Abstract
Background/Aims
Diffuse large B-cell lymphoma (DLBCL) in Koreans is frequently accompanied by extranodal (EN) disease at the time of autologous stem cell transplantation (ASCT). We sought to determine whether high EN involvement affected survival following ASCT in Koreans.
Methods
We reviewed 27 patients who had DLBCL with residual disease at ASCT: 13 with residual disease at nodal site(s) only and 14 with nodal and EN disease.
Results
Univariate analysis showed that disease status, lactate dehydrogenase (LDH), and performance status at ASCT were predictors of survival following ASCT. The number of EN sites, as categorized by the International Prognostic Index system, had no prognostic significance. When EN involvement at ASCT was classified as negative or positive, the 2-year overall survival for the negative group was 64%, significantly better than the 14% for the positive group (p=0.021), and the event-free survival for the negative group was 62%, significantly better than the 14% for the positive group (p=0.02).
Conclusions
Patients who had DLBCL with residual EN involvement at ASCT showed worse outcomes following ASCT compared to those without EN disease.
Keywords: Diffuse large B-cell lymphoma, Autologous stem cell transplantation, Extranodal involvement, Residual disease
INTRODUCTION
Non-Hodgkin's lymphoma (NHL) was the first tumor type to which high-dose therapy, supported by the reinfusion of autologous stem cells, was applied1), and dose intensification was shown to be able to cure some patients with relapsing or refractory disease2, 3). At present, NHL is the second most frequent indication for autologous stem cell transplantation (ASCT) because ASCT has been found to provide an increasing number of patients having the disease with the best opportunity for cure under certain circumstances. Despite recent therapeutic advances, however, many patients with NHL relapse after ASCT. Among the factors having significance in predicting how well patients will do after ASCT, the most predictive is the sensitivity of the lymphoma to chemotherapy4-8). Factors associated with a poor outcome include elevated lactate dehydrogenase (LDH)6), extensive previous treatment9), bulky disease10), poor performance status11), and high-grade histology9, 12).
The International Prognostic Index (IPI) is a well established scoring system that predicts the survival of patients with aggressive NHL13). IPI has also been evaluated in patients with NHL treated with high-dose therapy and ASCT14-19). Studies that have assessed the value of IPI for the result of ASCT in patients with selected pathologic subtypes typically use the age-adjusted IPI (AAIPI), rather than the IPI. The AAIPI lacks factors for extranodal (EN) involvement, as well as for age. In Korea, around two-thirds of patients who have NHL also have EN involvement at initial presentation20) and over 50% of patients have residual EN disease at ASCT21). We therefore hypothesized that EN involvement in NHL may affect survival after ASCT in Korean patients. We now report a Korean single institution study of 27 patients with diffuse large B-cell lymphoma (DLBCL) who had radiologically documented residual lymphoma at the time of high-dose therapy with ASCT.
MATERIALS AND METHODS
Patients
A registry showed that 27 patients with DLBCL and residual disease had been treated with ASCT at the Asan Medical Center between March 1996 and May 2005. Residual disease of at least 1×1 cm was documented by CT scan, performed just prior to high-dose therapy. Each residual EN involvement was documented by pathologic examination. Disease status was assessed by CT of the neck, chest, abdomen, and pelvis, performed just prior to high-dose therapy. Bone marrow biopsies were repeated to evaluate the lymphoma status. All patients were staged, according to the Ann Arbor system. Response was assessed using the International Working Group criteria22). Disease status of eligible patients was classified as initial partial response, chemotherapy-sensitive relapse, and primary refractory disease. Primary refractory disease was defined as stable or progressive disease documented at restaging, immediately after the completion of induction therapy23). To be eligible for ASCT, patients had to be 15-65 years old and have biopsy-proven DLBCL; adequate hepatic, renal, pulmonary, and cardiac function; negative serology for human immunodeficiency virus; and no history of other malignancies or central nervous system involvement.
All histological specimens were reviewed by one pathologist (JH). Before 1998, histopathological results had been classified according to the International Working Formulation24), but we retrospectively reclassified such specimens according to the World Health Organization/Revised European-American Lymphoma (WHO/REAL) system25). Since 1998, all histologic diagnoses have been performed using the WHO/REAL classification.
The ASCT protocol was approved by the institutional review board of Asan Medical Center. Written informed consent was obtained from each patient.
Peripheral blood progenitor cell mobilization
In patients assessed as candidates for ASCT, peripheral blood progenitor cell (PBPC) mobilization and collection were performed as described previously26). PBPCs were mobilized with chemotherapy, followed by lenograstim (Neutrogin, Choongwae Ltd., Seoul, Korea), both to reduce the tumor burden and to facilitate PBPC harvesting. The chemotherapy regimens for mobilization were 4 g/m2 cyclophosphamide or ESHAP (etoposide, methylprednisolone, cytarabine, cisplatin), with or without rituximab. All patients received 10 µg/kg/day lenograstim subcutaneously, starting on the day after the completion of mobilization chemotherapy and continuing until the last leukapheresis procedure. PBPCs were collected with a continuous-flow blood cell separator (Fenwal CS3000 plus, Baxter Healthcare, Deerfield, IL, USA). The total CD34+ cell count was monitored daily following each collection, with the target amount being 5×106 CD34+ cells per kilogram patient weight.
IPI and age-adjusted IPI at ASCT
The IPI consists of five risk factors: age older than 60 years, LDH greater than the upper limit of normal, ECOG performance status greater than 1, stage III or IV disease, and more than one EN site. The age-adjusted IPI (AAIPI) consists of three of the IPI risk factors: LDH, stage, and ECOG performance status13).
ASCT procedure
The BEAM15) or BEAC3) regimen was used as a high-dose conditioning regimen, and lenograstim was used to facilitate engraftment. Patients were cared for in a single room, with reverse isolation strictly maintained to prevent infectious complications. All patients were administered prophylactic antimicrobials, consisting of ciprofloxacin, fluconazole, and acyclovir. Patients received transfusions of red blood cells and platelets as clinically indicated. Generally, platelets were single donor transfusions, administered to keep the platelet count above 20,000/mm3 or to relieve clinical bleeding.
Statistical analysis
All continuous variables were analyzed using the Mann-Whitney test. Proportions were compared using the χ2 test or Fisher's exact test, as appropriate. Overall survival (OS) was defined as the time from the date of transplantation until death or the last follow-up. Event-free survival (EFS) was measured from the date of ASCT until the time of a relapse, evidence of disease progression, or death, regardless of cause. The date of the first event was used in calculating EFS. Survival curves were plotted using the product-limit method, according to Kaplan and Meier, and were compared using the log-rank test. The Cox proportional hazards regression model was used for multivariate analysis. Statistical analysis was performed with SPSS (version 12.0 for Windows, SPSS Inc., Chicago, IL, USA) and the significance level was set at 0.05.
RESULTS
Patient characteristics
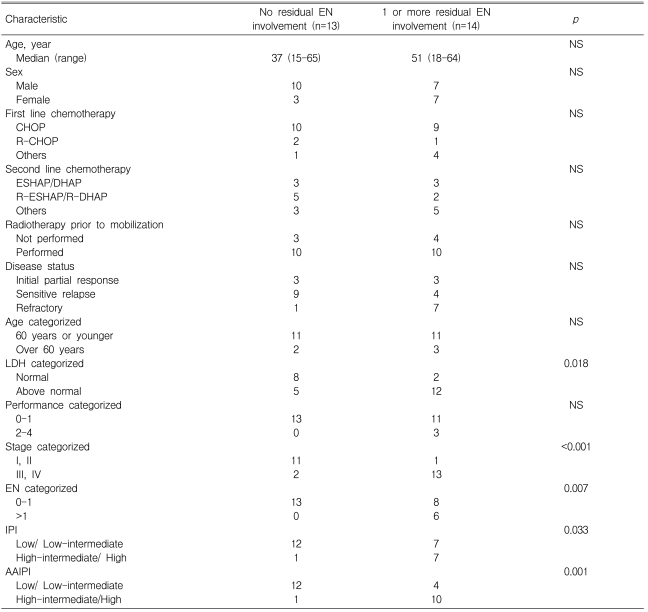
Twenty-seven patients with DLBCL were eligible for this analysis. Of them, 13 (48%) had no residual EN involvement and 14 (52%) had one or more residual EN disease at ASCT. Characteristics of the groups of patients at ASCT are listed in Table 1. Age and gender were not different between the groups. The most frequent first-line chemotherapy regimens were CHOP and rituximab plus CHOP. Dose-intense regimens, such as ESHAP, DHAP, and ICE, were administered to one patient in the no-EN group and four patients in the residual-EN group, who were found to have a significant tumor burden and poor prognostic features by their treating physicians. First-line chemotherapy regimens showed no statistically significant difference between the groups. For salvage regimens, ESHAP, DHAP, rituximab plus ESHAP, or rituximab plus DHAP were administered to the majority of patients. Three patients from the no-EN group and five patients from the residual-EN group were given aggressive regimens, such as CODOX-M/IVAC and GMALL, chosen by the treating physician. Two patients from the no-EN group and four patients from the residual-EN group were not administered second-line salvage chemotherapy because of their initial partial response (PR) status, and their PBPCs were mobilized by high-dose cyclophosphamide. Salvage chemotherapy regimens showed no difference between the groups. Radiotherapy history and disease status at ASCT were not different between the groups. Among the IPI factors, age and performance status were not significantly different between the groups. Patients showing LDH above the normal range were more frequent in the residual-EN group (86%) than in the no-EN group (39%; p=0.018). Advanced stage III/IV disease was observed in 13 patients (93%) in the residual-EN group (93%) and in one patient (15%) in the no-EN group (p<0.001). EN involvement score, categorized by the IPI system, was significantly worse in the residual-EN group than in the no-EN group (p=0.007). The IPI score was high-intermediate or high in seven patients (50%) in the residual-EN group and in one patient (8%) in the no-EN group (p=0.033). Ten patients (71%) in the residual-EN group had high-intermediate or high AAIPI scores and 12 patients (92%) in the no EN group showed low or low-intermediate risk (p=0.001).
Table 1.
Patient characteristics at autologous stem cell transplantation
EN, extranodal; NS, not significant; R, Rituximab; LDH, Lactate dehydrogenase; IPI, International Prognostic Index; AAIPI, Age-adjusted International Prognostic Index
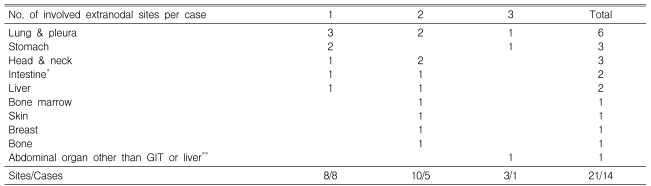
Details of the distribution of EN involvement sites at ASCT are presented in Table 2. Eight patients had involvement of one EN site, five had involvement of two EN sites, and one had involvement of three EN sites. Of the 21 total EN sites in these 14 patients with residual EN disease at ASCT, the most frequently observed were the lung and pleura (6 sites), followed by the stomach (3 sites), head and neck (3 sites), intestine (2 sites), and liver (2 sites).
Response to ASCT
Because no ASCT-related mortality occurred in the 27 patients, all were evaluable for response. Twelve patients (44%) achieved a complete response (CR) post-ASCT, seven patients (26%) a PR, and eight patients (30%) failed. Of 19 patients transplanted in chemosensitive status, 11 (58×) achieved CR with the ASCT. However, only one patient (13×) of the eight transplanted in chemoresistant status achieved a CR (p=0.03).
Survival
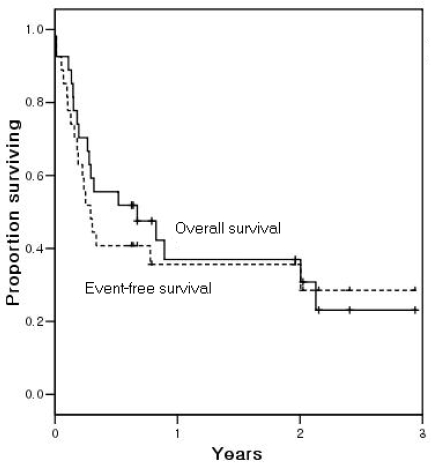
The median follow-up duration for survivors was 24 months (range, 7-36). Eighteen patients (67%) have died and nine patients are alive. Twelve patients died of disease progression and six patients died of other illnesses. No occurrence of secondary neoplasm was observed. The Kaplan-Meier estimate of the proportion of patients remaining alive at 2 years following ASCT was 37%, with an estimated EFS at 2 years of 36% (Figure 1).
Figure 1.
Overall survival and event-free survival for all 27 patients. The Kaplan-Meier curves show that the overall survival 2 years after autologous stem cell transplantation was 37% and the proportion of patients remaining event-free at 2 years was 36%.
Predictors of outcome following ASCT
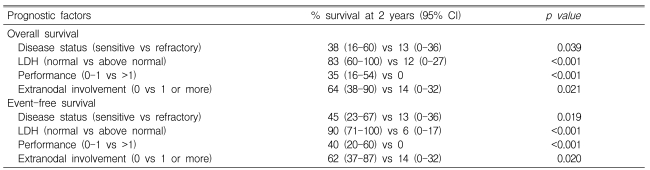
The univariate analysis for OS and EFS is shown in Table 3. A better outcome was associated with chemosensitive disease status at ASCT, normal LDH level, good performance, and no EN involvement at ASCT. However, based on a multivariate analysis, only normal LDH level [hazard ratio (HR) 0.05; 95& confidence interval (CI) 0.01-0.39; p=0.004 for OS; and HR 0.04; 95% CI 0.01-0.35; p=0.003 for EFS] was associated with superior survival.
Table 3.
Prognostic factors at autologous stem cell transplantation in univariate analysis
LDH, Lactate dehydrogenase
EN involvement as a predictor of outcome following ASCT
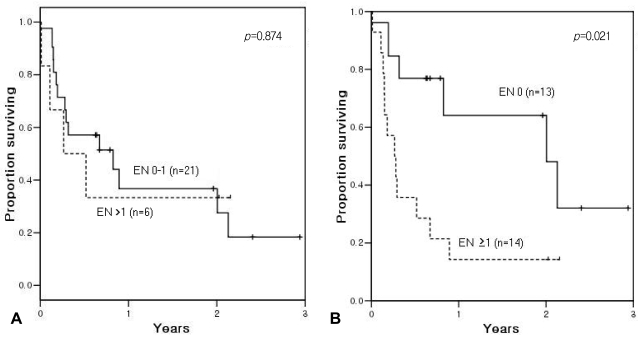
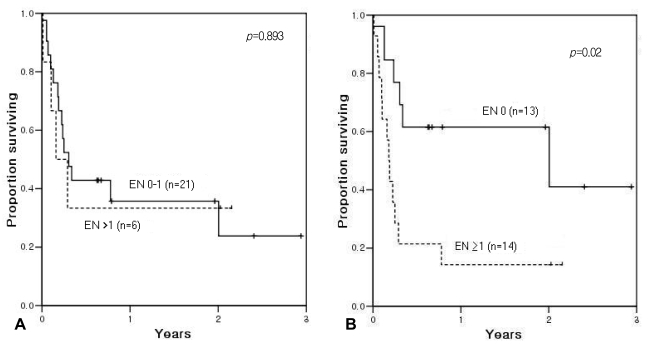
Note that when categorized according to the original IPI, the 2-year OS was 37% for patients with 0-1 EN sites and 33% for those with more than 1 EN involvement at ASCT (p=0.874; Figure 2A). When we applied a modified categorization of EN involvement, grouped as negative or positive, the 2-year OS was 64% for patients with no EN site at ASCT, significantly better than the 14% 2-year OS in patients with EN involvement at ASCT (p=0.021; Figure 2B). The 2-year EFS was 36% for patients with 0-1 EN site and 33% with more than 1 EN involvement at ASCT (p=0.893; Figure 3A). With the modified categorization of EN involvement, the 2-year EFS was 62% for patients with no residual EN site at ASCT, significantly better than the 14% 2-year EFS in patients with residual EN involvement at ASCT (p=0.02; Figure 3B).
Figure 2.
Overall survival according to extranodal (EN) disease status at autologous stem cell transplantation. (A) Comparison between 0-1 EN site and more than 1 EN site showed no difference in overall survival. (B) Modified categorization: patients with no remaining EN site had a 2-year overall survival rate of 64%, significantly better than the 14% rate observed in patients with remaining EN site(s) at autologous stem cell transplantation.
Figure 3.
Event-free survival according to extranodal (EN) disease status at autologous stem cell transplantation. (A) Comparison between 0-1 EN site and more than 1 EN site showed no difference in event-free survival. (B) Modified categorization: patients with no remaining EN site had a 2-year event-free survival rate of 62%, significantly better than the 14% rate observed in patients with remaining EN site(s) at autologous stem cell transplantation.
DISCUSSION
Patients with DLBCL can be cured by anthracycline-based chemotherapy. The recent introduction of rituximab and dose-dense chemotherapies has improved survival in these patients27, 28). However, at least 50% of patients with DLBCL fail to achieve a CR, or relapse after CR. Half of the relapsing or refractory patients respond to salvage chemotherapy, but cure is expected in fewer than 10%. Eventually, almost 50% of patients with DLBCL die of their disease29). High-dose therapy followed by ASCT has been widely used to improve the prognosis in these patients and has prolonged survival, especially in those with chemosensitive disease.
Over the past 10 years, ASCT for DLBCL performed at our institution resulted in poor outcomes for patients who were not in CR at ASCT, with a 37% rate of 2-year OS, and a 36% rate of 2-year EFS. The poor outcomes we observed may have been due to the possibly unique biology of DLBCL in Koreans or other unknown factors. We sought to discover factors predicting prognosis of these patients with residual disease at ASCT, especially focusing on the influence of EN involvement. Thus, all patients had to have had residual disease at the time of ASCT to join this study. When this condition was met, we included any patients with DLBCL for whom ASCT had been performed at our institution.
In the current study, disease status at the time of ASCT was a significant factor predicting OS and EFS. Among the five IPI factors, LDH and performance status had statistical significance as predictors for OS and EFS. Age and stage showed no significance, although that may have been the result of our small sample size. EN involvement categorized by the original IPI system did not show a survival difference.
Our initial expectation with this retrospective analysis was that EN involvement may have a significant prognostic value. While EN lymphomas represent 30~40% of all lymphoid neoplasms in Western countries, around two-thirds of Korean NHLs are classified as extranodal20). This high frequency of EN involvement may be associated with poorer survival rates in Korean patients. Fourteen of 27 patients (52%) in the current study had residual EN disease, even at ASCT. A univariate analysis, however, showed no significant difference between patients with 0-1 EN site and those with more than 1 EN sites based on the IPI system. When patients were stratified into those with or without residual EN sites at the time of ASCT, a statistically significant difference was observed in the 2-year OS (14% vs. 64%) and 2-year EFS (14% vs. 62%).
Almost all reports dealing with the clinical course and outcome following ASCT in NHL have classified EN involvement as 0-1 versus more than 1 site, as in the original IPI system. In a study categorizing EN disease as 0 versus 1 or more sites, a statistically significant difference in the 5-year OS (p=0.004) was detected30). Note that while they used a different categorization from the original IPI system, they did not provide an explanation for this. They analyzed 114 patients with DLBCL who did not achieve CR after induction chemotherapy and were set to be treated with ASCT. Seventy-one patients (62%) had EN involvement at diagnosis and 39 patients (35%) had residual EN disease at ASCT. The 5-year OS was 52% in patients with no residual EN disease, significantly better than the 25% for the 5-year OS in patients with residual EN involvement. Age-adjusted IPI, EN disease (0 versus ≥1) and disease status were significant prognostic factors for overall survival in a univariate analysis in that report.
Considering both the report from the GEL/TAMO group30) and the results of the current study, categorization into negative versus positive EN involvement had predictive value. Patients with DLBCL and residual EN disease at ASCT did poorly compared to those with no residual EN involvement. This categorization may be valid especially when the EN involvement is more prevalent. In the current study, EN involvement at ASCT categorized as absent or present showed a strong association with predictors of poor prognosis, such as LDH above a normal range, advanced stage, higher IPI, and higher AAIPI at ASCT. Why patients with residual EN disease at ASCT have poor survival remains to be answered.
Patients with DLBCL and residual EN disease at ASCT may have poor survival with ASCT and should be given other novel therapies. Because this was a retrospective study performed with a limited number of patients, the results should be interpreted cautiously. A small sample size and short follow-up period cause limitations in statistical power.
In conclusion, Korean patients with DLBCL and residual disease at ASCT have frequent EN involvement, and patients with residual EN involvement show poor outcomes following ASCT. A modified categorization of EN involvement into negative versus positive may be more useful in predicting outcome following ASCT, especially when EN disease is prevalent.
Table 2.
Distribution of extranodal involvement sites
GIT, Gastrointestinal tract
*EN sites of Intestine were duodenum and iluem. They were diagnosed by endoscopic biopsies.
**EN site of other abdominal organ was adrenal gland. Without pathologic confirm, it was possible to diagnose according to the radiologic finding.
Acknowledgement
We thank the nurses at ward 84 and the house staff members of the Department of Internal Medicine at Asan Medical Center for their dedication and excellent patient care.
References
- 1.Appelbaum FR, Herzig GP, Ziegler JL, Graw RG, Levine AS, Deisseroth AB. Successful engraftment of cryopreserved autologous bone marrow in patients with malignant lymphoma. Blood. 1978;52:85–95. [PubMed] [Google Scholar]
- 2.Moskowitz CH, Nimer SD, Glassman JR, Portlock CS, Yahalom J, Straus DJ, O'Brien JP, Elkin N, Bertino JR, Zelenetz AD. The International Prognostic Index predicts for outcome following autologous stem cell transplantation in patients with relapsed and primary refractory intermediate-grade lymphoma. Bone Marrow Transplant. 1999;23:561–567. doi: 10.1038/sj.bmt.1701624. [DOI] [PubMed] [Google Scholar]
- 3.Philip T, Guglielmi C, Hagenbeek A, Somers R, van der Lelie H, Bron D, Sonneveld P, Gisselbrecht C, Cahn JY, Harousseau JL, Coiffier B, Biron P, Mandelli F, Chauvin F. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 4.Colombat P, Gorin NC, Lemonnier MP, Binet C, Laporte JP, Douay L, Desbois I, Lopez M, Lamagnere JP, Najman A. The role of autologous bone marrow transplantation in 46 adult patients with non-Hodgkin's lymphomas. J Clin Oncol. 1990;8:630–637. doi: 10.1200/JCO.1990.8.4.630. [DOI] [PubMed] [Google Scholar]
- 5.Lazarus HM, Crilley P, Ciobanu N, Creger RJ, Fox RM, Shina DC, Bulova SI, Gucalp R, Cooper BW, Topolsky D, Soegiarso W, Brodsky I. High-dose carmustine, etoposide, and cisplatin and autologous bone marrow transplantation for relapsed and refractory lymphoma. J Clin Oncol. 1992;10:1682–1689. doi: 10.1200/JCO.1992.10.11.1682. [DOI] [PubMed] [Google Scholar]
- 6.Vose JM, Anderson JR, Kessinger A, Bierman PJ, Coccia P, Reed EC, Gordon B, Armitage J. High-dose chemotherapy and autologous hematopoietic stem-cell transplantation for aggressive non-Hodgkin's lymphoma. J Clin Oncol. 1993;11:1846–1851. doi: 10.1200/JCO.1993.11.10.1846. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler C, Strawderman M, Ayash L, Churchil WH, Bierer BE, Elias A, Gilliland DG, Antman K, Guinan EC, Eder JP, Weinstein H, Schwartz G, Ferrara J, Mazanet R, Rimm I, Tepler I, McCarthy P, Mauch P, Ault K, Gaynes L, McCauley M, Schnipper L. Prognostic factors for treatment outcome in autotransplantation of intermediate-grade and high-grade non-Hodgkin's lymphoma with cyclophosphamide, carmustine, and etoposide. J Clin Oncol. 1993;11:1085–1091. doi: 10.1200/JCO.1993.11.6.1085. [DOI] [PubMed] [Google Scholar]
- 8.Mills W, Chopra R, McMillan A, Pearce R, Linch DC, Goldstone AH. BEAM chemotherapy and autologous bone marrow transplantation for patients with relapsed or refractory non-Hodgkin's lymphoma. J Clin Oncol. 1995;13:588–595. doi: 10.1200/JCO.1995.13.3.588. [DOI] [PubMed] [Google Scholar]
- 9.Petersen FB, Appelbaum FR, Hill R, Fisher LD, Bigelow CL, Sanders JE, Sullivan KM, Bensinger WI, Witherspoon RP, Storb R, Clift R, Fefer A, Press O, Weiden P, Singer J, Thomas E, Buckner C. Autologous marrow transplantation for malignant lymphoma: a report of 101 cases from Seattle. J Clin Oncol. 1990;8:638–647. doi: 10.1200/JCO.1990.8.4.638. [DOI] [PubMed] [Google Scholar]
- 10.Rapoport AP, Lifton R, Constine LS, Duerst RE, Abboud CN, Liesveld JL, Packman CH, Eberly S, Raubertas RF, Martin BA, Flesher WR, Kouides PA, DiPersio JF, Rowe JM. Autotransplantation for relapsed or refractory non-Hodgkin's lymphoma (NHL): long-term follow-up and analysis of prognostic factors. Bone Marrow Transplant. 1997;19:883–890. doi: 10.1038/sj.bmt.1700772. [DOI] [PubMed] [Google Scholar]
- 11.Phillips GL, Fay JW, Herzig RH, Lazarus HM, Wolff SS, Lin HS, Shina DC, Glasgow GP, Griffith RC, Lamb CW, Herzig G. The treatment of progressive non-Hodgkin's lymphoma with intensive chemoradiotherapy and autologous marrow transplantation. Blood. 1990;75:831–838. [PubMed] [Google Scholar]
- 12.Weaver CH, Petersen FB, Appelbaum FR, Bensinger WI, Press O, Martin P, Sandmaier B, Deeg HJ, Hansen JA, Brunvand M, Rowley S, Benyunes K, Chauncey T, Fefer A, Hackman R, Gooley T, Schiffman K, Storb R, Sullivan K, Weiden P, Witherspoon R, Buckner C. High-dose fractionated total-body irradiation, etoposide, and cyclophosphamide followed by autologous stem-cell support in patients with malignant lymphoma. J Clin Oncol. 1994;12:2559–2566. doi: 10.1200/JCO.1994.12.12.2559. [DOI] [PubMed] [Google Scholar]
- 13.The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 14.Hamlin PA, Zelenetz AD, Kewalramani T, Qin J, Satagopan JM, Verbel D, Noy A, Portlock CS, Straus DJ, Yahalom J, Nimer SD, Moskowitz CH. Age-adjusted International Prognostic Index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2003;102:1989–1996. doi: 10.1182/blood-2002-12-3837. [DOI] [PubMed] [Google Scholar]
- 15.Caballero MD, Rubio V, Rifon J, Heras I, García-Sanz R, Vázquez L, Vidriales B, del Cañizo MC, Corral M, Gonzalez M, León A, Jean-Paul E, Rocha E, Moraleda JM, San Miguel JF. BEAM chemotherapy followed by autologous stem cell support in lymphoma patients: analysis of efficacy, toxicity and prognostic factors. Bone Marrow Transplant. 1997;20:451–458. doi: 10.1038/sj.bmt.1700913. [DOI] [PubMed] [Google Scholar]
- 16.Intragumtornchai T, Prayoonwiwat W, Numbenjapon T, Assawa-metha N, O'Charoen R, Swasdikul D. CHOP versus CHOP plus ESHAP and high-dose therapy with autologous peripheral blood progenitor cell transplantation for high-intermediate-risk and high-risk aggressive non-Hodgkin's lymphoma. Clin Lymphoma. 2000;1:219–225. doi: 10.3816/clm.2000.n.018. [DOI] [PubMed] [Google Scholar]
- 17.Blay J, Gomez F, Sebban C, Bachelot T, Biron P, Guglielmi C, Hagenbeek A, Somers R, Chauvin F, Philip T. The International Prognostic Index correlates to survival in patients with aggressive lymphoma in relapse: analysis of the PARMA trial. Blood. 1998;92:3562–3568. [PubMed] [Google Scholar]
- 18.Fanin R, Silvestri F, Geromin A, Infanti L, Sperotto A, Cerno M, Stocchi R, Savignano C, Rinaldi C, Damiani D, Baccarani M. Autologous stem cell transplantation for aggressive non-Hodgkin's lymphomas in first complete or partial remission: a retrospective analysis of the outcome of 52 patients according to the age-adjusted International Prognostic Index. Bone Marrow Transplant. 1998;21:263–271. doi: 10.1038/sj.bmt.1701081. [DOI] [PubMed] [Google Scholar]
- 19.Nademanee A, Molina A, O'Donnell M, Dagis A, Snyder D, Parker P, Stein A, Smith E, Plana's I, Kashyap A, Spielberger R, Fung H, Wong KK, Somlo G, Margolin K, Chow W, Sniecinski I, Vora N, Blume K, Niland J, Forman S. Results of high-dose therapy and autologous bone marrow/stem cell transplantation during remission in poor-risk intermediate and high-grade lymphoma: International Index high and high-intermediate risk group. Blood. 1997;90:3844–3852. [PubMed] [Google Scholar]
- 20.Ko YH, Kim CW, Park CS, Jang HK, Lee SS, Kim SH, Ree HJ, Lee JD, Kim SW, Huh JR. REAL classification of malignant lymphomas in the Republic of Korea: incidence of recently recognized entities and changes in clinicopathologic features. Cancer. 1998;83:806–812. [PubMed] [Google Scholar]
- 21.Suh C, Kim SH, Kim HJ, Jang G, Kim EK, Ko OB, Kim S, Sohn HJ, Lee JS, Kim M, Huh J. Prognostic factors in non-Hodgkin's lymphoma patients treated by autologous stem cell transplantation: a single center experience. Cancer Res Treat. 2005;37:294–301. doi: 10.4143/crt.2005.37.5.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JD, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 23.Vose JM, Zhang MJ, Rowlings PA, Lazarus HM, Bolwell BJ, Freytes CO, Pavlovsky S, Keating A, Yanes B, van Besien K, Armitage JO, Horowitz MM. Autologous transplantation for diffuse aggressive non-Hodgkin's lymphoma in patients never achieving remission: a report from the autologous blood and marrow transplant registry. J Clin Oncol. 2001;19:406–413. doi: 10.1200/JCO.2001.19.2.406. [DOI] [PubMed] [Google Scholar]
- 24.The Non-Hodgkin's Lymphoma Classification Project. National Cancer Institute sponsored study of classifications of non-Hodgkin's lymphomas: summary and description of a working formulation for clinical usage. Cancer. 1982;49:2112–2135. doi: 10.1002/1097-0142(19820515)49:10<2112::aid-cncr2820491024>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the clinical advisory committee meeting: Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 26.Lee JL, Kim S, Kim SW, Kim EK, Kim SB, Kang YK, Lee J, Kim MW, Park CJ, Chi HS, Huh J, Kim SH, Suh C. ESHAP plus G-CSF as an effective peripheral blood progenitor cell mobilization regimen in pretreated non-Hodgkin's lymphoma: comparison with high-dose cyclophosphamide plus G-CSF. Bone Marrow Transplant. 2005;35:449–454. doi: 10.1038/sj.bmt.1704798. [DOI] [PubMed] [Google Scholar]
- 27.Pfreundschuh M, Trumper L, Kloess M, Schmits R, Feller AC, Rudolph C, Reiser M, Hossfeld DK, Metzner B, Hasenclever D, Schmitz N, Glass B, Rube C, Loeffler M. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of young patients with good-prognosis (normal LDH) aggressive lymphomas: results of the NHL-B1 trial of the DSHNHL. Blood. 2004;104:626–633. doi: 10.1182/blood-2003-06-2094. [DOI] [PubMed] [Google Scholar]
- 28.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, van den Neste E, Salles G, Gaulard P, Reyes F, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 29.Caballero MD, Pérez-Simón JA, Iriondo A, Lahuerta JJ, Sierra J, Marín J, Gandarillas M, Arranz R, Zuazu J, Rubio V, de Sevilla F, Carreras E, García-Conde J, García-Laraña J, Grande C, Sureda A, Vidal MJ, Rifón J, Pérez-Equiza C, Varela R, Moraleda JM, García Ruíz JC, Albó C, Cabrera R, San Miguel JF, Conde E. High-dose therapy in diffuse large cell lymphoma: results and prognostic factors in 452 patients from the GEL-TAMO Spanish Cooperative Group. Ann Oncol. 2003;14:140–151. doi: 10.1093/annonc/mdg008. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez J, Caballero MD, Gutierrez A, Solano C, Arranz R, Lahuerta JJ, Sierra J, Gandarillas M, Perez-Simon JA, Zuazu J, Lopez-Guillermo A, Sureda A, Carreras E, Garcia-Laraña J, Marin J, Garcia JC, Fernandez de Sevilla A, Rifon J, Varela R, Jarque I, Albo C, Leon A, San Miguel J, Conde E. Autologous stem-cell transplantation in diffuse large B-cell non-Hodgkin's lymphoma not achieving complete response after induction chemotherapy: the GEL/TAMO experience. Ann Oncol. 2004;15:1504–1509. doi: 10.1093/annonc/mdh391. [DOI] [PubMed] [Google Scholar]