Abstract
Through the use of surrogate markers of efficacy, neoadjuvant studies may facilitate the implementation of new treatments into clinical practice. However, disease-free survival is the current standard outcome endpoint for registration of a novel treatment. The coupling of smaller neoadjuvant 'proof of principle' studies with larger adjuvant registration trials offers the promise of speeding up the time to market of new therapies. Clever new designs, such as the 'biological window' and 'learn on the way', can provide valuable insight regarding mechanisms of action and resistance of these novel drugs by identifying patients who are most likely to respond to a novel therapy early in the drug development process. Using the ongoing neoadjuvant trials with HER2 (human epidermal growth factor receptor 2)-directed therapy as a paradigm, this article discusses recent innovations in study design and the challenges of conducting translational research in the neoadjuvant setting.
Introduction
Neoadjuvant chemotherapy is the standard of care for patients with locally advanced and inflammatory breast cancer (IBC) [1], with the goal of improving operability and eradicating micrometastatic disease. In primary operable breast cancer, neoadjuvant chemotherapy increases the rate of breast conservation [2] and achieves similar overall survival (OS) as adjuvant chemotherapy [3] without compromising local control provided that careful multidisciplinary coordination is planned from the outset [4]. Currently, the same regimens used for adjuvant treatment are recommended for neoadjuvant therapy [5]. The initiation of chemotherapy prior to surgery allows for the identification of two distinct prognostic groups: patients able to attain a pathologic complete response (pCR) with a favourable long-term outcome and those with residual disease at surgery who are at a high risk of relapse [6]. Unfortunately, there is no additional therapy that has been shown to improve survival for patients failing to achieve a pCR to an anthracycline-taxane regimen [7] and this group of chemoresistant patients is desperately in need of novel therapeutic options.
There are many unresolved clinical questions regarding the use of neoadjuvant chemotherapy and additional locoregional treatment [5,6]. Well-designed neoadjuvant studies can quickly generate important preliminary data on the efficacy of novel therapies based on short-term endpoints such as pCR. They also offer an excellent opportunity to study the impact of systemic therapies on breast cancer biology, to explore surrogate markers of response (such as functional imaging), and to select promising biomarkers for future validation studies. The traditional model of conducting initial trials in metastatic breast cancer patients with refractory disease following standard therapy has been disappointing, as it demands a lot of effort and requires a long time before the drug reaches adjuvant registration. In addition, many new agents thought to be successful at controlling minimal residual disease have failed to demonstrate the efficacy for patients with advanced disease. As a result, a new pathway to accelerate the clinical development of emerging therapies is sorely needed.
The identification of the central role of the human epidermal growth factor receptor 2 (HER2) protein in the pathogenesis of HER2-overexpressing breast cancer is one of the greatest successes of modern oncology. Over the last 10 years, trastuzumab, a monoclonal antibody against the HER2 protein, has been approved for the treatment of HER2+ breast cancer, and a variety of exciting novel anti-HER2-directed therapies that are entering clinical testing have been developed. As such, HER2+ breast cancer represents the ideal paradigm for a discussion of targeted neoadjuvant breast cancer therapy. The purpose of this article is to review ongoing neoadjuvant randomised clinical trials in HER2+ breast cancer evaluating novel HER2-directed agents, with an emphasis on recent innovations in trial design, platforms for the evaluation of surrogate endpoints and translational research, and the challenges in conducting neoadjuvant research.
Search strategy
Ongoing clinical trials were identified using the ClinicalTrials.gov database on 22 May 2008 [8]. With the search terms 'neoadjuvant breast cancer' and 'HER2 positive', 29 studies were recognised; with the terms 'preoperative' and 'HER2 positive', no additional studies were identified. The selection was limited to neoadjuvant randomised clinical trials that include HER2+ breast cancer. Trials investigating trastuzumab as the only HER2 agent were excluded from the review as we wished to focus on the neoadjuvant trials introducing novel therapies for early breast cancer. With this strategy, nine studies were identified and these are listed in Table 1 and Figure 1. Information regarding clinical trial design was largely collected from the ClinicalTrials.gov library; additional data (where available) were retrieved from the ClinicalTrials.gov (PDQ®) database of the National Cancer Institute (Bethesda, MD, USA) and through personal communication with the principal investigators of individual studies.
Table 1.
Ongoing randomised neoadjuvant studies targeting HER2 with new agents
| ClinicalTrials.gov identifier | Sponsor and participating groups | Investigational drugs | Phase | Estimated enrolment | Status | Start/Completion | Primary endpoint | Translational research | Patient eligibility (all HER2+) |
| NCT00486668 [16] | NSABP | Lapatinib Trastuzumab | III | 522 | Recruiting | July 2007 to July 2014a | - pCR (breast only) | GEP | ER+/- PR+/- |
| NCT00499681 [17] | Vanderbilt-Ingram Cancer Center (Nashville, TN, USA) NCI | Lapatinib Letrozole | II | 36 | Recruiting | July 2007 to July 2010 | - Change in percentage of Ki67+ tumour cells – pCR | Biomarkersb Imaging | ER+ and/or PR+ |
| NCT00450892 [18] | EORTC | Lapatinib Trastuzumab | I–II | 114 | Recruiting | Feb. 2007 to Dec. 2009 | - pCRc – Safety and tolerability – RR – Phdinam. |
GEP Phdinam. | ER+/- PR+/- Inflammatory BC Included |
| NCT00567554 GeparQuinto [19] | GBG AGO Ovarian Cancer Study Group | Lapatinib Trastuzumab | III | 2,547 (all patients) | Recruiting | Oct. 2007 to Dec. 2015 | - pCR | Biomarkers CTCs Imaging Phgen. | ER+/- PR+/- Inflammatory BC Included |
| NCT00674414 [20] | Fédération Nationale des Centres de Lutte Contre le Cancer (Paris, France) | Everolimus Trastuzumab | II | 120 | Recruiting | Apr. 2008 to Apr. 2014 | - RR (clinical and echographic) | Phgen. Phdinam. Proteomics Biomarkers | ER+/- PR+/- |
| NCT00545688 [21] | Hoffmann-La Roche (Grenzach-Wyhlen, Germany) | Pertuzumab Trastuzumab | II | 400 | Recruiting | Dec. 2007 to Oct. 2014 | - pCR | NR | ER+/- PR+/- Inflammatory BC Included |
| NCT00524303 [22] | GSK | Trastuzumab Lapatinib | II | 99 | Recruiting | Sept. 2007 to July 2013 | - Pathologic RR | Biomarkers | ER+/- PR+/- Inflammatory BC Included |
| NCT0053358 [23] | GSK BIG Grupo Espaniol de Estudio Tratamiento y Otras Estrategias Experimentales en Tumores Solidos (Madrid, Spain) | Trastuzumab Lapatinib | III | 450 | Recruiting | Sept. 2007 to Sept. 2019 | - pCR (breast only) | Biomarkers Imaging CTCs | |
| NCT00548184 [24] | Baylor Breast Cancer Center (Houston, TX, USA) GSK | Lapatinib Trastuzumab | II | 64 | Not yet recruiting | Jan. 2008 to Jan. 2012 | Biomarkers | Biomarkers | ER+/- PR+/- |
aIncluding the follow-up period; btranslational research described as biomarkers in which no additional information about the techniques was given; conly for phase II part. AGO, Arbeitsgemeinschaft Gynäkologische Onkologie; BC, breast cancer; BIG, Breast International Group (Brussels, Belgium); CTCs, circulating tumour cells; EORTC, European Organisation for Research and Treatment of Cancer; ER, oestrogen receptor; GBG, German Breast Group (Neu-Isenburg, Germany); GEP, gene expression profiling; GSK, GlaxoSmithKline (Uxbridge, Middlesex, UK); HER2, human epidermal growth factor receptor 2; NCI, National Cancer Institute (Bethesda, MD, USA); NSABP, National Surgical Adjuvant Breast and Bowel Project; NR, not reported; pCR, pathologic complete response; Phdinam., pharmacodynamics; Phgen., pharmacogenetics; PR, progesterone receptor; RR, response rate.
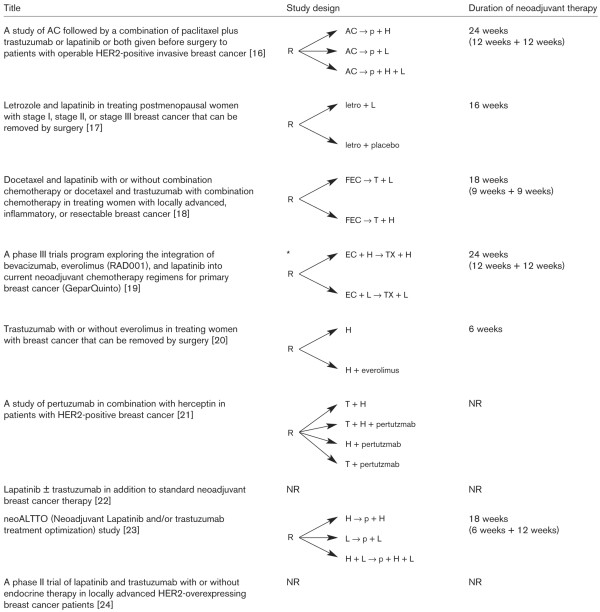
Figure 1.
Trial design of ongoing randomised neoadjuvant studies targeting HER2 with new agents. *Only the randomization for HER2 positive patients is presented. AC, doxorubicin-cyclophosphamide; EC, epirubicin-cyclophosphamide; FEC, 5-fluoruracil-epirubicin-cyclophosphamide; H, trastuzumab; HER2, human epidermal growth factor receptor 2; L, lapatinib; letro, letrozole; NR, not reported; p, paclitaxel; R, randomisation, T, docetaxel; X, capecitabine.
Prior randomised neoadjuvant studies in HER2+ disease
Even in the absence of targeted therapy against the HER2 signalling axis, HER2+ breast cancer demonstrates a higher rate of pCR to traditional neoadjuvant chemotherapy [9]. A retrospective single-series study suggests that patients with HER2+ disease who experience a pCR to neoadjuvant chemotherapy (without anti-HER2 therapies) may experience a better disease-free survival (DFS) with long-term follow-up [10]. Three randomised studies in the neoadjuvant setting have evaluated the additional of trastuzumab to standard therapy (Table 2). After 42 of a planned 165 patients had been accrued, the M. D. Anderson study was initially stopped because the pCR rate with trastuzumab added to paclitaxel followed by 5-fluoruracil-epirubicin-cyclophosphamide (P → FEC) chemotherapy was 65% versus 25% with chemotherapy alone [11,12]. The larger NeOAdjuvant Herceptin (NOAH) trial reported similar findings with trastuzumab added to doxorubicin-paclitaxel followed by paclitaxel followed by cyclophosphamide-methotrexate-5-fluoruracil (AP → P → CMF) chemotherapy [13]. Interestingly, both of these studies administered anthracycline chemotherapy concurrently with trastuzumab and did not report a high rate of observed cardiac toxicity. However, the 16% rate of clinical grade 3/4 congestive heart failure observed in the pivotal first-line metastatic trial with concurrent trastuzumab and doxorubicin-cyclophosphamide (AC) would suggest that this approach should not be employed outside of a clinical trial setting [14]. More recently, the GeparQuattro study evaluating epirubicin, cyclophosphamide, and docetaxel with or without capecitabine and/or trastuzumab before surgery reported a similar doubling in the observed pCR rate with the addition of trastuzumab as seen in the NOAH study [15], using a more conventional schedule of initiating trastuzumab after the completion of anthracycline therapy.
Table 2.
Reported randomised phase III trials with neoadjuvant trastuzumab
| pCR rate, percentage (95% CI) | |||||||
| Reference | Number of patients | Patient population | Design | HER2 assessment | No H | With H | P value |
| Buzdar et al., 2005 [11], 2007 [12] | 42 | 65% T2 40% N0/57% N1 | P → FEC vs. P + H → FEC + H | IHC 3+ or FISH+ | 26 (9–51) | 65 (43–84) | NS |
| Gianni et al., 2007 [13] | 228 | 60% T4 85% N+ | AP → P → CMF vs. AP + H → P + H → CMF + H | IHC 3+ or FISH | 23 (NR) | 43 (NR) | 0.002 |
| Untch et al., 2008 [15] | 453 | NA | EC → D or EC → DX or EC → D → X vs. EC → D + H or EC → DX + H or EC → D + H → X + H | NA | 20 (NR) | 41 (NR) | <0.001 |
C, cyclophosphamide; CI, confidence interval; D, docetaxel; E, epirubicin; F, 5-fluoruracil; FISH, fluorescence in situ hybridization; H, trastuzumab; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; M, methotrexate; N, nodal status; NA, not applicable; NR, not reported; NS, not significant; P, paclitaxel; pCR, pathologic complete response; T, tumour size; X, capecitabine.
Designs of the ongoing randomised neoadjuvant studies in HER2+ disease
The majority of ongoing HER2-targeted trials are investigating the efficacy of lapatinib in the neoadjuvant setting for HER2+ breast cancer (Table 1) [16-24]. Most of these studies use pCR as a primary endpoint. pCR has been proven to correlate with survival endpoints (DFS and OS) in neoadjuvant chemotherapeutic trials [25,26] but its precise definition is still debated. Whereas early trials regarded pCR as the absence of tumour on pathologic slides in breast and axillary lymph nodes, some of the later trials defined pCR as the absence of tumour in breast only without considering nodal evaluation in the operative specimen. In addition, definitions of pCR in neoadjuvant trials do not consistently account for the presence of minimal residual cellularity and residual in situ carcinoma. Recently, it was shown that the extent of residual breast cancer burden, calculated as a continuous index based on primary tumour measurements (size and cellularity) and lymph node metastases (number and size), correlates with survival outcomes [27]. Therefore, it is clear that a unified definition of pathologic response for neoadjuvant trials is required, much like the recent consensus statement regarding standard efficacy endpoints (STEEP) for adjuvant trials in early-stage disease [28]. Unfortunately, the definitions of response endpoints in the ongoing neoadjuvant trials with novel anti-HER2 agents are inconsistent (Table 1). It should be noted that there is even less evidence regarding the correlation between the extent of residual breast cancer burden following neoadjuvant targeted therapy (with or without chemotherapy) and survival [12,29].
At the present time, pCR is not robust enough to replace survival as an endpoint for the registration of novel therapies. Even though pCR appears to identify a subgroup with a favourable prognosis [6,25,30], therapies that improve the rate of pCR do not necessarily translate into long-term differences in survival, as demonstrated by the addition of taxane therapy in the NSABP (National Surgical Adjuvant Breast and Bowel Project) B-27 trial [6]. However, the evaluation of pCR in neoadjuvant studies can provide a critical early marker of efficacy, especially if a neoadjuvant study is coupled with a larger adjuvant registration trial using survival as its primary endpoint, such as in the case of the ongoing neoALTTO (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) and the ALTTO (Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) trials. A similar model has been employed by the recently completed neo-tAnGo and tAnGo trials in nonselected populations [31]. Adjuvant registration trials require an enormous financial and patient investment to detect small differences in long-term outcome. A well-designed neoadjuvant study can rapidly provide a 'go/no go' decision for an emerging therapy. Invaluable data for the appropriate selection of patients and the evaluation of endpoints for a subsequent adjuvant registration trial can be generated by a well-designed neoadjuvant pilot trial: this concept is currently being developed and explored by the Breast International Group (Brussels, Belgium).
The proliferation marker Ki67 is another marker of interest to be used as a surrogate marker for efficacy, especially with endocrine therapy [17,32,33]. Declines in Ki67 after 2 weeks of neoadjuvant treatment showed no difference between tamoxifen and the combination of tamoxifen and anastrozole in the IMPACT (Immediate Preoperative 'Arimidex' [anastro-zole], Tamoxifen, or Arimidex Combined with Tamoxifen) trial, while a significantly greater drop was found in the anastrazole-alone arm [33], mirroring the DFS results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial [34]. If these findings had been known before the launch of the ATAC trial, the combination arm likely would have been dropped from the study design from the outset, thereby saving considerable financial and patient resources.
The eligibility criteria for the ongoing neoadjuvant anti-HER2 studies are similar. With the exception of the National Cancer Institute study [35] that includes only patients with hormone receptor-positive (HR+) and HER2+ breast cancer, all other studies include HR+ and HR-disease. Although HR+ and HR- HER2+ breast cancer may exhibit different clinical behaviour [36], the joint inclusion of HER2+disease regardless of HR status in the evaluation of novel anti-HER2 therapy is justified, as trastuzumab has been shown to be effective regardless of the expression of hormonal receptors [37].
Perhaps more controversial is the joint inclusion of IBC together with locally advanced disease in the same study, as IBC represents a separate entity with distinct epidemiology, biology, and long-term outcome [38]. Although gene expression profiling (GEP) has identified the same five subtypes of IBC as originally described for noninflammatory breast cancer, differences in several key pathways and proteins do exist [39]. IBC should be evaluated in separate clinical trials or at least stratification should be planned at the time of randomisation.
In contrast to the classical design, in which an experimental therapy is compared with a standard therapy, examples of novel designs are available amongst the ongoing anti-HER2 neoadjuvant studies: the so-called 'biological window' design and 'learn on the way' design. The biological window design exposes the patients to a short period of therapy with the drug of interest alone to allow for the evaluation of biologic endpoints. This design can also provide valuable insight regarding drug pharmacodynamics and early evidence of drug activity and highlight potential mechanisms of resistance. As this phase of design is purely for research purposes and does not offer patients direct therapeutic benefit, there are ethical concerns that must be respected [40]. A short biological window period can be followed by a more classical design comparing standard neoadjuvant therapy with new combinations, including targeted therapy with curative intent, such as in the neoALTTO trial (Figure 2). In the future, these studies may lead to a reduction in overtreatment with chemotherapy, as they may identify patients with excellent response to targeted therapy alone that can be evaluated in a future chemotherapy-sparing study. Though feasible, such neoadjuvant studies require careful planning, enormous logistical support for material collection, and efficient screening systems to identify appropriate patients, all of which significantly increase the complexity and cost of conducting such research.
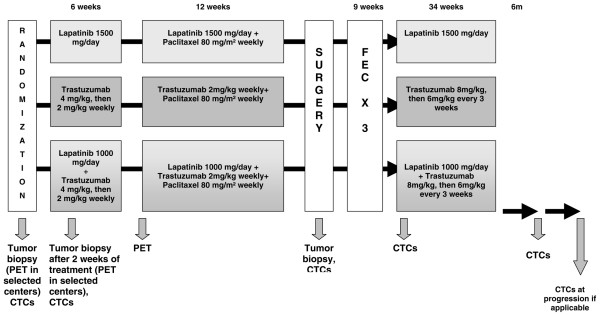
Figure 2.
neoALTTO (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) study design. CTCs, circulating tumour cells; FEC, 5-fluoruracil-epirubicin-cyclophosphamide; PET, positron emission tomography. Note: In the combined Lapatinib + Trastuzumab + Paclitaxel arm, there is a protocol amendment pending approval to reduce the Lapatinib dose to 750 mg/day because of concerns regarding excess diarrhea. Reprinted with permission from GlaxoSmithKline and SOLTI (Spanish Breast Cancer Cooperative Group).
The 'learn on the way' design uses information gained from an initial treatment period to guide decisions regarding further therapy. A patient who does not demonstrate early clinical response at interim evaluation is unlikely to experience pCR with completion of standard neoadjuvant chemotherapy [41,42]. Thus, early-response evaluation can identify a subgroup of patients with poor long-term outcome, providing the opportunity to explore a switch to an alternative therapy to improve the likelihood of pCR. To date, studies evaluating neoadjuvant taxane [41] and capecitabine-vinca alkaloid [42] combinations in nonselected populations have failed to demonstrate a benefit for patients who do not respond to anthracycline-based therapy. The GeparQuinto study described in Figures 3, 4, 5 is an example of one such 'learn on the way' approach [7]. The 'learn on the way' design is an excellent opportunity to reduce overtreatment and validate surrogate markers of response. Unfortunately, in the GeparQuinto study, only the nonresponding HER2- cohort will undergo a second randomisation based upon early response, whereas in the HER2+ cohort early response is not used to inform further decision-making. In the future, 'learn on the way' designs based upon biomarker endpoints rather than tumour shrinkage may be employed, although there are important challenges regarding the standardisation of cutoff values and interlaboratory reproducibility, along with the need for prompt assessment must be addressed using such a dynamic approach.
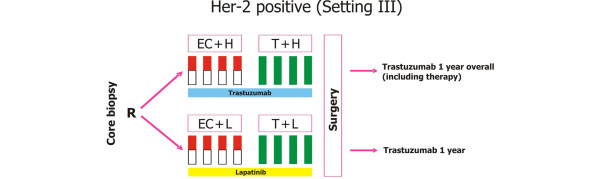
Figure 3.
Decision tree from GeparQuinto study. B, bevacizumab; EC, epirubicin-cyclophosphamide; H, trastuzumab; Her-2, human epidermal growth factor receptor 2; L, lapatinib; Pw, paclitaxel weekly; T, docetaxel. Reprinted with permission from GBG (German Breast Group).
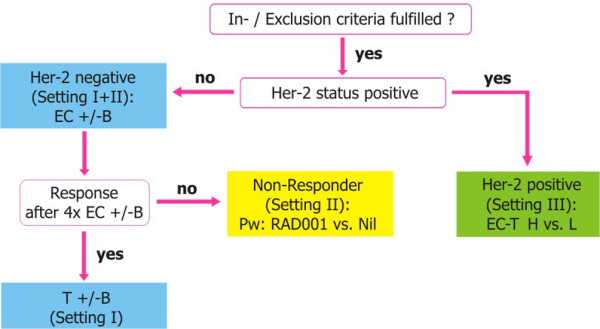
Figure 4.
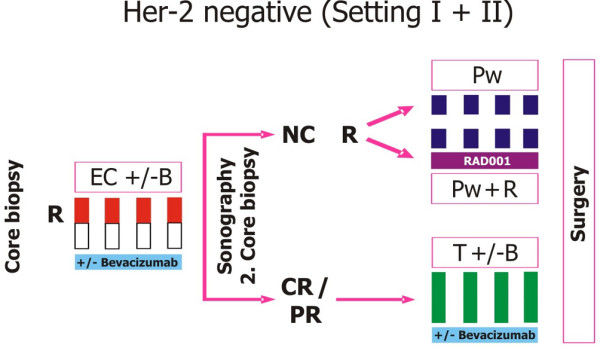
GeparQuinto study design for HER2-negative cohort. B, bevacizumab (15 mg/kg intravenously: day 1 q day 21 for eight cycles); C, cyclophosphamide (600 mg/m2: day 1 q day 21 for four cycles); CR, complete response; E, epirubicin (90 mg/m2: every 3 weeks for four cycles); Her-2, human epidermal growth factor receptor 2; NC, no change; PR, partial response; Pw, paclitaxel weekly (80 mg/m2: weekly for 12 weeks total); Pw + R, paclitaxel weekly + RAD001 (5 mg daily); R, randomisation; T, docetaxel (100 mg/m2: day 1 q day 21 for four cycles). Reprinted with permission from GBG (German Breast Group).
Figure 5.
GeparQuinto study design for HER2-positive cohort. C, cyclophosphamide (600 mg/m2: day 1 q day 21 for four cycles); E, epirubicin (90 mg/m2: every 3 weeks for four cycles); H, trastuzumab (8 mg/kg: loading dose, 6 mg/kg: every 3 weeks); Her-2, human epidermal growth factor receptor 2; L, lapatinib (1,250 mg daily for 24 weeks: run-in phase cycles 1 and 5: 1,000 mg daily); R, randomisation; T, docetaxel (100 mg/m2: every 3 weeks for four cycles). Reprinted with permission from GBG (German Breast Group).
Translational research
The neoadjuvant setting is an ideal platform to evaluate the predictive value of biomarkers using emerging technologies like GEP, proteomics, functional imaging, circulating tumour cells (CTCs), and the role of the host in the response to therapy (pharmacodynamic and pharmacogenetic substudies) (Table 1). Traditionally, translational research in breast cancer has been characterised by efforts to validate single predictive biomarkers using available tumour blocks from completed clinical trials, with largely disappointing results. With new techniques like GEP and biomarker models [43], several putative markers can be linked together to obtain a more powerful prognostic or predictive tool. The biological window design provides an especially valuable opportunity for the rapid evaluation of pharmacodynamic endpoints with biologic therapy.
Imaging modalities have not reliably been shown to predict the response to neoadjuvant therapy. This is, in part, because previous validation studies have been conducted in an incomplete and piecemeal manner. Given the rapidly expanding array of new imaging modalities, well-planned neoadjuvant studies with prospective integration of imaging endpoints are required to define and compare the role of molecular and functional imaging as early-response predictors. In contrast to traditional imaging modalities based upon anatomic evaluation of response, functional modalities such as magnetic resonance imaging [44-47], magnetic resonance spectroscopy [48], positron emission tomography [49,50], single photon emission computed tomography [51], ultrasound with enhancement [52], and optical imaging [53] have all shown the ability to predict early response and warrant prospective evaluation in phase III clinical trials. In particular, their ability to predict an early response using anti-HER2 therapy still needs to be demonstrated prospectively.
CTCs can be identified in the blood of 10% to 30% of patients with early breast cancer and their detection is associated with poor long-term DFS [54-56]. Currently, their prognostic and predictive value is also being investigated in neoadjuvant trials exploring the efficacy of novel anti-HER2 drugs (Table 1). Several methods of CTC detection have been described, although reverse transcription-polymerase chain reaction and immunomagnetic/fluorescent approaches are the most advanced [57,58].
Studies exploring the efficacy of novel anti-HER2 drugs not only are tumour-orientated but also will evaluate the role of the host with prospective pharmacogenetic and pharmacodynamic translational research studies. These studies will provide us with valuable knowledge regarding interindividual differences in drug metabolism and the efficacy of novel agents [59,60].
Translational research rarely leads directly to the registration of novel therapies, making it difficult to justify the high costs of adequately powered translational research to industrial trial sponsors [61]. However, innovative neoadjuvant translational research may identify patients likely to benefit from novel therapies that may ultimately reduce the number of participants needed for a subsequent registration study in the adjuvant setting as well as increase the probability of detecting a beneficial effect due to more accurate patient selection. As the examples of trastuzumab for breast cancer and gefitinib for lung cancer treatment illustrate, knowledge of the appropriate patient population for a novel targeted therapy can make the difference between a blockbuster drug and a drug that never makes it to market [62-66].
Conclusion
Ongoing neoadjuvant studies exploring the efficacy of novel anti-HER2 agents with innovative designs, like the 'biological window' and 'learn on the way', promise to deliver new knowledge of breast cancer biology and treatment. In the future, a greater collaborative effort between research groups is required to translate the new insights regarding the molecular heterogeneity of breast cancer into individualised therapies. Past failures have been marred by duplicative trial designs, poorly planned translational research, and overestimation of the benefit of experimental therapy in unselected populations. A new partnership between academic investigators, industry, patients, and policy-makers is needed, with a common understanding that properly conducted innovative neoadjuvant research can transform the dream of tailored therapy for breast cancer into reality.
Abbreviations
ATAC: Arimidex, Tamoxifen, Alone or in Combination; CTC: circulating tumour cell; DFS: disease-free survival; GEP: gene expression profiling; HER2: human epidermal growth factor receptor 2; HR: hormone receptor; IBC: inflammatory breast cancer; neoALTTO: Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimisation; NOAH: NeOAdjuvant Herceptin; OS: overall survival; pCR: pathologic complete response.
Competing interests
FC has received unrestricted research grants from Hoffmann-La Roche (Grenzach-Wyhlen, Germany) and GlaxoSmithKline (Uxbridge, Middlesex, UK) and has participated in advisory boards for GlaxoSmithKline. MP has received honoraria from Hoffmann-La Roche, GlaxoSmithKline, AstraZeneca (London, UK), and Novartis (Basel, Switzerland). The other authors declare that they have no competing interests.
References
- Chia S, Swain SM, Byrd DR, Mankoff DA. Locally advanced and inflammatory breast cancer. J Clin Oncol. 2008;26:786–790. doi: 10.1200/JCO.2008.15.0243. [DOI] [PubMed] [Google Scholar]
- Bonadonna G, Valagussa P, Brambilla C, Ferrari L, Moliterni A, Terenziani M, Zambetti M. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol. 1998;16:93–100. doi: 10.1200/JCO.1998.16.1.93. [DOI] [PubMed] [Google Scholar]
- Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- Buchholz TA, Lehman CD, Harris JR, Pockaj BA, Khouri N, Hylton NF, Miller MJ, Whelan T, Pierce LJ, Esserman LJ, Newman LA, Smith BL, Bear HD, Mamounas EP. Statement of the science concerning locoregional treatments after preoperative chemotherapy for breast cancer: a National Cancer Institute conference. J Clin Oncol. 2008;26:791–797. doi: 10.1200/JCO.2007.15.0326. [DOI] [PubMed] [Google Scholar]
- Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, Buzdar AU, Smith IE, Symmans WF, Singh B, Winer EP. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008;26:814–819. doi: 10.1200/JCO.2007.15.3510. [DOI] [PubMed] [Google Scholar]
- Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, Tamkus D, King KM, Pajon ER, Wright MJ, Robert J, Paik S, Mamounas EP, Wolmark N. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- von Minckwitz G, Blohmer JU, Raab G, Löhr A, Gerber B, Heinrich G, Eidtmann H, Kaufmann M, Hilfrich J, Jackisch C, Zuna I, Costa SD, German Breast Group In vivo chemosensitivity-adapted preoperative chemotherapy in patients with early-stage breast cancer: the GEPARTRIO pilot study. Ann Oncol. 2005;16:56–63. doi: 10.1093/annonc/mdi001. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov homepage http://clinicaltrials.gov
- Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, Morandi P, Fan C, Rabiul I, Ross JS, Hortobagyi GN, Pusztai L. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- Penault-Llorca F, Abrial C, Mouret-Reynier M-A, Raoelfils I, Durando X, Leheurteur M, Gimbergues P, Tortochaux J, Cure H, Chollet P. Achieving higher pathological complete response rates in HER-2-positive patients with induction chemotherapy without trastuzumab in operable breast cancer. Oncologist. 2007;12:390–396. doi: 10.1634/theoncologist.12-4-390. [DOI] [PubMed] [Google Scholar]
- Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano SH, Cristofanilli M, Frye DK, Smith TL, Hunt KK, Singletary SE, Sahin AA, Ewer MS, Buchholz TA, Berry D, Hortobagyi GN. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- Buzdar AU, Valero V, Ibrahim NK, Francis D, Broglio KR, Theriault RL, Pusztai L, Green MC, Singletary SE, Hunt KK, Sahin AA, Esteva F, Symmans WF, Ewer MS, Buchholz TA, Hortobagyi GN. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13:228–233. doi: 10.1158/1078-0432.CCR-06-1345. [DOI] [PubMed] [Google Scholar]
- Gianni L, Semiglazov V, Manikhas GM, Eiermann W, Lluch A, Tjulandin S, Feyereislova A, Vanhauwere B, Valagussa P, Baselga J. Neoadjuvant trastuzumab in locally advanced breast cancer (NOAH): antitumour and safety analysis. 2007 ASCO Annual Meeting Proceedings 43rd American Society of Clinical Oncology Annual Meeting; 1–5 June 2007; Chicago, IL. Abstract 532.
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, Bauerfeind I, Hilfrich J, Mehta K, von Minckwitz G. Neoadjuvant treatment of HER2 overexpressing primary breast cancer with trastuzumab given concomitantly to epirubicin/cyclophosphamide followed by docetaxel ± capecitabine. First analysis of efficacy and safety of the GBG/AGO multicenter intergroup-study 'GeparQuattro'. Presented at: 6th European Breast Cancer Conference; 15–19 April 2008; Berlin, Germany. Abstract 1LB.
- Schmid P, Untch M, Kossé V, Bondar G, Vassiljev L, Tarutinov V, Lehmann U, Maubach L, Meurer J, Wallwiener D, Possinger K. Leuprorelin acetate every-3-months depot versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant treatment in premenopausal patients with node-positive breast cancer: the TABLE study. J Clin Oncol. 2007;25:2509–2515. doi: 10.1200/JCO.2006.08.8534. [DOI] [PubMed] [Google Scholar]
- Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE, Jr, Wickerham DL, Wolmark N. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- Hudis C, Modi S. Preoperative chemotherapy for breast cancer: miracle or mirage? JAMA. 2007;298:2665–2667. doi: 10.1001/jama.298.22.2665. [DOI] [PubMed] [Google Scholar]
- Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC., Jr American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- Trastuzumab with or without everolimus in treating women with breast cancer that can be removed by surgery http://www.clinicaltrials.gov/ct2/show/NCT00674414?term=nct 00674414&rank=1
- Minna JD, Girard L, Xie Y. Tumor mRNA expression profiles predict responses to chemotherapy. J Clin Oncol. 2007;25:4329–4336. doi: 10.1200/JCO.2007.12.3968. [DOI] [PubMed] [Google Scholar]
- Roché H, Fumoleau P, Spielmann M, Canon JL, Delozier T, Serin D, Symann M, Kerbrat P, Soulié P, Eichler F, Viens P, Monnier A, Vindevoghel A, Campone M, Goudier MJ, Bonneterre J, Ferrero JM, Martin AL, Genève J, Asselain B. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol. 2006;24:5664–5671. doi: 10.1200/JCO.2006.07.3916. [DOI] [PubMed] [Google Scholar]
- Goldstein L, O'Neill A, Sparano J, Perez E, Shulman L, Martino S, Davidson N. E2197: Phase III AT (doxorubicin/docetaxel) vs. AC (doxorubicin/cyclophosphamide) in the adjuvant treatment of node positive and high risk node negative breast cancer. 2005 ASCO Annual Meeting Proceedings 41st American Society of Clinical Oncology Annual Meeting; 13–17 May 2005; Orlando, FL. Abstract 512.
- Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, Ingle JN, Cooper MR, Hayes DF, Tkaczuk KH, Fleming G, Holland JF, Duggan DB, Carpenter JT, Frei E, 3rd, Schilsky RL, Wood WC, Muss HB, Norton L. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- Bear HD, Anderson S, Smith RE, Geyer CE, Jr, Mamounas EP, Fisher B, Brown AM, Robidoux A, Margolese R, Kahlenberg MS, Paik S, Soran A, Wickerham DL, Wolmark N. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24:2019–2027. doi: 10.1200/JCO.2005.04.1665. [DOI] [PubMed] [Google Scholar]
- Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, Margolese RG, Cruz AB, Jr, Hoehn JL, Lees AW, Dimitrov NV, Bear HD. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M, Buzdar AU, Singletary SE, Hortobagyi GN, Pusztai L. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman JA, Sparano JA, Hunsberger S, Enos RA, Gelber RD, Zujewski JA. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- Coudert BP, Largillier R, Arnould L, Chollet P, Campone M, Coeffic D, Priou F, Gligorov J, Martin X, Trillet-Lenoir V, Weber B, Bleuse JP, Vasseur B, Serin D, Namer M. Multicenter phase II trial of neoadjuvant therapy with trastuzumab, docetaxel, and carboplatin for human epidermal growth factor receptor-2-overexpressing stage II or III breast cancer: results of the GETN(A)-1 trial. J Clin Oncol. 2007;25:2678–2684. doi: 10.1200/JCO.2006.09.9994. [DOI] [PubMed] [Google Scholar]
- Thomas E, Holmes FA, Smith TL, Buzdar AU, Frye DK, Fraschini G, Singletary SE, Theriault RL, McNeese MD, Ames F, Walters R, Hortobagyi GN. The use of alternate, non-cross-resistant adjuvant chemotherapy on the basis of pathologic response to a neoadjuvant doxorubicin-based regimen in women with operable breast cancer: long-term results from a prospective randomized trial. J Clin Oncol. 2004;22:2294–2302. doi: 10.1200/JCO.2004.05.207. [DOI] [PubMed] [Google Scholar]
- Poole CJ, Hiller L, Howard HC, Dunn JA, Canney P, Wardley AM, Kennedy MJ, Coleman RE, Leonard RC, Earl HM, tAnGo trial collaborators tAnGo: a randomized phase III trial of gemcitabine (gem) in paclitaxel-containing, epirubicin/cyclophosphamide-based, adjuvant chemotherapy (CT) for women with early-stage breast cancer (EBC) 2008 ASCO Annual Meeting Proceedings 44th American Society of Clinical Oncology Annual Meeting; 1–5 June 2008; Chicago, IL. Abstract 532.
- Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, A'Hern R, Salter J, Detre S, Hills M, Walsh G. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007;99:167–170. doi: 10.1093/jnci/djk020. [DOI] [PubMed] [Google Scholar]
- Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, Griffith C, Boeddinghaus I, Salter J, Detre S, Hills M, Ashley S, Francis S, Walsh G, IMPACT Trialists Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res. 2005;11(2 Pt 2):951s–958s. [PubMed] [Google Scholar]
- Baum M, Buzdar A, Cuzick J, Forbes J, Houghton J, Howell A, Sahmoud T. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of post-menopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer. 2003;98:1802–1810. doi: 10.1002/cncr.11745. [DOI] [PubMed] [Google Scholar]
- Letrozole and lapatinib in treating postmenopausal women with stage I, stage II, or stage III breast cancer that can be removed by surgery http://clinicaltrials.gov/ct2/show/NCT00499681
- Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, Bellon JR, Wong JS, Smith BL, Harris JR. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- Untch M, Gelber RD, Jackisch C, Procter M, Baselga J, Bell R, Cameron D, Bari M, Smith I, Leyland-Jones B, de Azambuja E, Wermuth P, Khasanov R, Feng-Yi F, Constantin C, Mayordomo JI, Su CH, Yu SY, Lluch A, Senkus-Konefka E, Price C, Haslbauer F, Suarez Sahui T, Srimuninnimit V, Colleoni M, Coates AS, Piccart-Gebhart MJ, Goldhirsch A, HERA Study Team Estimating the magnitude of trastuzumab effects within patient subgroups in the HERA trial. Ann Oncol. 2008;19:1090–1096. doi: 10.1093/annonc/mdn005. [DOI] [PubMed] [Google Scholar]
- Anderson WF, Chu KC, Chang S. Inflammatory breast carcinoma and noninflammatory locally advanced breast carcinoma: distinct clinicopathologic entities? J Clin Oncol. 2003;21:2254–2259. doi: 10.1200/JCO.2003.07.082. [DOI] [PubMed] [Google Scholar]
- Bertucci F, Finetti P, Rougemont J, Charafe-Jauffret E, Cervera N, Tarpin C, Nguyen C, Xerri L, Houlgatte R, Jacquemier J, Viens P, Birnbaum D. Gene expression profiling identifies molecular subtypes of inflammatory breast cancer. Cancer Res. 2005;65:2170–2178. doi: 10.1158/0008-5472.CAN-04-4115. [DOI] [PubMed] [Google Scholar]
- Agulnik M, Oza AM, Pond GR, Siu LL. Impact and perceptions of mandatory tumor biopsies for correlative studies in clinical trials of novel anticancer agents. J Clin Oncol. 2006;24:4801–4807. doi: 10.1200/JCO.2005.03.4496. [DOI] [PubMed] [Google Scholar]
- Evans TR, Yellowlees A, Foster E, Earl H, Cameron DA, Hutcheon AW, Coleman RE, Perren T, Gallagher CJ, Quigley M, Crown J, Jones AL, Highley M, Leonard RC, Mansi JL. Phase III randomized trial of doxorubicin and docetaxel versus doxorubicin and cyclophosphamide as primary medical therapy in women with breast cancer: an angloceltic cooperative oncology group study. J Clin Oncol. 2005;23:2988–2995. doi: 10.1200/JCO.2005.06.156. [DOI] [PubMed] [Google Scholar]
- von Minckwitz G, Kümmel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, Gerber B, Huober J, Costa SD, Jackisch C, Loibl S, Mehta K, Kaufmann M, German Breast Group Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: phase III randomized GeparTrio trial. J Natl Cancer Inst. 2008;100:542–551. doi: 10.1093/jnci/djn085. [DOI] [PubMed] [Google Scholar]
- Ellis MJC. ASCO Educational Book, Spring 2008. Alexandria, VA: American Society of Clinical Oncology; 2008. Neoadjuvant therapy for breast cancer; pp. 55–57. [Google Scholar]
- Chen JH, Feig B, Agrawal G, Yu H, Carpenter PM, Mehta RS, Nalcioglu O, Su MY. MRI evaluation of pathologically complete response and residual tumors in breast cancer after neoadjuvant chemotherapy. Cancer. 2008;112:17–26. doi: 10.1002/cncr.23130. [DOI] [PubMed] [Google Scholar]
- Esserman L, Kaplan E, Partridge S, Tripathy D, Rugo H, Park J, Hwang S, Kuerer H, Sudilovsky D, Lu Y, Hylton N. MRI phenotype is associated with response to doxorubicin and cyclophosphamide neoadjuvant chemotherapy in stage III breast cancer. Ann Surg Oncol. 2001;8:549–559. doi: 10.1007/s10434-001-0549-8. [DOI] [PubMed] [Google Scholar]
- Padhani AR, Hayes C, Assersohn L, Powles T, Makris A, Suckling J, Leach MO, Husband JE. Prediction of clinicopathologic response of breast cancer to primary chemotherapy at contrast-enhanced MR imaging: initial clinical results. Radiology. 2006;239:361–374. doi: 10.1148/radiol.2392021099. [DOI] [PubMed] [Google Scholar]
- Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Tripathy D, Wolverton DS, Rugo HS, Hwang ES, Ewing CA, Hylton NM. MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR Am J Roentgenol. 2005;184:1774–1781. doi: 10.2214/ajr.184.6.01841774. [DOI] [PubMed] [Google Scholar]
- Meisamy S, Bolan PJ, Baker EH, Bliss RL, Gulbahce E, Everson LI, Nelson MT, Emory TH, Tuttle TM, Yee D, Garwood M. Neoadjuvant chemotherapy of locally advanced breast cancer: predicting response with in vivo (1)H MR spectroscopy – a pilot study at 4 T. Radiology. 2004;233:424–431. doi: 10.1148/radiol.2332031285. [DOI] [PubMed] [Google Scholar]
- Schelling M, Avril N, Nährig J, Kuhn W, Römer W, Sattler D, Werner M, Dose J, Jänicke F, Graeff H, Schwaiger M. Positron emission tomography using [(18)F]Fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol. 2000;18:1689–1695. doi: 10.1200/JCO.2000.18.8.1689. [DOI] [PubMed] [Google Scholar]
- Smith IC, Welch AE, Hutcheon AW, Miller ID, Payne S, Chilcott F, Waikar S, Whitaker T, Ah-See AK, Eremin O, Heys SD, Gilbert FJ, Sharp PF. Positron emission tomography using [(18)F]-fluorodeoxy-D-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol. 2000;18:1676–1688. doi: 10.1200/JCO.2000.18.8.1676. [DOI] [PubMed] [Google Scholar]
- Dunnwald LK, Gralow JR, Ellis GK, Livingston RB, Linden HM, Lawton TJ, Barlow WE, Schubert EK, Mankoff DA. Residual tumor uptake of [99mTc]-sestamibi after neoadjuvant chemotherapy for locally advanced breast carcinoma predicts survival. Cancer. 2005;103:680–688. doi: 10.1002/cncr.20831. [DOI] [PubMed] [Google Scholar]
- Bloch SH, Dayton PA, Ferrara KW. Targeted imaging using ultrasound contrast agents. Progess and opportunities for clinical and research applications. IEEE Eng Med Biol Mag. 2004;23:18–29. doi: 10.1109/MEMB.2004.1360405. [DOI] [PubMed] [Google Scholar]
- Tromberg BJ, Cerussi A, Shah N, Compton M, Durkin A, Hsiang D, Butler J, Mehta R. Imaging in breast cancer: diffuse optics in breast cancer: detecting tumors in pre-menopausal women and monitoring neoadjuvant chemotherapy. Breast Cancer Res. 2005;7:279–285. doi: 10.1186/bcr1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos EN, Sanidas E, Kafousi M, Mavroudis D, Askoxylakis J, Bozionelou V, Perraki M, Tsiftsis D, Georgoulias V. Detection of CK-19 mRNA-positive cells in the peripheral blood of breast cancer patients with histologically and immunohistochemically negative axillary lymph nodes. Ann Oncol. 2005;16:240–246. doi: 10.1093/annonc/mdi043. [DOI] [PubMed] [Google Scholar]
- Xenidis N, Perraki M, Kafousi M, Apostolaki S, Bolonaki I, Stathopoulou A, Kalbakis K, Androulakis N, Kouroussis C, Pallis T, Christophylakis C, Argyraki K, Lianidou ES, Stathopoulos S, Georgoulias V, Mavroudis D. Predictive and prognostic value of peripheral blood cytokeratin-19 mRNA-positive cells detected by real-time polymerase chain reaction in node-negative breast cancer patients. J Clin Oncol. 2006;24:3756–3762. doi: 10.1200/JCO.2005.04.5948. [DOI] [PubMed] [Google Scholar]
- Stathopoulou A, Ntoulia M, Perraki M, Apostolaki S, Mavroudis D, Malamos N, Georgoulias V, Lianidou ES. A highly specific real-time RT-PCR method for the quantitative determination of CK-19 mRNA positive cells in peripheral blood of patients with operable breast cancer. Int J Cancer. 2006;119:1654–1659. doi: 10.1002/ijc.22017. [DOI] [PubMed] [Google Scholar]
- Ghossein RA, Carusone L, Bhattacharya S. Review: polymerase chain reaction detection of micrometastases and circulating tumor cells: application to melanoma, prostate, and thyroid carcinomas. Diagn Mol Pathol. 1999;8:165–175. doi: 10.1097/00019606-199912000-00001. [DOI] [PubMed] [Google Scholar]
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL, Flockhart DA, Desta Z, Perez EA, Ingle JN. Pharmacogenetics of tamoxifen biotrans-formation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23:9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- Punglia RS, Burstein HJ, Winer EP, Weeks JC. Pharmacogenomic variation of CYP2D6 and the choice of optimal adjuvant endocrine therapy for postmenopausal breast cancer: a modeling analysis. J Natl Cancer Inst. 2008;100:642–648. doi: 10.1093/jnci/djn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccart M, Goldhirsch A, Wood W, Pritchard K, Baselga J, Reaby L, Kossler I, Kyriakides S, Norton L, Coates A. Keeping faith with trial volunteers. Nature. 2007;446:137–138. doi: 10.1038/446137a. [DOI] [PubMed] [Google Scholar]
- Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, Natale RB, Schiller JH, Von Pawel J, Pluzanska A, Gatzemeier U, Grous J, Ochs JS, Averbuch SD, Wolf MK, Rennie P, Fandi A, Johnson DH. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial – INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, Scagliotti G, Rosell R, Oliff I, Reeves JA, Wolf MK, Krebs AD, Averbuch SD, Ochs JS, Grous J, Fandi A, Johnson DH. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial – INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- Cufer T, Vrdoljak E, Gaafar R, Erensoy I, Pemberton K. Phase II, open-label, randomized study (SIGN) of single-agent gefitinib (IRESSA) or docetaxel as second-line therapy in patients with advanced (stage IIIb or IV) non-small-cell lung cancer. Anticancer Drugs. 2006;17:401–409. doi: 10.1097/01.cad.0000203381.99490.ab. [DOI] [PubMed] [Google Scholar]
- Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, Eek R, Horai T, Noda K, Takata I, Smit E, Averbuch S, Macleod A, Feyereislova A, Dong RP, Baselga J. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol. 2003;21:2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8:303–306. doi: 10.1634/theoncologist.8-4-303. [DOI] [PubMed] [Google Scholar]