Abstract
Background
We investigated whether the presence of diabetes mellitus (DM) was related to the degree of the anemia in predialytic patients with renal failure and what was the most relevant factor for anemia in patients with chronic kidney disease (CKD) from DM (DM-CKD).
Methods
Seventy seven patients (47 predialytic patients with long-term type 2 DM (DM-CKD) and 30 predialytic patients whose disease was due to other causes (non DM-CKD)) were enrolled in this study. The blood hemoglobin (Hb) and hematocrit, and the creatinine, ferritin, vitamin B12, folate, iron, LDH, albumin, hs-CRP, intact-PTH, erythropoietin, leptin and Insulin-like growth factor I (IGF-1) levels were measured using standard methods. The estimated GFR was calculated using the abbreviated MDRD equation.
Results
The two groups did not significantly differ as to age, gender, the serum creatinine level and the inflammatory status. The Hb level was significantly lower in the DM-CKD patients than that in the non DM-CKD patients (8.5±1.7 g/dL vs 9.6±1.6 g/dL, respectively, p=0.01). The Hb level was significantly lower in the DM-CKD patients who were being treated with ACE inhibitors (the DM-ACE patients) than that in the non DM-CKD patients who were being treated with ACE inhibitors (the non DM-ACE patients) (8.5±1.5 g/dL vs 10.8±1.6 g/dL, respectively, p=0.001). Multiple regression analysis indicated that serum IGF-1 concentration was independently associated with the Hb level (β=0.425, p=0.02) in the DM-CKD patients.
Conclusions
The Hb concentration was significantly lower in the DM-CKD patients than that in the non DM-CKD patients. It was independently associated with the serum IGF-1 concentration in the DM-CKD patients.
Keywords: Diabetes mellitus, Kidney failure, Anemia, Insulin-like growth factor I, ACE inhibitor
INTRODUCTION
Cardiovascular complications are important factors that contribute to the increased risk of death for patients with CKD (chronic kidney disease), and especially for those patients with CKD and diabetic nephropathy. Therefore, the combination of diabetes and CKD has become a major health problem. Anemia coupled with the presence of diabetes is thought to play a major role in augmenting the cardiovascular risk profile of CKD patients1). Anemia appears at an earlier stage of CKD in patients with diabetic nephropathy than that in patients without diabetes2). Furthermore, Ishimura and colleagues3) have demonstrated that for a given level of renal dysfunction, anemia is more severe in patients with type 2 diabetes than that in matched, non-diabetic, control patients. Although the serum erythropoietin (EPO) concentrations were within the normal range in both groups, the Hb concentration of anemic diabetic patients was significantly lower than that of the patients with non-diabetic renal disease. These findings led the authors to conclude that the presence of diabetes is an independent risk factor for the development of anemia. On the contrary, there was a study that diabetes itself does not have an influence on the development of anemia4).
Anemia is a serious and frequent complication of CKD, and even in patients who are not receiving dialysis5). This has been thought to be mainly caused by a relative or an absolute deficiency of EPO production by the kidney6, 7). Recombinant EPO has been useful for the treatment of anemia of CKD patients8, 9), but some of these patients respond poorly10, 11). The anemia in such cases may be due to the short survival of red blood cells (RBC)12), the presence of unknown inhibitors of erythropoiesis in uremia patients13), hyperparathyroidism, the accumulation of aluminum and a nutritional deficiency such as iron, vitamin B12, and folate14) in CKD patients. Further, the data from several studies has indicated that factors other than EPO, such as IGF-1, can promote erythropoiesis in vitro15) and correct the anemia of chronic kidney disease in vivo16). In addition, recent data has shown that the adipokine leptin influences the EPO sensitivity in patients with ESRD17).
Therefore, we investigated whether the presence of DM was related to the severity of the anemia observed in the patients with renal failure and who were not receiving dialysis, and we also determined what was the most relevant factor for inducing anemia from diabetes in patients with CKD.
MATERIALS AND METHODS
Seventy seven Korean patients with CKD (41 males and 36 females, aged 29 to 86, mean age: 61 years), and who were regularly followed at the outpatient clinic of Chung-Ang University Hospital were enrolled in this study between March 2002 and September 2005. None of them had evidence of clinical gastrointestinal bleeding such as a history of tarry stool or a positive stool occult blood test, an established infection and/or severe microinflammation above a 5 mg/dL level of high-sensitivity C-reactive protein (hs-CRP). None of the patients had received blood transfusions or recombinant human EPO therapy for anemia. None of the patients had taken drugs that could affect erythropoiesis (such as aluminum-containing phosphate binders and immunosuppressive agents).
The serum creatinine concentration (Scr) was determined on a multiparameter analyzer (Olympus AU 640; Olympus Optical, Tokyo, Japan) with using Jaffe's method. We then estimated the glomerular filtration rate (GFR) using the abbreviated MDRD equation (aMDRD)18).
aMDRD = 186×Scr-1.154×age-0.203×1.212 (×0.742 if female).
Forty seven patients (28 males and 19 females) had CKD that was caused by long-term type 2 DM (mean age: 62±12 years, age range: 37 to 86 years) (the DM-CKD patients). The remaining 30 CKD patients (14 males and 16 females) had CKD that was due to other causes (the non DM-CKD patients) such as chronic glomerulonephritis (n=14), hypertension (n=10), polycystic kidney disease (n=1) and renal disease of an unknown cause (n=5), according to the clinical documents in the medical records.
Blood samples were taken in the morning during checkups at the outpatient clinic. The serum was frozen at -70℃ until assay. We measured patients' blood Hb and hematocrit (Hct) using an automatic blood cell counter (Coulter STKS, Coulter Electronics, Hialeah, FL, USA), and the chemistry profiles such as iron, the total iron binding capacity (TIBC), the ferritin, lactate dehydrogenase (LDH) and albumin levels were determined using an Hitachi 747 automatic analyzer (Hitachi, Tokyo, Japan). Intact parathyroid hormone (i-PTH) was measured using a commercial immunoradiometric assay (Diagnostic products corporation, Los Angeles, CA, USA) with the normal range set at 12-72 pg/mL. hs-CRP was measured using the N high sensitivity CRP kit (Dade Bering, Marburg, Germany).
Serum EPO was measured by RIA using a commercially available kit (Diagnostic Systems Laboratories, Webster, TX, USA) with rabbit anti-human EPO that was purified from urine and calibrated according to the World Health Organization 2nd International Reference guidelines. The normal range was 16.6-37.5 mU/mL, according to the manufacturer's instructions. The serum IGF-1 levels were also determined by performing RIA with a commercially available kit (Nichols Institute Diagnostics, San Juan Capistrano, CA, USA), and the kit used an acid-ethanol extraction method to dissociate IGF-1 from the IGF-1 binding proteins.
The normally distributed data was expressed as the mean±standard deviation (SD) or the mean standard error of the mean (SEM). Differences between the two groups were compared by the unpaired student t-test. The dats with a skewed distribution was expressed as medians (and the range). Differences between the two groups were compared by the Man-Whitney test with Welch's correction. Differences between multiple groups were determined by one-way ANOVA testing. Multiple regression analysis with a forward elimination procedure was used to assess the combined influence of the variables on the Hb levels. The following variables were used in a model of the analysis: age, gender, the GFR as expressed by the aMDRD, the presence of DM, the transferrin saturation (TS), the ferritin, vitamin B12, folate, LDH, albumin and hs-CRP levels, the usage of angiotensin converting enzyme (ACE) inhibitor and the serum EPO, IGF-1, leptin and i-PTH levels. Gender, the presence of DM and the usage of ACE inhibitor were represented by dummy variables (1: male, 2: female, 1: presence, 2: absence, 1: usage, 2: non-usage). Analysis was done using the Instat version 3.0 Statistical System.
RESULTS
Comparison of the parameters regarding erythropoiesis between the DM-CKD and non DM-CKD patients
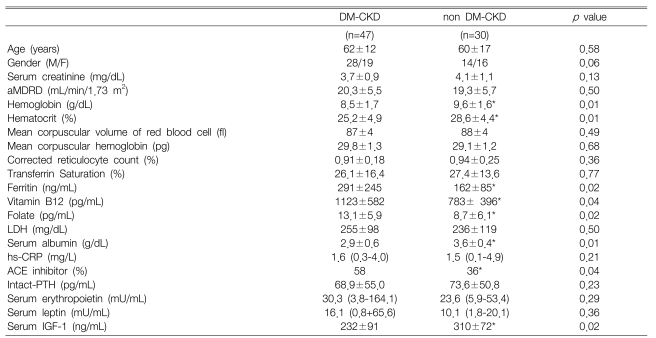
The Hb concentration and Hct were significantly lower in the DM-CKD patients than those levels in the non DM-CKD patients (8.5±1.7 g/dL vs 9.6±1.6 g/dL, p=0.01 and 25.2±4.9% vs 28.6±4.4%, p=0.01, respectively). The mean corpuscular volume and mean corpuscular hemoglobin level of the RBCs were within the normal range in both the DM and non DM-CKD patients (87±4 fl vs 88±4 fl, p=0.49 and 29.8±1.3 pg vs 29.1±1.2 pg, p=0.68, respectively), and there were no significant differences between the groups. The serum ferritin, vitamin B12 and folate levels were significantly higher in the DM-CKD patients than those in the non DM-CKD patients (291±245 mg/mL vs 162±85 ng/mL, p=0.02 and 1123±582 pg/mL vs 783±396 pg/mL, p=0.04 and 13.1±5.9 pg/mL vs 8.7±6.1 pg/mL, p=0.02, respectively). The serum EPO levels were similar in both groups [30.3 (3.8-164.1) mU/mL vs 23.6 (5.9-53.4) mU/mL, p=0.29]. On the contrary, the serum IGF-1 levels were significantly lower in the DM-CKD patients than those the non DM-CKD patients (232±91 mU/mL vs 310±72 mU/mL, respectively, p=0.02) (Table 1).
Table 1.
Comparison of the clinical characteristics between the DM CKD and non DM CKD patients
Data are means±SDs, or medians (range).
M, male; F, female; DM-CKD, chronic renal failure from diabetes mellitus; non DM-CKD, chronic renal failure from a disease other than diabetes mellitus; aMDRD, the abbreviated MDRD equation; LDH, lactate dehydrogenase; hs-CRP, high sensitivity C-reactive protein; ACE, angiotensin converting enzyme; PTH, parathyroid hormone; IGF-1, insulin-like growth factor 1.
*p<0.05 vs. DM-CKD
Comparison of Hb according to the usage of ACE inhibitors
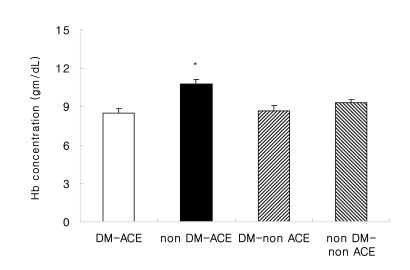
The Hb concentration was significantly lower in the DM-ACE patients than that in the non DM-ACE patients (8.5±1.5 g/dL vs 10.8±1.6 g/dL, respectively, p=0.001) (Figure 1). There was no difference of the Hb concentration in the non DM-CKD patients.
Figure 1.
Hemoglobin concentration levels with or without ACE inhibitors. Abbreviations are: DM-ACE (n=27), chronic renal failure due to diabetes mellitus receiving ACE inhibitor treatment; non DM-ACE (n=17), chronic renal failure due to a disease other than diabetes mellitus receiving ACE inhibitor treatment.; DM-non ACE (n=20), chronic renal failure due to diabetes mellitus without ACE inhibitor treatment; non DM-non ACE (n=13), chronic renal failure due to a disease other than diabetes mellitus without ACE inhibitor treatment, p=0.001 vs. DM-ACE.
Risk factors affecting the Hb levels in the DM-CKD patients and the non DM-CKD patients
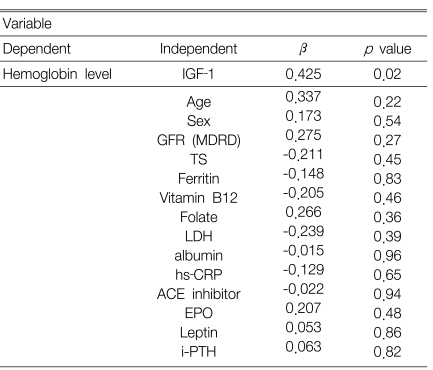
Multiple regression analysis showed that serum IGF-1 concentration was independently associated with the Hb level in the DM-CKD patients (β=0.425, p=0.02) (Table 2). However, the serum IGF-1 concentration was not associated with the Hb level in the non DM-CKD patients and in all the patients (DM-CKD and non DM-CKD) when they were grouped together (data not shown).
Table 2.
Risk factors affecting the hemoglobin levels in DM CKD patients
The following variables were used in a model of the analysis: age, gender, GFR, transferrin saturation (TS), the ferritin, vitamin B12 and folate, LDH, albumin, hs-CRP levels, usage of ACE inhibitor, and the serum EPO, IGF-1, leptin and i-PTH leves.
DISCUSSION
There have been several reports that anemia appears at an earlier stage of CKD in the patients with diabetic nephropathy than in the CKD patients without diabetes, and the anemia in DM-CKD patients becomes more severe, regardless of the type of DM2, 3). The reason why anemia occurs at an earlier stage of CKD in the patients with DM is currently unclear. A few explanations have been suggested by some authors. Up to 90% of EPO is produced by the peritubular fibroblasts of the kidneys. Interstitial damage has been observed even at an early stage in patients with diabetic nephropathy. Further, the deterioration of the renal function in DM-CKD patients is more progressive compared to the non DM-CKD patients. This results in the reduction of the renal interstitium, which correlates well with early appearance of anemia in DM-CKD patients19, 20). Hence, it is possible that EPO deficiency anemia may reflect the early renal interstitial damage that occurs in patients with diabetes. Alternatively, as the release of EPO is modulated by the sympathetic nervous system, the coexistence of autonomic neuropathy may be relevant21, 22). EPO deficiency anemia has also been reported in conjunction with autonomic neuropathy in diabetes. That is, many authors have agreed that the etiology of early and severe anemia in DM-CKD patients is related to the relative deficiency of EPO. On the contrary, another group of researchers concluded that both serum creatinine and the presence of diabetes are independent risk factors for the development of anemia, and this is irrespective of the serum EPO concentration3). According to our study that enrolled groups of patients with similar renal function, there was no significant difference of the serum EPO concentration in both groups despite the lower Hb concentration in the DM-CKD patients.
There was no evidence of chronic inflammatory disease and no clinical evidence of gastrointestinal occult bleeding in all the patients. The mean corpuscular volume and the mean corpuscular hemoglobin in the red blood cells were within the normal ranges for all the patients, suggesting that their anemia was related to chronic renal failure, but it was unrelated to a deficiency of iron, folate or vitamin B12. In addition, it was observed that the DM-CKD patients had higher levels of ferritin, vitamin B12 and folate. Metabolic and functional abnormalities of RBCs have been reported in DM patients, such as a reduction in the membrane fluidity and a decrease in Na/K ATPase23, 24). In those studies, this led to an increase in cell size, and increased osmotic fragility and hemolysis. However, we could not find any hemolytic evidence in that there were no significant differences of the serum LDH levels and the corrected reticulocyte counts between the DM-CKD patients and the non DM-CKD patients.
IGF-1 is produced by the liver and this is under the control of growth hormone (GH), as well as being influenced by other sources, including the kidney25). However, this process is dependent on appropriate insulin levels25). Thus, as a likely consequence of insulin deficiency, the patients with type 1 DM exhibit abnormalities of the GH/IGF/IGF-binding protein (IGF-BP) axis (including GH hypersecretion), reduced circulating levels of IGF-1 and elevated levels of IGFBP-127). The IGFBPs modulate IGF-1 by inhibiting its activity26). Garay-Sevilla et al27) have also reported that the free IGF-1 was lower in type 2 DM patients suffering with complications such as retinopathy and nephropathy. In our study, the serum IGF-1 level was significantly lower in the DM-CKD patients than that in the non DM-CKD patients. In addition, multiple regression analysis indicated that the serum level of IGF-1 independently contributed to the Hb level in the DM-CKD patients. A previous in-vitro study suggested an important role for IGF-1, as well as for EPO, in erythropoiesis28). Brox et al28) reported that the response to subtherapeutic dosages of both EPO and IGF-1 was comparable to the maximal response obtained with a single therapeutic dose of EPO in CKD mice. Shih et al29) have demonstrated that the serum IGF-1 levels in patients with end-stage renal disease (ESRD) and erythrocytosis were significantly increased as compared with those levels in normal subjects or ESRD patients with anemia, and this was despite there being no difference of the serum EPO level. Taken together, we can suggest that the earlier and more severe anemia in DM-CKD patients is related to their serum IGF-1 concentration. But, the serum IGF-1 concentration was not associated with the Hb level in the non DM-CKD patients and all the patients (the DM-CKD and non DM-CKD patients). This discrepancy between the DM-CKD patients and the non DM-CKD patients can not be explained from our results. So, we need to perform further studies regarding the exact role of IGF-1 in DM-CKD patients.
ACE inhibitors have been reported to reduce the Hct levels in a number of clinical settings, and use of ACE inhibitors and the reduced HCT levels has even led to an exacerbation of anemia among dialysis patients and renal transplant recipients30-32). There was a report that IGF-1 seemed to play a role in the ACE inhibitors-related decrease of the Hct in patients with posttransplant erythrocytosis, and this mainly occurred in patients without any modification of their EPO serum levels33). In our results, the DM-CKD patients receiving ACE inhibitors had a lower Hb concentration compared to the non DM-CKD patients taking ACE inhibitors. However, there was no difference of the serum IGF-1 levels in both groups (data not shown). ACE inhibitors have been routinely used to decrease proteinuria in patients with diabetic nephropathy. So, the mechanism of the low hemoglobin concentration in the patients with DM-CKD and who were taking ACE inhibitors remains to be determined.
From our results, we can conclude that the Hb concentration was significantly lower in the DM-CKD patients, and this was independently associated with the serum IGF-1 concentration, and especially in the predialysis patients. Prospective and multi-center based studies are needed to investigate the effectiveness of IGF-1 treatment in diabetic pre-dialysis patients.
Footnotes
This study was financially supported by a Chung-Ang University Research Grant, 2006.
References
- 1.Foley RN, Culleton BF, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Cardiac disease in diabetic end-stage renal disease. Diabetologia. 1997;40:1307–1312. doi: 10.1007/s001250050825. [DOI] [PubMed] [Google Scholar]
- 2.Bosman DR, Winkler AS, Marsden JT, Macdougall IC, Watkins PJ. Anemia with erythropoietin deficiency occurs early in diabetic nephropathy. Diabetes Care. 2001;24:495–499. doi: 10.2337/diacare.24.3.495. [DOI] [PubMed] [Google Scholar]
- 3.Ishimura E, Nishizawa Y, Okuno S, Matsumoto N, Emoto M, Inaba M, Kawagishi T, Kim CW, Morii H. Diabetes mellitus increases the severity of anemia in non-dialyzed patients with renal failure. J Nephrol. 1998;11:83–86. [PubMed] [Google Scholar]
- 4.European Best Practice Guidelines for the management of anemia in patients with chronic renal failure. Working Party for European Best Practice Guidelines for the Management of Anaemia in Patients with Chronic Renal Failure. Nephrol Dial Transplant. 1999;14(Suppl 5):1–50. [PubMed] [Google Scholar]
- 5.Eschbach JW, Kelly MR, Haley NR, Abels RI, Adamson JW. Treatment of the anemia of progressive renal failure with recombinant human erythropoietin. N Engl J Med. 1989;321:158–163. doi: 10.1056/NEJM198907203210305. [DOI] [PubMed] [Google Scholar]
- 6.Spivak JL. Serum immunoreactive erythropoietin in health and disease. J Perinat Med. 1995;23:13–17. doi: 10.1515/jpme.1995.23.1-2.13. [DOI] [PubMed] [Google Scholar]
- 7.Adamson JW, Eschbach J, Finch CA. The kidney and erythropoiesis. Am J Med. 1968;44:725–733. doi: 10.1016/0002-9343(68)90254-4. [DOI] [PubMed] [Google Scholar]
- 8.Winearls CG. Historical review on the use of recombinant human erythropoietin in chronic renal failure. Nephrol Dial Transplant. 1995;10(Suppl 2):3–9. doi: 10.1093/ndt/10.supp2.3. [DOI] [PubMed] [Google Scholar]
- 9.Eschbach JW, Adamson JW. Recombinant human erythropoietin: implications for nephrology. Am J Kidney Dis. 1988;11:203–209. doi: 10.1016/s0272-6386(88)80150-1. [DOI] [PubMed] [Google Scholar]
- 10.Danielson B. R HuEPO hyporesponsiveness: - who and why? Nephrol Dial Transplant. 1995;10(Suppl 2):69–73. doi: 10.1093/ndt/10.supp2.69. [DOI] [PubMed] [Google Scholar]
- 11.Drueke TB. R HuEPO hyporesponsiveness: - who and why? Nephrol Dial Transplant. 1995;10(Suppl 2):62–68. doi: 10.1093/ndt/10.supp2.62. [DOI] [PubMed] [Google Scholar]
- 12.Chaplin H, Jr, Mollison PL. Red cell life span in nephritis and in hepatic cirrhosis. Clin Sci. 1953;12:351–360. [PubMed] [Google Scholar]
- 13.Fukushima Y, Nakamoto Y, Miura AB, Miyagata S, Tsuchida S. The inhibitory factors of hematopoiesis in chronic hemodialysis patients treated with recombinant human erythropoietin. Tohoku J Exp Med. 1990;161:217–225. doi: 10.1620/tjem.161.217. [DOI] [PubMed] [Google Scholar]
- 14.Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin: results of a combined phase I and II clinical trial. N Engl J Med. 1987;316:73–78. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- 15.Correa PN, Axelrad AA. Production of erythropoietic bursts by progenitor cells from adult human peripheral blood in an improved serum-free medium: role of insulin like growth factor 1. Blood. 1991;78:2823–2833. [PubMed] [Google Scholar]
- 16.Deicher R, Horl WH. Hormonal adjuvants for the treatment of renal anemia. Eur J Clin Invest. 2005;35(Suppl 3):75–84. doi: 10.1111/j.1365-2362.2005.01533.x. [DOI] [PubMed] [Google Scholar]
- 17.Axelsson J, Qureshi AR, Heimburger O, Lindholm B, Stenvinkel P, Barany P. Body fat mass and serum leptin levels influence epoetin sensitivity in patients with ESRD. Am J Kid Dis. 2005;46:628–634. doi: 10.1053/j.ajkd.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.White KE, Bilous RW. Type 2 diabetic patients with nephropathy show structural-functional relationships that are similar to type 1 disease. J Am Soc Nephrol. 2000;11:1667–1673. doi: 10.1681/ASN.V1191667. [DOI] [PubMed] [Google Scholar]
- 20.Keane WF, Brenner BM, de Zeeuw D, Grunfeld JP, McGill J, Mitch WE, Ribeiro AB, Shahinfar S, Simpson RL, Snapinn SM, Toto R. The risk of developing end stage renal disease in patients with type 2 diabetes and nephropathy. Kidney Int. 2003;63:1499–1507. doi: 10.1046/j.1523-1755.2003.00885.x. [DOI] [PubMed] [Google Scholar]
- 21.Hoeldtke RD, Streeten DH. Treatment of orthostatic hypotension with erythropoietin. N Engl J Med. 1993;329:611–615. doi: 10.1056/NEJM199308263290904. [DOI] [PubMed] [Google Scholar]
- 22.Ghirlanda G, Cotroneo P, Todaro L, Pitocco D, Manto A, Storti S, Caputo S, Ricerca BM. Erythropoietin depletion and anemia in diabetes mellitus. Diabet Med. 2000;17:410. doi: 10.1046/j.1464-5491.2000.00277.x. [DOI] [PubMed] [Google Scholar]
- 23.Ranmani Jourdheuil D, Mourayre Y, Vague P, Boyer J, Juhan-Vague I. In vivo insulin effect on ATPase activities in erythrocyte membrane from insulin dependent diabetics. Diabetes. 1987;36:991–995. doi: 10.2337/diab.36.9.991. [DOI] [PubMed] [Google Scholar]
- 24.Bryszewska M, Watala C, Torzecka W. Changes in fluidity and composition of erythrocyte membranes and in composition of plasma lipids in type I diabetes. Br J Haematol. 1986;62:111–116. doi: 10.1111/j.1365-2141.1986.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 25.Sonksen PH, Russell-Jones D, Jones RH. Growth hormone and diabetes mellitus: a review of sixty-three years of medical research and a glimpse into the future? Horm Res. 1993;40:68–79. doi: 10.1159/000183770. [DOI] [PubMed] [Google Scholar]
- 26.Thraikill KM. Insulin-like growth factor-1 in diabetes mellitus: its physiology, metabolic effects, and potential clinical utility. Diabetes Technol Ther. 2000;2:69–80. doi: 10.1089/152091599316775. [DOI] [PubMed] [Google Scholar]
- 27.Garay-Sevilla ME, Nava LE, Malacara JM, Wróbel K, Wróbel K, Pėrez U. Advanced glycosylation end products, insulin-like growth factor-1 and IGF-binding protein-3 in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2000;16:106–113. doi: 10.3904/kjim.2007.22.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brox AG, Zhang F, Guyda H, Gagnon RF. Subtherapeutic erythropoietin and insulin-like growth factor-1 correct the anemia of chronic renal failure in the mouse. Kidney Int. 1996;50:937–943. doi: 10.1038/ki.1996.394. [DOI] [PubMed] [Google Scholar]
- 29.Shih LY, Huang JY, Lee CT. Insulin-like growth factor 1 plays a role in regulating erythropoiesis in patients with end-stage renal disease and erythrocytosis. J Am Soc Nephrol. 1999;10:315–322. doi: 10.1681/ASN.V102315. [DOI] [PubMed] [Google Scholar]
- 30.Onoyama K, Sanoi T, Motomura K, Fujishima M. Worsening of anemia by angiotensin converting inhibitors and its prevention by antiestrogenic steroid in chronic hemodialysis patients. J Cardiovasc Pharmacol. 1989;13(Suppl 3):S27–S30. doi: 10.1097/00005344-198900133-00007. [DOI] [PubMed] [Google Scholar]
- 31.Fyhrquist F, Karppinen K, Honkanen T, Saijonmaa O, Rosenlof K. High serum erythropoietin levels are normalized during treatment of congestive heart failure with enalapril. J Intern Med. 1989;226:257–260. doi: 10.1111/j.1365-2796.1989.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 32.Vlahakos DV, Canzanello VJ, Madaio MP, Madias NE. Enalapril-associated anemia in renal transplant patients treated for hypertension. Am J Kidney Dis. 1991;17:199–205. doi: 10.1016/s0272-6386(12)81129-2. [DOI] [PubMed] [Google Scholar]
- 33.Morrone LF, Di Paolo S, Logoluso F, Schena A, Stallone G, Giorgino F, Schena FP. Interference of angiotensin-converting inhibitors on erythropoiesis in kidney transplant recipients: role of growth factors and cytokines. Transplantation. 1997;64:913–918. doi: 10.1097/00007890-199709270-00021. [DOI] [PubMed] [Google Scholar]