Abstract
Infrared radiation is increasingly and uncritically used for cosmetic and wellness purposes, despite the poorly understood biologic effects of such treatments on humans. In the present study, we investigated the effects of infrared radiation on collagen and elastin production in dermal fibroblasts, as well as the clinical and histopathologic effects of infrared radiation on photo-aged facial skin lesions. In order to determine the effects of infrared radiation on collagen and elastin production, dermal fibroblasts were exposed to infrared radiation for varying lengths of time and collagen and elastin contents were subsequently determined. Additionally, 20 patients with mild to moderate facial wrinkles and hyperpigmented lesions received daily treatments of far infrared radiation (900 to 1000 µm) for six-months. During the treatment, patients and a medical observer conducted independent photographic and clinical evaluations every 4 weeks, and skin biopsies were obtained for histological analysis at baseline and one month post-treatment. We found that the content of collagen and elastin produced by the fibroblasts increased after infrared radiation, and that this increase was proportional to the duration of irradiation exposure. Following 6 months of treatment, all patients reported good (51-75%) improvements in skin texture and roughness. Additionally, patients noted fair (25-50%) improvement in color tone of the skin; however, improvements in hyperpigmented lesions were not observed. Objective medical evaluation of the patients indicated that roughness and laxity were fairly improved, but there was no significant improvement in hyperpigmented lesions. Histological examination failed to reveal any differences as well. These results suggest that infrared radiation may have beneficial effects on skin texture and wrinkles by increasing collagen and elastin contents from the stimulated fibroblasts. Therefore, skin treatment with infrared radiation may be an effective and safe non-ablative remodeling method, and may also be useful in the treatment of photo-aged skin.
Keywords: Infrared radiation, fibroblasts, photo-aging
INTRODUCTION
As the outermost barrier, human skin is in direct contact with numerous environmental factors including solar radiation, which is one cause of photo-aging. Characterized clinically by wrinkles, mottled pigmentation, rough skin, and loss of skin tone, the major histologic alterations associated with photo-aging lie in dermal connective tissue. A variety of highly effective, ablative methods have been used to treat the facial skin lesions associated with photo-aging; however, most patients complain of discomfort during the treatment and must also be concerned with the risk of infection and scarring, as ablative methods completely disrupt or remove the epidermis in a direct manner. The primary mechanism for treatment of photo-aged skin lesions by these methods appears to be through the deposition of new extracellular matrix elements.1-3 Nonablative dermal remodeling (NDR), a recently developed procedure also called non-ablative skin rejuvenation or subsurfacing, does not disrupt the epidermis, and is a better tolerated alternative to ablative laser resurfacing. NDR is designed to selectively confine thermal injuries of the papillary and upper reticular dermis without causing epidermal damage, and thus induce fibroblast activation and synthesis of new collagen and extracellular matrix material.4 In contrast to detailed studies of ultraviolet radiation responses, little is known about the biologic effects of infrared (IR) radiation. IR radiation exists in the invisible portion of the electromagnetic spectrum, and is adjacent to the long wavelength of the visible light range that extends up to the microwave range. Several studies have shown that IR radiation produces a temperature-independent stimulatory effect on the proliferation of human fibroblasts and collagen synthesis in vivo.5,6 However, since IR radiation is increasingly and uncritically used for cosmetic and wellness purposes, the present study was undertaken to investigate the effects of IR radiation on collagen and elastin production in dermal fibroblasts and determine its clinical and histologic effects on photo-aged facial skin lesions including facial wrinkles, roughness, tightness, and hyperpigmented lesions.
MATERIALS AND METHODS
Culture of human dermal fibroblasts
Primary human dermal fibroblast cultures, established by explanting tissue specimens obtained from neonatal foreskin, were utilized in passages.3-8 All cell cultures were maintained in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal calf serum and 2 mM glutamine.
IR treatment
Human dermal fibroblasts were exposed in vitro to infrared (IR) radiation, and an IR radiation emitting device (MG Care®, Seoul, Korea) was used as the source. This device emits far IR radiation (900 to 1000 µm) with an energy flux of 35 mW/cm2. Human dermal fibroblasts were exposed to IR radiation for 1 to 5 hours. Collagen and elastin assays were performed after a 24-hour post-irradiation incubation period. Results were compared with a control group which was not exposed to IR radiation.
Collagen and elastin assay
Collagen assays were performed using the Sircol collagen assay kit (Biocolor, UK) according to manufacturer's instructions. Briefly, the collected supernatant was centrifuged at 1,500 rpm for 4 min to isolate the extracellular matrix, and 100 µl of the resulting supernatant was mixed with 1 ml of Sircol dye for 30 min and centrifuged at 10,000 rpm for 5 min to isolate the formed collagen-dye complex. After decanting the suspension, droplets were dissolved in 1 ml Sircol alkali reagent and assessed at 513 nm by spectrophotometry. Total soluble collagen was expressed as a concentration (µg/mL) relative to control fibroblasts that did not receive infrared radiation.
Similarly, soluble elastin content was determined according to Fastin Elastin Assay instructions7 and with the use of a commercial kit containing the dye label 5,10,15,20-tetraphenyl-21-23 porphrine sulphonate (Biocolor Ltd, Belfast, England).
Both assays were performed 3 times and results were obtained by averaging the data.
Patients and methods
Twenty females (ages 35-61; mean 44.3 years, skin phototypes III-IV) with facial wrinkles and hyperpigmented lesions were enrolled in the study from March to October, 2004. Patients received 15-20 minute daily treatments (on 5 weekdays) of far IR radiation for a period of 6 months. The emitting device, which was made of medical silicon, covered the entire face except for the eyes, mouth, and nostrils; the temperature of the device was approximately 32.0℃ to 35.0℃ and the radiation energy was 35 mW/cm2. Photographic documentation was taken after 0, 3, and 6 months of treatment, and clinical improvement scores were determined every 4 weeks. All of the patients completed the six-month treatment session and were able to return for follow-up evaluations. Each patient and a medical observer independently performed clinical assessments using a well-established grading scale of 0 = < 25% (minimal), 1 = 26-50% (fair), 2 = 51-75% (good), 3 = 75-90% (excellent), 4 = 91-100% (clear) improvement. Assessment categories included improvements in small wrinkles, color tone, brown spots, roughness and tightness. Average improvement scores were calculated as the mean of the grading scales of all categories. Categories of the clinical assessment by the medical observer included fine wrinkles, coarse wrinkles, roughness, mottled hyperpigmentation, laxity, and skin tone. Standardized photographs and dermoscopic observations (Coscam CCL-205, Sometech Cosmetic, Seoul, Korea) were reviewed to help the medical observers identify each clinical grade. Skin biopsies with a 2-mm punch were performed on the cheeks for hyperpigmented lesions and on the lateral sides of eyes for wrinkles prior to treatment and 1 month after final treatment. In addition to hematoxylin-eosin stain, special stains such as elastic stain and Masson-trichrome stain were performed to verify the difference in amount of elastic and collagen fibers in the dermis. Side effects were recorded and rated in severity (0 = none, 1 = mild, 2 = moderate, 3 = severe) at each follow-up visit. We performed a Wilcoxon rank sum test (SAS version 8.2) to compare the treatment effects between the two groups and a Shapiro-Wilk test (SAS version 8.2) to compare baseline and post-treatment measurements.
RESULTS
Collagen and elastin assay
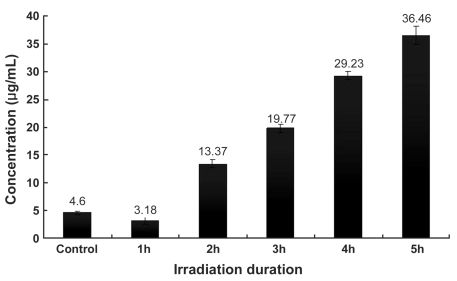
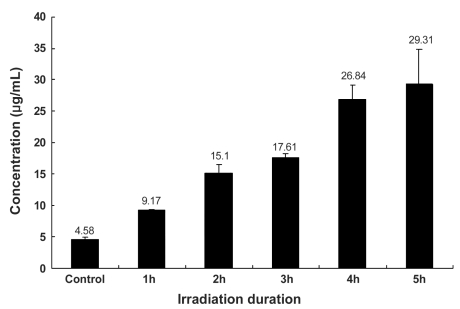
Total soluble collagen was increased 2 hours after IR radiation exposure, relative to controls, and total content increased with the duration of IR radiation exposure (Fig. 1); the results were an average of three measurements. The concentration of total soluble collagen in the control was 4.6 µg/mL, and in treatment groups the concentrations increased to 3.18 µg/mL, 13.37 µg/mL, 19.77 µg/mL, 29.23 µg/mL, and 36.46 µg/mL with 1, 2, 3, 4, and 5 hours of IR treatment, respectively. Soluble elastin also increased 1 hour after infrared radiation compared to the control. Similar to collagen, the content of soluble elastin increased with longer durations of irradiation (Fig. 2). The concentration of soluble elastin in the control was 4.58 µg/mL, and in treatment groups the concentrations of soluble elastin increased to 9.17 µg/mL, 15.1 µg/mL, 17.61 µg/mL, 26.84 µg/mL, and 29.31 µg/mL, with 1, 2, 3, 4, and 5 hours of irradiation treatment, respectively.
Fig. 1.
Concentrations of total soluble collagen after IR irradiation. Total soluble collagen increased after 2 hours of infrared radiation compared to control. Concentrations increased with irradiation duration.
Fig. 2.
Concentration of soluble elastin after IR irradiation. Soluble elastin increased after 1 hour of infrared radiation compared to control. Concentration of soluble elastin increased with irradiation duration.
Clinical evaluation
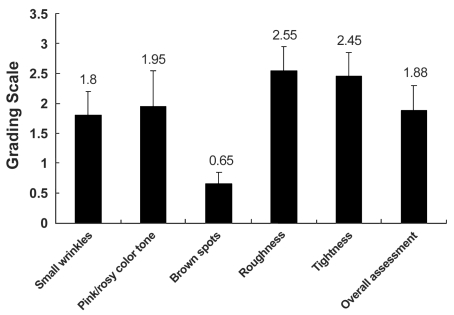
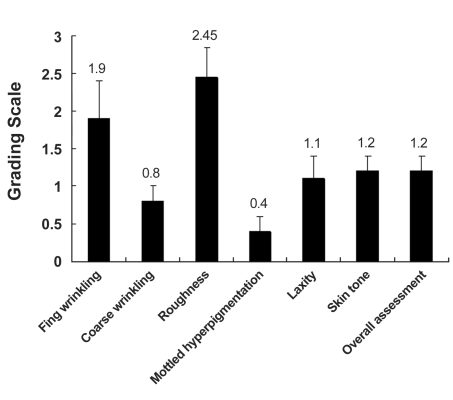
The average improvement score, as evaluated by patients, was 1.88, which was statistically significant (p < 0.05). The grading scale in the category of roughness and tightness was 2.55 and 2.45, respectively, indicating a 51-75% improvement. The grading scale of skin color tone was 1.95. The grading scale of small wrinkles was 1.8, indicating fair improvement (26-50%). Unlike the previous categories, however, hyperpigmented lesions showed minimal improvement (Fig. 3). The average grading scale, as evaluated by a medical observer, was 1.31, which was also statistically significant (p < 0.05). Roughness was the most improved category, with a grading scale of 2.45 (Fig. 4). Fine wrinkles were at least fairly improved (26-50%) in all patients with the grading scale of 1.9; however, coarse wrinkle showed minimal improvement (Fig. 5). Skin tone and laxity, with a grading scale of 1.1 and 1.2, respectively, were fairly improved (26-50%) in all 20 patients, and hyperpigmented lesions did not show any statistically significant improvement.
Fig. 3.
Clinical improvement scores (Patient evaluation). The average improvement score as determined by the patient was 1.88. Roughness and tightness were the most improved categories with a grading scale of 2.55 and 2.45, respectively. Hyperpigmented lesions, however, showed minimal improvement.
Fig. 4.
Clinical improvement scores (Medical observer evaluation). The average improvement score as determined by a medical observer was 1.2. Roughness was the most improved category with a grading scale of 2.45. Fine wrinkles were at least fairly improved in all patients, with the grading scale of 1.9. There was minimal improvement in the coarse wrinkle category.
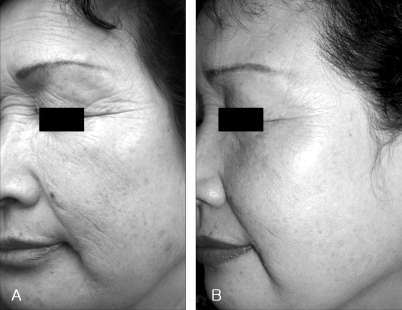
Fig. 5.
Photographic evaluation of facial wrinkles. Improvement of facial wrinkles after 6 months (A: Baseline, B: after 6 months).
Histolopathologic evaluation
Histopathologic examinations indicated that there was no significant difference in the basal hyperpigmentation after 6 months of treatment relative to the control group. Neither the depth nor the amount of solar elastosis in the dermis was significantly different after 6-months of treatment with IR radiation. There were no differences noted between the two groups using special stains such as elastin stain or Masson-trichrome stain.
Side effects
In general, any side effects of infrared radiation treatment were minimal and transient. Of the 20 patients treated, 80% developed mild transient erythema, which lasted only a few hours after the treatment and was not a significant problem for the patients. Other complaints included mild dryness (3 patients) and scaling of the face (2 patients). One patient experienced exacerbation of perioral dermatitis, which subsided without any treatment. There were no instances of pigmentary alteration or burns due to treatment, and all of the treatment sessions were generally well tolerated with minimal complaints.
DISCUSSION
Infrared (IR) radiation is an invisible portion of the electromagnetic spectrum adjacent to the long wavelength of the visible light range and extends to the microwave range. IR radiation consists of wavelength ranging from 0.75 to 1000 µm (0.75 µm = 750 nm),8 and can be subdivided into near (0.75 to 3 µm), middle (3 to 30 µm), and far (30 to 1000 µm). The device used in this study emits far IR radiation that elevates the skin temperature to a pleasant 32 to 35℃. Recently, nonablative collagen remodeling techniques have emerged as a means to avoid side effects such as oozing and erythema due to the complete removal of the epidermis in direct ablative methods. There is some evidence that the underlying mechanism of this action is the induction of new collagen growth due to thermal damage of the dermis.9,10 Using this concept, the need for gross damage of the epidermis merely to improve the surface may be unnecessary, as thermal effects to the dermis, without subsequent injury to the epidermis, can be induced with IR radiation.9 Therefore, we investigated the effects of IR radiation on collagen and elastin production in fibroblasts as well as the clinical and histopathologic effects of infrared radiation on facial skin, especially on photo-aged skin lesions such as roughness, tightness, wrinkles, and hyperpigmentation.
Our study showed that IR radiation increased the amount of total soluble collagen and soluble elastin in fibroblasts and demonstrated that could result in clinical improvement in skin texture. The clinical effects were, however, gradual, with a mean improvement of 25% to 50% achieved after 6 months of treatment, and roughness and tightness of the skin was improved in all of the 20 patients enrolled in the study. Fine wrinkles were at least fairly improved in all patients, but hyperpigmented lesions of the skin were not affected with IR radiation. This finding was further supported by histopathologic examination, which did not reveal any discernable differences in basal hyperpigmentation. IR irradiation has been reported to cause skin changes similar to those found in solar UV irradiation-induced elastosis;10,11 however, in the present study, there was no difference in the depth or amount of solar elastosis after 6 months of treatment with IR radiation.
Many reports have indicated that that IR radiation may have a stimulatory effect on the proliferation of human fibroblasts and collagen synthesis during wound healing.3,5,9 Toyokawa9 et al. reported that greater collagen regeneration and infiltration of fibroblasts expressing transforming growth factor-β1 (TGF-β1) is observed in rat skin after irradiation with far infrared radiation. The cytokine TGF-β1 is well known to accelerate wound healing12 by stimulating fibroblasts to produce extracellular matrix proteins, including collagen and fibronectin, and to also facilitate their deposition.13,14 Several in vivo studies have also demonstrated that near-IR rays and IR radiation emitting lasers can have therapeutic effects on wound healing by promoting collagen synthesis, cell proliferation, and keratinocyte motility.15,16 The production of collagen fibers from activated fibroblasts by IR radiation has been suggested as a possible mechanism for the therapeutic effect on wound healing; however, there are no reports as of yet, other than for wound healing, that explain the mechanisms of treatment effects of IR radiation on aging or wrinkling. While our culture results may or may not extrapolate to clinical results, our study showed that infrared radiation may have proliferative effects of fibroblasts in either an in vivo or in vitro environment. The results of this study, however, are limited, as the in vivo clinical results do not prove any changes in fibroblastic activity. We evaluated fibroblastic activity in vivo by comparing the histopathology using collagen and elastin stains; however, this method may have been insufficient for comparing minute differences of collagen and elastin production in fibroblasts. The results suggest that the improvement of texture of the skin may have resulted from increased collagen and elastin in the dermis. Further, thermal effects of infrared radiation may have a role in activating fibroblasts, but additional studies regarding the abundance of cytokines in proliferating fibroblasts are needed.
The side effects of treatment with the IR radiation were minimal in this study. The severity and duration of erythema observed post-treatment was mild and resolved by 30 minutes post- treatment. In addition, there was no histopathologic evidence of remarkable inflammation, IR radiation burns, or increased solar elastosis. This suggested that IR radiation treatment at an ambient temperature is safe and does not cause harmful thermal injuries. Our results further suggest that IR radiation may result in beneficial effects on skin texture and wrinkles by increasing collagen and elastin in the dermis through stimulation of fibroblasts. Thus, treatment with IR radiation may be an effective and safe non-ablative remodeling method of the skin, and it may have some use as a supportive method in the treatment of photo-aged skin. Elucidation of the exact photophysical and photochemical mechanisms triggered by IR radiation as well as future studies of the practicality of IR radiation treatment may reveal novel therapeutic applications of IR radiation in clinical dermatology.
References
- 1.Stuzin JM, Baker TJ, Baker TM, Kligman AM. Histologic effects of the high-energy pulsed CO2 laser on photoaged facial skin. Plast Reconstr Surg. 1997;99:2036–2055. doi: 10.1097/00006534-199706000-00034. [DOI] [PubMed] [Google Scholar]
- 2.Ross EV, McKinlay JR, Sajben FP, Miller CH, Barnette DJ, Meehan KJ, et al. Use of a novel erbium laser in a Yucatan minipig: a study of residual thermal damage, ablation, and wound healing as a function of pulse duration. Lasers Surg Med. 2002;30:93–100. doi: 10.1002/lsm.10030. [DOI] [PubMed] [Google Scholar]
- 3.Seckel BR, Younai S, Wang KK. Skin tightening effects of the ultrapulse CO2 laser. Plast Reconstr Surg. 1998;102:872–877. doi: 10.1097/00006534-199809030-00040. [DOI] [PubMed] [Google Scholar]
- 4.Nelson JS, Majaron B, Kelly KM. What is nonablative photorejuvenation of human skin? Semin Cutan Med Surg. 2002;21:238–250. doi: 10.1053/sder.2002.36764. [DOI] [PubMed] [Google Scholar]
- 5.Mester E, Mester AF, Mester A. The biomedical effects of laser application. Lasers Surg Med. 1985;5:31–39. doi: 10.1002/lsm.1900050105. [DOI] [PubMed] [Google Scholar]
- 6.Castro D, Abergel R, Meeker C, Dwyer R, Lesavoy M, Uitto J. Effects of the Nd:YAG laser on DNA synthesis and collagen production in human skin fibroblasts cultures. Ann Plast Surg. 1983;11:214–222. [PubMed] [Google Scholar]
- 7.Pawlicka E, Bankowski E, Jaworski S. Elastin of the umbilical cord arteries and its alterations in EPH gestosis (preeclampsia) Biol Neonate. 1999;75:91–96. doi: 10.1159/000014083. [DOI] [PubMed] [Google Scholar]
- 8.Phillips M. The new encyclopedia britannica. 15th ed. vol 6. Chicago: Encyclopedia Britannica Inc; 1985. Electromagnetic radiation; pp. 644–665. [Google Scholar]
- 9.Khan MH, Sink RK, Manstein D, Eimeri D, Anderson RR. Intradermally focused infrared laser pulses: thermal effects at defined tissue depths. Lasers Surg Med. 2005;36:270–280. doi: 10.1002/lsm.20142. [DOI] [PubMed] [Google Scholar]
- 10.Toyokawa H, Matsui Y, Uhara J, Tsuchiya H, Teshima S, Nakanishi H, et al. Promotive effects of far-infrared ray on full-thickness skin wound healing in rats. Exp Biol Med. 2003;228:724–729. doi: 10.1177/153537020322800612. [DOI] [PubMed] [Google Scholar]
- 11.Kligman LH. Intensification of ultraviolet-induced dermal damage by infrared radiation. Arch Dermatol Res. 1982;272:229–238. doi: 10.1007/BF00509050. [DOI] [PubMed] [Google Scholar]
- 12.Yu W, Naim JO, Lanzafame RJ. Expression of growth factors in early wound healing in rat skin. Lasers Surg Med. 1994;15:281–289. doi: 10.1002/lsm.1900150308. [DOI] [PubMed] [Google Scholar]
- 13.Postlethwaite AE, Keski-Oja J, Moses HL, Kang AH. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor β. J Exp Med. 1987;165:251–256. doi: 10.1084/jem.165.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raghow R, Postlethwaite AE, Keski-Oja J, Moses HL, Kang AH. Transforming growth factor-β increases steady-state levels of type 1 procollagen and fibronectin messenger RNAs posttranscriptionally in cultured human dermal fibroblasts. J Clin Invest. 1987;79:1285–1288. doi: 10.1172/JCI112950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 16.O'kane S, Ferguson MW. Transforming growth factor βs and wound healing. Int J Biochem Cell Biol. 1997;29:63–78. doi: 10.1016/s1357-2725(96)00120-3. [DOI] [PubMed] [Google Scholar]