-up estimated for patients in the various groups. Survival curves were compared between groups using the log-rank test, and differences were considered statistically significant for p values<0.05.
RESULTS
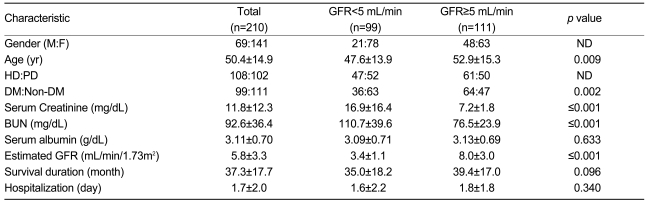
A total of 210 patients who began dialysis because of chronic kidney disease were recruited in the study. Of these, 108 (51.4%) were hemodialysis patients and 102 (48.6%) were peritoneal dialysis patients. There were 69 men (32.9%) and 141 women (67.1%); the mean age was 50.4±14.9 years. The mean serum creatinine and BUN levels were 11.8±12.3 mg/dL and 92.6±36.4 mg/dL, respectively. The mean eGFR calculated using a four-variable MDRD equation was 5.8±3.3 mL/min/1.73m2, and the mean survival period was 37.3±17.7 months.
The study subjects were grouped based on eGFR at the initiation of dialysis. Ninety-nine patients had an eGFR<5 mL/min/1.73m2; their mean eGFR was 3.4±1.1 mL/min/1.73m2, and their mean age was 47.6±13.9 years. Of the 99 patients, 47 (47.5%) were hemodialysis patients and 52 (52.5%) were peritoneal dialysis patients. Their mean albumin level was 3.09±0.71 g/dL, and the mean survival duration was 35.0±18.2 months (Table 1).
Table 1.
Basic characteristics of patients classified by estimated glomerular filtration rate
GFR, glomerular filtration rate; ND, not done; HD, hemodialysis; PD, peritoneal dialysis; DM, diabetes mellitus.
In the group with an eGFR>5 mL/min/1.73m2, there were 111 (52.8%) patients: 61 hemodialysis patients (54.9%) and 50 peritoneal dialysis patients (45.1%). Their mean eGFR was 8.0±3.0 mL/min/1.73m2, and their mean age was 52.9±15.3 years. Their mean serum albumin level was 3.13±0.69 g/dL (Table 1).
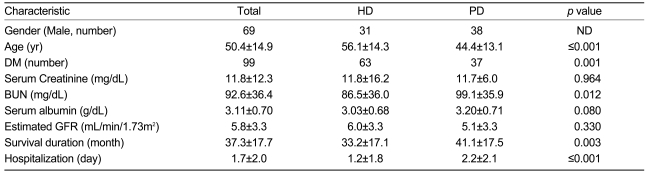
Classified based on the modality of RRT, the hemodialysis group had a mean age of 56.1±14.3 years, a mean eGFR of 6.0±3.3 mL/min/1.73m2, a mean albumin level of 3.03±0.68 g/dL, and a mean survival duration of 33.2±17.1 months. For the peritoneal dialysis patients, the mean age was 44.4±13.1 years, the mean eGFR was 5.6±3.3 mL/min/1.73m2, and the mean survival duration was 41.1±17.5 months (Table 2).
Table 2.
Basic characteristics of patients receiving renal replacement therapy
GFR, glomerular filtration rate; ND, not done; HD, hemodialysis; PD, peritoneal dialysis; DM, diabetes mellitus.
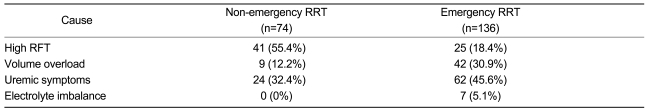
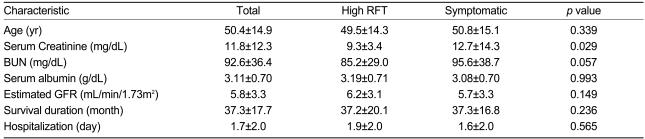
The reasons for initiating RRT were high serum creatinine and/or BUN levels (n=66, 31.4%); volume overload (n=51, 24.3%); uremic symptoms such as nausea, vomiting, poor appetite, or encephalopathy (n=86, 41%); or electrolyte imbalances (n=7, 3.3%). Emergency RRT was performed in 136 (64.8%) patients, primarily for symptoms of uremia (n=62, 45.6%) (Table 3). There were no differences between the asymptomatic and symptomatic patients in serum albumin level (3.19±0.71 vs. 3.08±0.70 g/dL; p=0.993), eGFR (6.2±3.1 vs. 5.7±3.3 mL/min/1.73m2, p=0.149), and survival duration (37.2±20.1 vs. 37.3±16.8 months, p=0.236) (Table 4).
Table 3.
The causes of the initiation of dialysis
RRT, renal replacement therapy; RFT, renal function test (serum creatinine and/or BUN).
Table 4.
Basic characteristics of patients classified by the cause of the initiation of dialysis
RFT, renal function test (serum creatinine and/or BUN); GFR, glomerular filtration rate.
A total of 33 patients died during the follow-up period; 11 (33.3%) of these expired due to cardiovascular causes. Other causes of death included infection (n=7, 21.2%), malignancy (n=3, 9%), bleeding (n=3, 9%), other causes (i.e., seizures, suicide, gastrointestinal perforation, and traffic accident; n=4), and unknown causes (n=5, 15.1%) (Table 5).
Table 5.
Causes of death
RRT, renal replacement therapy; GFR, glomerular filtration rate; TA, traffic accident.
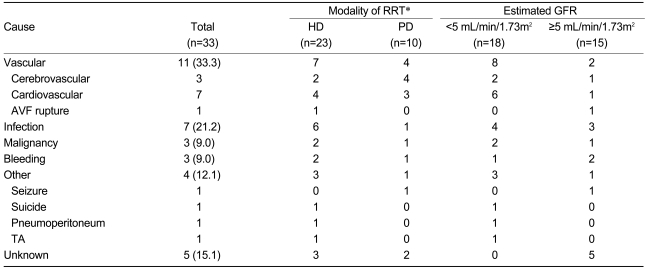
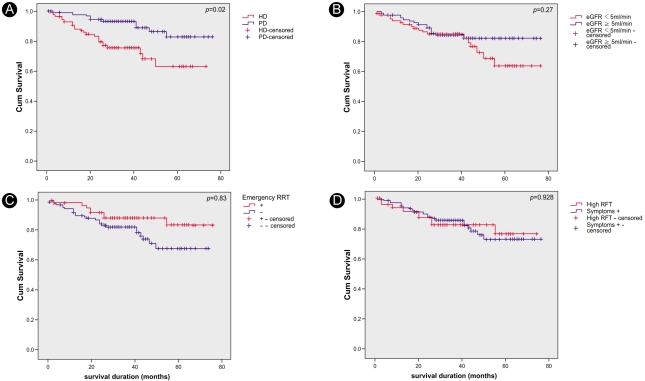
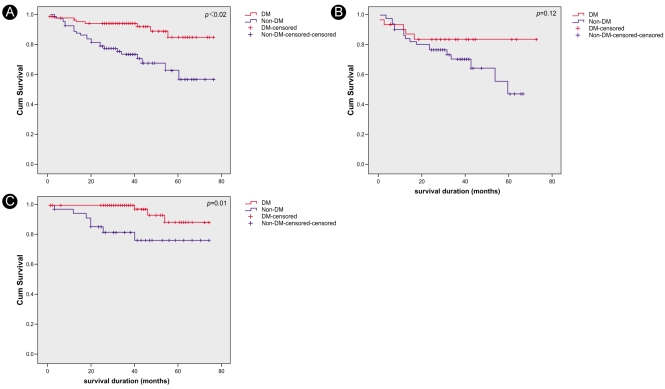
According to the Kaplan-Meier survival curves, hemodialysis patients had poorer survival rates than peritoneal dialysis patients (p=0.002). There was no difference in survival between the groups based on eGFRs at the initiation of dialysis (p=0.27). Furthermore, there were no differences in survival based on the indications for starting RRT or whether emergency RRT was performed (Fig. 1). However, in the subgroup analysis, the hemodialysis patients with higher eGFRs (>5 mL/min/1.73 m2) at the initiation of dialysis showed higher survival rates compared with those with lower eGFRs (<5 mL/min/1.73m2) at initiation (Fig. 2). In addition, among these patients, there was no significant difference in survival between the diabetic and non-diabetic patients. In the peritoneal dialysis patient group, there was no significant difference in survival between those with higher and those with lower eGFRs; however, the diabetic patients had significantly lower survival rates than the non-diabetic patients (Fig. 4).
Figure 1.
Kaplan-Meier survival curves. Hemodialysis patients showed higher mortality rates than peritoneal dialysis patients (p=0.02, A); there were no significant differences in survival between patients according to estimated GFR (p=0.27, B), emergency dialysis (p=0.08, C), or symptoms (p=0.93, D).
Figure 2.
Kaplan-Meier survival curves. There were higher mortality rates for hemodialysis patients compared with peritoneal dialysis patients in the lower estimated GFR group (p<0.01, A); however, there were no differences in the higher estimated GFR group (p=0.384, B). Hemodialysis patients with lower estimated GFR had higher mortality rates than hemodialysis patients with higher estimated GFR (p<0.05, C); however, there were no differences for peritoneal dialysis patients (p=0.50, D).
Figure 4.
Kaplan-Meier survival curves. Overall, diabetic patients showed lower survival rates compare with non-diabetic patients (p<0.01, A). Diabetic patients also showed lower survival rates in the peritoneal dialysis group (p=0.01, C), but there were no significant differences in survival rates between diabetic and non-diabetic patients in the hemodialysis group (p=0.12, B).
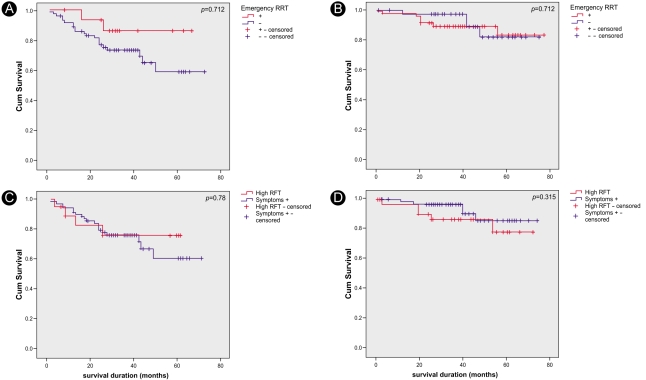
There was no significant difference in survival between the patient groups with and without emergency RRT. The survival rates in hemodialysis and peritoneal dialysis patients did not differ regardless of the cause for initiating RRT (Fig. 3).
Figure 3.
Kaplan-Meier survival curves. There was no significant survival difference between the groups receiving emergency RRT and those receiving non-emergency RRT (A and B) in either the hemodialysis (p=0.184, A) or the peritoneal dialysis patients (p=0.712, B). The survival rates in neither the hemodialysis (p=0.78, C) nor the peritoneal dialysis (p=0.315, D) groups differed based on the cause for initiating RRT.
DISCUSSION
The decision to initiate dialysis in end-stage renal disease is not an easy one for either patients or physicians. The main reasons for initiating chronic dialysis are symptoms relating to reduced renal function, including nausea, vomiting, poor appetite, over-hydration, encephalopathy, and electrolyte imbalance. Some guidelines recommend that chronic dialysis be initiated at a specified eGFR. However, in patients with chronic renal failure, the clinical features and laboratory indices that are used as guidelines for initiating RRT do not always correlate with survival [4]. Our study revealed that the survival of patients did not depend on the indications used in deciding to initiate chronic dialysis. For example, patients who underwent chronic dialysis because of high serum creatinine and/or BUN levels did not have better survival rates than patients who underwent chronic dialysis for symptoms related to uremia, volume overload, or electrolyte imbalances.
In several reports and guidelines, the baseline eGFR used to initiate early dialysis ranges from 5 to 15 mL/min [5]. In the present study, the criterion used to define the high eGFR group was an eGFR>5 mL/min.
The suggestion that early initiation of dialysis is beneficial was first supported by Bonomini et al. [6]. The Canada-USA (CANUSA) study reported significantly poorer survival rates for patients with lower levels of residual renal function at the initiation of dialysis [7]; similar results were found by Tattersall et al. [8]. Other studies, however, failed to take into account the effect of lead-time bias. Jamie et al. reported that a Cox proportional hazards model demonstrated a significant inverse relationship between the estimated creatinine clearance rate at the start of dialysis and survival, so that patients who started dialysis with lower estimated creatinine clearance rates tended to survive longer [8]. This relationship became significant when parameters such as gender, age, weight, presence of diabetes, mode of first dialysis, initial dialysis access, hemoglobin level, serum albumin level, blood leukocyte count, and Wright/Khan index were taken into account at the start of dialysis.
The major causes of death in patients undergoing dialysis are cardiovascular disease, infection, and withdrawal from treatment [10]. In general, cardiovascular disease accounts for approximately 50% of the deaths. However, only 11 patients (33.3%) died due to cardiovascular causes in our study, although there were five cases of death from unknown causes, which might have included death due to undetected cardiovascular disease. Furthermore, the follow-up period was relatively short in our study, which might have resulted in an apparently lower mortality rate.
Our data show that hemodialysis patients had a higher mortality rate compared with those treated with peritoneal dialysis. The hemodialysis patients were also significantly older than the peritoneal dialysis patients (mean age: 56.1±14.3 vs. 44.4±13.1 yr, respectively; p<0.0001). Diabetes significantly affected the survival rate of peritoneal dialysis patients overall, but it did not significantly affect survival in the hemodialysis group (Fig. 4). Fewer peritoneal dialysis patients developed end-stage renal disease due to diabetes. Because diabetes is a major factor in the development of cardiovascular disease, and fewer peritoneal dialysis patients had cardiovascular disease, the survival rates between the two modalities could not be compared.
In subgroup analyses, lower mortality rates were observed for early initiation of chronic dialysis in endstage renal disease among the hemodialysis patients, whereas no differences were observed among the peritoneal dialysis patients. In the hemodialysis group, 23 (21.3%) patients died; only 10 (9.8%) peritoneal dialysis patients died. The lower mortality rate and small sample size that was based on the eGFR at the start of dialysis may underlie the lower mortality among the peritoneal dialysis patients. In contrast to our findings, the CANUSA study showed higher mortality in peritoneal dialysis patients with lower creatinine clearance and a much lower mortality rate for peritoneal dialysis patients than that reported in our study. One possible reason for this difference is the younger mean age of the patients in the peritoneal dialysis group in our study (mean age: 54.3 vs. 44.4 yr, CANUSA study vs. our study). As age is an important prognostic factor, it may explain the higher mortality in the older patient group in the CANUSA study. Moreover, no mortality based on the residual renal function at the beginning of dialysis was observed in our peritoneal dialysis patients.
In conclusion, this study fails to support the view that early initiation of dialysis can prolong the survival of patients with end-stage renal disease. Furthermore, there were no differences in survival based on the indications for initiation of dialysis. However, higher mortality rates were observed for patients treated with hemodialysis compared with peritoneal dialysis, and higher mortality rates were observed for late initiation of treatment in hemodialysis patients. Further research with a greater sample size is required to verify whether early initiation of treatment could improve survival among patients with end-stage renal disease; this will be defined by the results of the Initiating Dialysis Early and Late Study [11].
References
- 1.Churchill DN, Blake PG, Jindal KK, Toffelmire EB, Goldstein MB. Clinical practice guidelines for initiation of dialysis. J Am Soc Nephrol. 1999;10(Suppl 13):S289–S291. [PubMed] [Google Scholar]
- 2.National Kidney Foundation, authors. K/DOQI clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis. 1997;30(Suppl 2):S70–S73. doi: 10.1016/s0272-6386(97)70028-3. [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation, authors. KDOQI clinical practice guidelines and clinical practice recommendations for 2006 updates: Hemodialysis adequacy, peritoneal dialysis adequacy and vascular access. Am J Kidney Dis. 2006;48(Suppl 1):S13–S16. [Google Scholar]
- 4.Ifudu O, Dawood M, Homel P, Friedman EA. Timing of initiation of uremia therapy and survival in patients with progressive renal disease. Am J Nephrol. 1998;18:193–198. doi: 10.1159/000013336. [DOI] [PubMed] [Google Scholar]
- 5.Kazmi WH, Gilbertson DT, Obrador GT, et al. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis. 2005;46:887–896. doi: 10.1053/j.ajkd.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Bonomini V, Feletti C, Scolari MP, Stefoni S. Benefits of early initiation of dialysis. Kidney Int Suppl. 1985;17:S57–S59. [PubMed] [Google Scholar]
- 7.Canada-USA (CANUSA) Peritoneal Dialysis Study Group, authors. Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. J Am Soc Nephrol. 1996;7:198–207. doi: 10.1681/ASN.V72198. [DOI] [PubMed] [Google Scholar]
- 8.Tattersall J, Greenwood R, Farrington K. Urea kinetics and when to commence dialysis. Am J Nephrol. 1995;15:283–289. doi: 10.1159/000168850. [DOI] [PubMed] [Google Scholar]
- 9.Traynor JP, Simpson K, Geddes CC, Deighan CJ, Fox JG. Early initiation of dialysis fails to prolong survival in patients with endstage renal failure. J Am Soc Nephrol. 2002;13:2125–2132. doi: 10.1097/01.asn.0000025294.40179.e8. [DOI] [PubMed] [Google Scholar]
- 10.United States Renal Data System, authors. Excerpts from USRDS 2005 Annual Data Report: US Department of Health and Human Services. Am J Kindey Dis. 2006;47(Suppl):S1. [Google Scholar]
- 11.Cooper BA, Branley P, Bufone L, et al. The Initiating Dialysis Early and Late (IDEAL) study: study rational and design. Perit Dial Int. 2004;24:176–181. [PubMed] [Google Scholar]