Abstract
In the present study, whether the ADAM-8, -9, -10, -12, -15, -17, and ADAMTS-1 proteins might play a role in mouse uterus during periimplantation period was investigated. Immunoblotting analyses demonstrated that all ADAM proteins consistently appeared throughout days 1 to 8 of pregnancy but with a variation depending on the species of ADAM gene, the progression of pregnancy, and the site of the uterus. Immunohistochemical analyses indicated that ADAM proteins were localized in the luminal or glandular epithelial layers with a varying intensity depending on the species of ADAM and the progression of pregnancy. Particularly ADAM-8, -12, and -15, were predominantly located in the implantation site of the uterine tissues, whereas little or no protein was localized in the interimplantation site. Based upon these observations, it is suggested that the ADAMs might play an important role in the remodeling of the mouse uterus during the periimplantation period.
Keywords: ADAM, uterus, implantation, remodeling
INTRODUCTION
ADAMs (a disintegrin and metalloprotease) is a unique family of protein members consisting of a prodomain, metalloprotease, disintegrin-like and cysteine-rich domains, and, in most cases, epidermal growth factor-like, transmembrane, and cytoplasmic domains. Their disintegrin and cysteine- rich domains can interact with integrins. Disintegrin domains of ADAM-9/MDC-9/meltrin-γ can function as an adhesion molecule by interacting with an αvβ5 integrin.1 The disintegrin and cysteine-rich domains of ADAM-12/meltrin-α represent two functional ligands for an α7β1 integrin, and adhesion to each of these two ligands via the α7β1 integrin triggers different cellular responses.2 Recombinant ADAMs-12 and ADAM-15/metargidin/MDC15 disintegrin domains specifically interact with integrin α9β1 in an RGD-independent manner.3 Due to these properties, ADAMs play many important roles in cell-cell and cell-matrix interactions. ADAMTS (a disintegrin and metalloprotease with thrombospondin motifs) is a relatively new family of ADAM-related proteins. They differ from the other ADAMs by a unique character - a thrombospondin type 1-repeat motif lying between the disintegrin and the cysteine-rich domains. Of the currently known 30 members, ADAMTS-1/METH-1 is the prototype and plays an important role in normal growth, fertility, and organogenesis.4 ADAMTS-5/aggrecanase-2 and ADAMTS-6 are exclusively expressed in the placenta suggesting their possible role during implantation in mice.5 In light of these findings and others, a vast amount of studies attribute the proteolytic and/or cell adhesion activities of ADAM and ADAMTS proteins to the remodeling of various tissues.
Mammalian uterus is a dynamic organ that undergoes a cyclic degradation and renewal during the reproductive cycle and a drastic remodeling during pregnancy. At the beginning of implantation, endometrial fibroblasts surrounding the embryo transform into an epithelioid cell type, in a reaction called decidualization, which is dependent on the priming by ovarian steroid hormones. Decidualization in murine endometrium involves cell growth and a severe reduction of the extracellular spaces accompanying modifications of many extracellular matrix (ECM) components. In mice, progressive loss of laminin and type IV collagen in the uterine luminal epithelial basement membrane occurs in a consistent spatiotemporal pattern following the onset of blastocyst implantation, which is closely correlated with the area occupied by decidualized endometrial stroma and occurs in areas not yet in contact with trophoblast cells.6 High levels of collagen type VI protein are present in the endometrium and myometrium of mouse uterus up to day 4.5 of pregnancy. After implantation, reduction in collagen type VI protein within the decidualizing endometrium correlates with the reduced levels of alpha 1, 2, and 3 chain mRNAs. During decidualization of the endometrium, differences in the nature and the amount of proteoglycans are observed suggesting the changes of collagen fibril thickness observed.7 The profile of small leucine-rich proteoglycans in the uterine ECM alters the differentiation of endometrial stromal cells.8 During the periimplantation period, perlecan in the extracellular spaces of the endometrial stroma is lost. Beginning soon before implantation, syndecan-4 increases in the fibroblasts of the subepithelial stroma, and after implantation, syndecan-4 is pronounced in predecidual and mature decidual cells.8 These observations indicate that the mouse uterus undergoes a considerable degree of remodeling during implantation.
Among the factors involved in the rearrangement of ECM components accompanying decidualization, matrix metalloproteinases (MMPs) and tissue inhibitors of matrix metalloproteinase (TIMPs) have been studied extensively and are known to play important roles in the uteri of many mammals.9 In mice, early pregnancy is associated with the expression of mRNA and positive immunoreactivity for multiple MMPs, including MMP-2, -3, -7, -9, -11, -13, and membrane type-MMP-1. Recently we observed that cycling mouse uteri exhibited differential mRNA expression of ADAM genes identical to those in the present study depending on the oestrous stage suggesting their roles in remodeling during the cycle.10
In the present study, immunoblotting and immunohistochemistry techniques were used to determine whether ADAM-8, -9, -10, -12, -15, -17, and ADAMTS-1 might play a role in uterine remodeling during implantation in mice.
MATERIALS AND METHODS
Animals
ICR mice were supplied from Daehan Biolink (Daejeon, Korea). Animals were kept under conditions that followed the institutional guidelines of Seoul Women's University for the care and use of experimental animals, under controlled lighting (12 h light-dark cycle). Sexually mature (6-8 week old) female mice were used. Females were mated with fertile males of the same strain and checked for vaginal plugs on the following morning. The day of vaginal plug formation was regarded as day 1 of pregnancy. Whole uteri were collected from pregnant mice on days 1-5. From days 6 to 8 of pregnancy, when implantation and interimplantation sites were visualized, uterine tissues of each site were separately collected.
Chemicals
Acrylamide, bisacrylamide, and N,N,N',N'-tetramethylethylenediamine were purchased from Bio-Rad (Hercules, CA, USA). Rabbit polyclonal antibodies against mouse ADAM-9, -10, -12, -15, and -17 were purchased from Chemicon (Temecula, CA, USA). Goat polyclonal antibodies against mouse ADAM-8, -10, -12, -15, and ADAMTS-1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Goat polyclonal antibody against mouse ADAM-9 was purchased from R & D Systems (Minneapolis, MN, USA). All other chemicals were supplied by Sigma (St. Louis, MO, USA) unless specified elsewhere.
Immunoblotting
Mouse uterine tissue was homogenized in a lysis buffer (0.125 M Tris-HCl, pH 6.8) containing 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM phenylmethylsulfonyl fluoride, and 1 µg/µL soybean trypsin inhibitor. Insoluble materials were precipitated by centrifugation at 12,000 × g for 15 min at 4℃. The supernatants were regarded as total cell lysates. The protein concentration of each sample was determined using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA). Samples were diluted with equal volumes of a reducing sample buffer (0.125 M Tris-HCl, pH 6.8, 4% sodium dodecyl sulfate (SDS), 10% mercaptoethanol, 20% glycerol, and 0.004% bromophenol blue), and then boiled for 3 min at 95℃. Fifty micrograms of protein was loaded into each lane. Proteins were separated by SDS-polyacrylamide gel electrophoresis on an 8% acrylamide gel in parallel with a prestained protein molecular marker. Subsequently, gels were soaked in a transfer buffer composed of 25 mM Tris (pH 8.4), 192 mM glycine, and 10% methanol for 15-30 min. To hydrate the polyvinylidene difluoride (PVDF) membranes (Immobilon-P, Millipore, Bedford, MA, USA), they were soaked in absolute methanol for 15 s, soaked in distilled water for 2 min, and then equilibrated in transfer buffer for 5 min. Proteins on the gel were electrotransferred onto a PVDF membrane for 60 min at 4℃ and 100 V. To saturate non-specific binding sites after transfer, membranes were incubated at 37℃ for 1 h in a washing buffer containing 0.8% NaCl, 0.02% KCl, 0.144% Na2HPO4 · 2H2O, 0.02% KH2PO4, 0.2% Tween 20, 10 mM sodium azide, and 5% bovine serum albumin (BSA). Membranes were then incubated for 1 h in washing buffer containing 1% normal goat serum and 1 µg/mL rabbit polyclonal antibody against mouse ADAM-9, -10, -12, -15, or -17. Following several washes with the washing buffer containing 0.1% BSA, membranes were incubated for 2 h in washing buffer containing 1:100 diluted gold-labeled goat anti-rabbit IgG. After reaction, the signal was visualized using an IntenSE BL kit (Amersham, Buckinghamshire, England) according to the manufacturer's instructions. Immunoblotting results were confirmed by three independent experiments. For ADAM-8 and ADAMTS-1, membranes were incubated for 1 h in washing buffer containing 1% normal donkey serum and 1 µg/mL goat polyclonal antibody against mouse ADAM-8 or ADAMTS-1. They were then incubated for 1 h in washing buffer containing 2 µg/mL biotinylated donkey antibody against goat IgG. After several washes with the washing buffer containing 0.1% BSA, membranes were incubated for 2 h in washing buffer containing 1:100 diluted streptavidin-conjugated 10-nm gold particles. Signals were detected using the same IntenSE BL kit.
Immunohistochemistry
Mouse uterine tissue was fixed with 4% paraformaldehyde in PBS at 4℃, processed into paraffin, and then sectioned at 4 µm. The sections on silane coating slides (Corning, NY, USA) were deparaffinized using xylene and rehydrated with ethyl alcohol and distilled water. To quench endogenous peroxidase, the sections were incubated in 3% hydrogen peroxide for 10 min at room temperature. Following several washes with Trisbuffered saline (TBS: pH 7.4, 5 mM Tris, 0.15 M NaCl, and 0.05% Tween 20), the sections were soaked in 10 mM citrate buffer solution (pH 6.0) and heated with a microwave twice for 8 min each. After cooling the sections for 20 min at room temperature, they were incubated with 1:25 diluted goat polyclonal antibody against mouse ADAM-8, -10, or ADAMTS-1 overnight at 4℃, with goat polyclonal antibody against mouse ADAM-9, -12 (diluted to 1:50), or -15 (diluted to 1:100) for 1 h at room temperature. After gentle agitation in TBS three times for 5 min each, the sections were incubated with 1:300 diluted biotinylated anti-goat IgG (Dako, Carpinteria, CA, USA) for 30 min at room temperature. After being rinsed, the sections were incubated with streptavidin-biotin peroxidase complex (Dako) for 10 min. For ADAM-17, the sections were incubated with 1:100 diluted rabbit polyclonal antibody against mouse ADAM-17 for 1 h, and then washed several times with TBS. The sections were incubated with EnVison™ containing horseradish peroxidase anti-rabbit IgG (Dako) for 40 min at room temperature. Immunoreactivity for ADAM proteins was visualized utilizing 3,3'-diaminobenzidine tetrahydrochloride and counterstained with Mayer's haematoxylin. Finally the sections were photographed under bright-field illumination using a microscope (LSM410, Carl Zeiss, Oberkochen, Germany).
RESULTS
Protein expression of ADAM-8
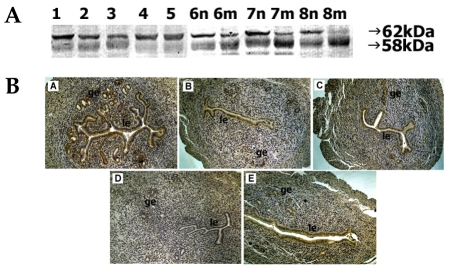
In mouse uterine extracts, proteins of ADAM-8 were detectable as two bands of 62 kDa and a smaller 58 kDa. During days 1-5 of pregnancy, the 62-kDa protein was observed with strong intensity on day 1 and weak intensity was observed on day 3. Intensity of the 58-kDa protein was strong on days 1-3 but decreased thereafter. During days 6-8 of pregnancy, the 62-kDa intensity was constantly strong at interimplantation sites but it decreased and was almost undetectable on day 8 of pregnancy. Staining intensity of the 58-kDa protein increased at both implantation and interimplantation sites as pregnancy progressed (Fig. 1A).
Fig. 1.
Spatial and temporal expression of ADAM-8 in mouse uterus on days 1-8 of pregnancy. A, immunoblot results of uterine tissue extracts. Numerical numbers indicate the day of pregnancy; n and m indicate the type of uterine tissues obtained from implantation (m) or interimplantation (n) sites. B, immunolocalization of ADAM-8 in mouse uterus during early pregnancy. Uterine cross sections are shown for pregnant day 1 (A), pregnant day 3 (B), pregnant day 5 (C), interimplantation site (D), and implantation site (E) on day 6 of pregnancy. le, luminal epithelium; ge, glandular epithelium. × 100.
On day 1, intense staining of ADAM-8 protein was observed in both the luminal and glandular epithelia, but the intensity weakened on day 3. By day 5, distinct signal was uniformly distributed in the luminal epithelium. On day 6, intense staining was observed in the luminal epithelium but not in the glandular epithelium of the implantation site. No discernable staining was observed at the interimplantation site.
Protein expression of ADAM-9
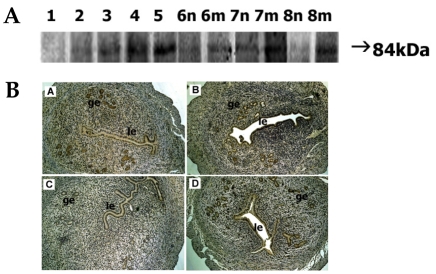
Protein expression of 84-kDa ADAM-9 gradually increased from day 1 to day 5 and strong expression was observed on day 5. On days 6-8, intense staining was observed at the implantation site whereas little or no signal was seen at the interimplantation site (Fig. 2A).
Fig. 2.
Spatial and temporal expression of ADAM-9 in mouse uterus on days 1-8 of pregnancy. A, immunoblot results of uterine tissue extracts. Numerical numbers indicate the day of pregnancy; n and m indicate the type of uterine tissues obtained from implantation (m) or interimplantation (n) sites. B, immunolocalization of ADAM-9 in mouse uterus during early pregnancy. Uterine cross sections are shown for pregnant day 3 (A), pregnant day 5 (B), interimplantation site (C), and implantation site (D) on day 6 of pregnancy. le, luminal epithelium; ge, glandular epithelium. × 100.
ADAM-9 protein on day 3 was mostly located in the glandular epithelium. Only weak staining was seen in the luminal epithelium. On day 5, a high level of ADAM-9 protein was detected in both the luminal epithelium and the glandular epithelium. On days 6-8, intense staining was seen in the same area of the implantation site but little or no staining was observed at interimplantation site (Fig. 2B).
Protein expression of ADAM-10
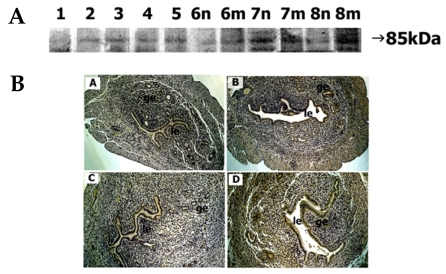
Low intensity of the 85-kDa ADAM-10 protein was observed in the tissue extract of day 1 uterus. On days 2-5, ADAM-10 protein signal was discernable and the intensity remained constant. Tissues on days 6-7 exhibited a similar intensity of staining. However, tissue from day 8 showed stronger staining at the implantation site compared to the interimplantation site (Fig. 3A).
Fig. 3.
Spatial and temporal expression of ADAM-10 in mouse uterus on days 1-8 of pregnancy. A, immunoblot results of uterine tissue extracts. Numerical numbers indicate the day of pregnancy; n and m indicate the type of uterine tissues obtained from implantation (m) or interimplantation (n) sites. B, immunolocalization of ADAM-10 in mouse uterus during early pregnancy. Uterine cross sections are shown for pregnant day 1 (A), pregnant day 3 (B), interimplantation site (C), and implantation site (D) on day 6 of pregnancy. le, luminal epithelium; ge, glandular epithelium. × 100.
Discernable staining of ADAM-10 protein was seen in both the luminal and glandular epithelia of day 3 uterus. On day 5, the intensity markedly increased, particularly in the luminal epithelium. On day 6, strong staining was observed in both epithelia of the implantation site, whereas positive staining was seen only in the luminal epithelium of the interimplantation site (Fig. 3B).
Protein expression of ADAM-12
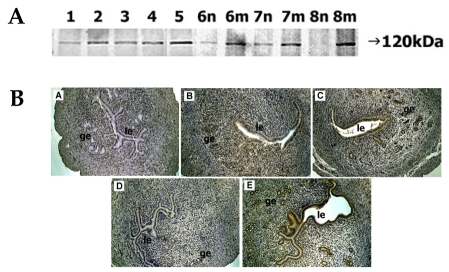
The staining intensity of the 120-kDa ADAM-12 protein on day 1 was weak but gradually increased, reaching a peak on day 5. During days 6-8, the intensity of ADAM-12 at the implantation site became strong whereas that at the interimplantation site became very faint such that on day 8 (Fig. 4A).
Fig. 4.
Spatial and temporal expression of ADAM-12 in mouse uterus on days 1-8 of pregnancy. A, immunoblot results of uterine tissue extracts. Numerical numbers indicate the day of pregnancy; n and m indicate the type of uterine tissues obtained from implantation (m) or interimplantation (n) sites. B, immunolocalization of ADAM-12 in mouse uterus during early pregnancy. Uterine cross sections are shown for pregnant day 1 (A), pregnant day 3 (B), pregnant day 5 (C), interimplantation site (D), and implantation site (E) on day 6 of pregnancy. le, luminal epithelium; ge, glandular epithelium. × 100.
Localization of ADAM-12 protein was not seen in the uterus at day 1. On day 3, localization was observed in the luminal epithelium. By day 5, the staining intensity in both luminal and glandular epithelia became more pronounced. On day 6, very intense staining was observed in both epithelia of the implantation site, while little staining was observed in the interimplantation site (Fig. 4B).
Protein expression of ADAM-15
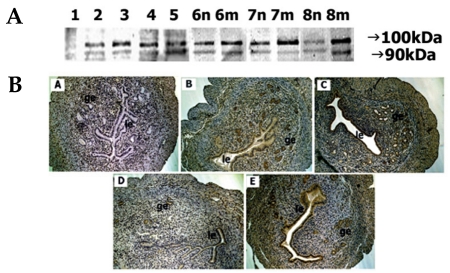
On day 1, a low ADAM-15 protein signal was observed on immunoblot analysis. On day 2 and afterwards, the presence of ADAM-15 protein was detected as two bands, 100 kDa and 90 kDa, and the intensity of the 100-kDa band was always stronger than that of the 90-kDa band. As pregnancy continued up to day 5, the staining intensity of both proteins markedly increased. On days 6-8, the staining intensity of both proteins at the implantation site progressively increased while that of interimplantation site, on the contrary, gradually decreased. On day 8, strong staining of both proteins was observed at the implantation site while weak staining was observed at the implantation site (Fig. 5A).
Fig. 5.
Spatial and temporal expression of ADAM-15 in mouse uterus on days 1-8 of pregnancy. A, immunoblot results of uterine tissue extracts. Numerical numbers indicate the day of pregnancy; n and m indicate the type of uterine tissues obtained from implantation (m) or interimplantation (n) sites. B, immunolocalization of ADAM-15 in mouse uterus during early pregnancy. Uterine cross sections are shown for pregnant day 1 (A), pregnant day 3 (B), pregnant day 5 (C), interimplantation site (D), and implantation site (E) on day 6 of pregnancy. le, luminal epithelium; ge, glandular epithelium. × 100.
No immunostaining of ADAM-15 protein was observed on day 1. However, positive staining was detected on day 3, particularly in the glandular epithelium. On day 5, very strong staining was observed in both sites. By day 6, strong staining was seen in the epithelia of the implantation site only. No distinct staining was observed at the interimplantation site (Fig. 5B).
Protein expression of ADAM-17
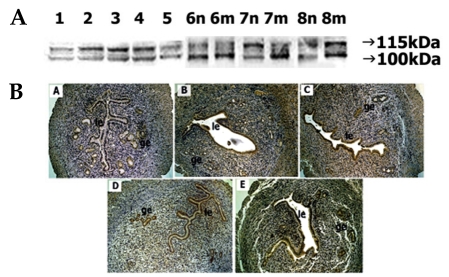
Immunoblot analyses showed the appearance of ADAM-17 protein as two reactive bands of 115 kDa and 100 kDa. Both protein levels were noted strongly on days 2-4 of pregnancy but the intensity of the 115 kDa band was stronger than that of the 100 kDa. On day 5, both proteins were weakly stained. On day 6, a strong intensity of 100-kDa protein was noted at both implantation and interimplantation sites. By day 7, a strong 115-kDa and weak 100-kDa bands were observed at the interimplantation site, but a weak 115 kDa-and strong 100-kDa bands at the implantation site were demonstrated. On day 8, a strong 115-kDa and weak 100-kDa band was observed in the interimplantation site but both proteins were intensely stained at the implantation site (Fig. 6A).
Fig. 6.
Spatial and temporal expression of ADAM-17 in mouse uterus on days 1-8 of pregnancy. A, immunoblot results of uterine tissue extracts. Numerical numbers indicate the day of pregnancy; n and m indicate the type of uterine tissues obtained from implantation (m) or interimplantation (n) sites. B, immunolocalization of ADAM-17 in mouse uterus during early pregnancy. Uterine cross sections are shown for pregnant day 1 (A), pregnant day 4 (B), pregnant day 5 (C), interimplantation site (D), and implantation site (E) on day 6 of pregnancy. le, luminal epithelium; ge, glandular epithelium. × 100.
On day 1 of pregnancy, little staining of ADAM-17 protein was observed (Fig. 6B). On days 4 and 5, a relatively high level of signal was found in both epithelia. On day 6, the luminal and glandular epithelia of both interimplantation and implantation sites exhibited strong staining.
Protein expression of ADAMTS-1
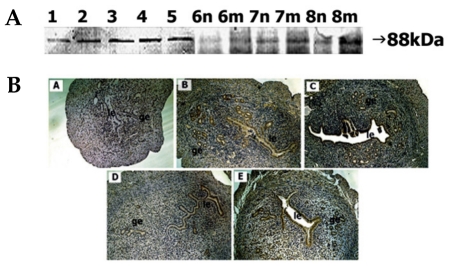
Weak immunostaining of the 88-kDa ADAMTS-1 protein was seen on day 1. The staining intensity of the protein remarkably increased until day 5. During days 6-8, the staining intensity at the implantation site was greater than that of the interimplantation site (Fig. 7A).
Fig. 7.
Spatial and temporal expression of ADAM-TS1 in mouse uterus on days 1-8 of pregnancy. A, immunoblot results of uterine tissue extracts. Numerical numbers indicate the day of pregnancy; n and m indicate the type of uterine tissues obtained from implantation (m) or interimplantation (n) sites. B, immunolocalization of ADAM-TS1 in mouse uterus during early pregnancy. Uterine cross sections are shown for pregnant day 1 (A), pregnant day 4 (B), pregnant day 5 (C), interimplantation site (D), and implantation site (E) on day 6 of pregnancy. le, luminal epithelium; ge, glandular epithelium. × 100.
Localization of ADAMTS-1 protein was not discernable on day 1. On day 4, distinct staining was observed in the glandular epithelium. Weak staining was seen in the luminal epithelium. On day 5, intense staining was observed in both epithelia. By day 6, weak signal was observed in both epithelia at the interimplantation site, whereas intense staining was observed in both epithelia at the implantation site (Fig. 7B).
DISCUSSION
The present study demonstrates that the ADAM-8, -9, -10, -12, -15, -17, and ADAMTS-1 proteins might play important roles in the remodeling of the mouse uterus during the periimplantation period using immunoblotting and immunohistochemistry techniques.
Increasing evidence has suggested the importance of the ADAM family of proteins in various tissues. ADAM-8 is involved in neurite outgrowth and the suppression of neuronal death, the upregulation of IgE production, and the induction of inflammatory cytokines in B cell lines.11 While its role in these cells is mediated by proteolytic activity, it also displays adhesive activity. The importance of adhesive activity has been shown in the neurodegeneration observed in wobbler mutant mice12 and in later stages of osteoclast differentiation from monocytic precursors.13 In mouse uterus, ADAM-8 protein exhibited differential expression from day 1 throughout day 5 of pregnancy. After the onset of implantation, ADAM-8 expression was even spatially restricted to the epithelial layers of the implantation site. These spatiotemporal expressions imply that ADAM-8 expression might be related to the initiation and maintenance of implantation. Western blot analyses of ADAM-8 protein showed two proteins, 62-kDa and 58-kDa forms. Since the 62-kDa form is known to generate the 58-kDa form by removal of a carbohydrate,14 the 58-kDa form observed in mouse uterus is likely to be a further processed form of the 62-kDa. In the implantation site, the 62-kDa protein gradually diminished whereas the 58-kDa protein greatly increased as pregnancy progressed, implying that the latter form might be related to the implantation process. Both the 62-kDa and 58-kDa forms, however, have been shown to lack metalloprotease activity but to function via adhesive activity.15 Taken together, these observations suggest that ADAM-8 might play a role in remodeling of the mouse uterus before and after implantation, and act via an adhesive interaction with apposed epithelial cells, stromal cells, and/or embryos.
Immunohistochemical signaling of ADAM-12 protein in mouse uterus remarkably increased from the beginning of pregnancy, but the localization was spatially restricted to the implantation site. Western blot results demonstrated ADAM-12 proteins as a ~120-kDa proform, which lacks proteolytic activity and a ~90-kDa mature, processedform, which lacks a prodomain but possesses proteolytic activity. In fibroblasts and myoblasts, the ~120-kDa is the predominant form with minor expression of the ~90-kDa form.15 However, the amount of the proteins in these cell lysates is too small to detect, unless they are concentrated by immunoprecipitation or affinity chromatography.15 In contrast, pregnant mouse uterine tissues synthesized a vast amount of the ~120-kDa form, as revealed by the fact that the protein was visualized without concentration. The ~90-kDa was not detected probably because of a minute quantity. For the proliferation of myoblasts and reserve cells, and the differentiation of early adipocytes,16 ADAM-12 acts via its cell-cell and/or cell-matrix adhesion activity. In cardiomyocytes, COS-1 cells, osteoblasts and other cells,17 ADAM-12 exhibits proteolytic activity by releasing HB-EGF, insulin-like growth factor-binding protein (IGFBP)-3, or IGFBP-5. Participation of ADAM-12 in proliferation and differentiation of uterine tissues from the beginning of pregnancy is thus suggested to be due to one or both of these activities.
ADAM-15 protein is initially synthesized as a ~110-kDa protein but is soon transformed into the 90-kDa form by removal of the prodomain.18 In mouse tissues such as heart, lung, muscle, brain, spleen, and testis, the amount of ADAM-15 protein is so small that only the 90-kDa form has been found even after concentration of the tissue lysates.18 In the present study, however, both the 100-kDa and 90-kDa proteins were easily detected in lysates of uterine tissues that were prepared without concentration, indicating that mouse uterus actively synthesizes and processes ADAM-15 proteins. While the amount of 90-kDa protein preferentially increased during days 1-5, both the 100-kDa and 90-kDa proteins increased after the onset of implantation and, particularly, at the implantation site. During early pregnancy in mice, progressive loss of laminin and type IV collagen in the uterine luminal epithelial basement membrane occurs in the area occupied by decidualized endometrial stroma and in the area not yet in contact with trophoblast cells.6 Since ADAM-15 can cleave type IV collagen and gelatin,19 its role in mouse uterus appears to be the reconstruction of ECM components during implantation. ADAM-15 has also been implicated in cartilage destruction found in inflammatory joint disease, in the restructuring of the mesangial matrix in mesangiocapillary glomerulonephritis, and in endothelial functions.19 The potential role of ADAM-15 in these events appears to be mediated via its specific interaction with integrin αvβ3 in an RGD-dependent manner, with integrin α9β1 in an RGD-independent manner,3 or ectodomain sheddase activity.11 Revealing the action mode of ADAM-15 will give an insight about its specific role in the mouse uterus during the periimplantation period.
In many mammals, the apical surface of the uterine epithelium is covered by a mucin glycocalyx. In rabbits, the presence of blastocysts in the uterine lumen resulted in a localized reduction of Muc1 at the implantation site of the luminal epithelium; higher expression of ADAM-9 was shown to correlate with the implantation site by an in situ hybridization study.20 Thus ADAM-9 was suggested to play a role as a MUC1 sheddase during the implantation window for rabbit embryos. Human uterine epithelial cells cultured in vitro also exhibited a local loss of MUC1 at the site of blastocyst adhesion. In the receptive phase of human endometrium, luminal and glandular uterine epithelial cells was the predominant site of MUC1 localization and ADAM-17 protein has been localized to this area.21 These findings suggested that in humans, ADAM-17 appears to be responsible for the shedding of MUC1.21 In mice, MUC-1 expression was higher in the proestrous and oestrous stages, and its protein declined to barely detectable levels by day 4 of pregnancy, i.e. before the time of blastocyst attachment.22 In earlier studies conducted with the same experiment system, genes of ADAM-8, -12, and -15 began to increase in expression from the beginning of pregnancy or on day 3 until day 5, which is around the time of implantation.10 Thus one or more of these ADAMs can be candidates for mouse MUC1 sheddase. In the present study, we monitored the expression of ADAM proteins. Unlike ADAM-9 and -12, ADAM-8, -10, -15, and -17 did not show any significant change in the level of protein expression during this period. However, after initiation of implantation, differential expression was observed such that strong expression was seen on day 8 at the implantation site and significantly lower expression occurred at the interimplantation site. Therefore these ADAMs seem to be involved in uterine remodeling after the initiation of implantation rather than before implantation.
ADAM-9 has been shown to play an either adhesive or proteolytic role. The disintegrin domain of ADAM-9 can function as an adhesion molecule by interacting with an αvβ5 integrin in an RGD-independent manner or an α6β1 integrin.1 Its metalloprotease domain has an alpha-secretase-like activity that cleaves amyloid precursor protein, acts as an insulin-like growth factor binding protein-5 protease in human osteoblasts, or degrades gelatin, β-casein, and fibronectin.23 It would be interesting to determine which type of ADAM-9 activity is involved in the remodeling of uterine tissues. Many studies have attributed the role of ADAM-10 to an ectodomain sheddase releasing a soluble fragment from Delta1 ligand, to an amyloid precursor protein cleaving enzyme 1 sheddase, to a vesicle-based protease targeting L1, or to a sheddase of EGF and betacellulin.24 Considering that there is no known adhesive activity of ADAM-10, ADAM-10 is believed to function in the mouse uterus via its proteolytic activity. ADAM-17 is produced as an inactive zymogen of 100-115 kDa in size, which is subsequently proteolytically processed to the catalytically active form of 85-100 kDa size.25 Thus, the 110 kDa and 100 kDa bands observed in the mouse uterus represent an inactive proform and an active processed form, respectively. Since ADAM-17 is well known to act as a sheddase for various cell signaling molecules and their receptors,26 uterine ADAM-17 is suggested to function via its proteolytic activity.
Unlike other ADAMs in the present study, ADAMTS-1 does not contain a transmembrane domain and after synthesis, it is secreted into ECM. It binds to the heparin-binding domain of vascular endothelial cell growth factor, inhibiting endothelial cell proliferation.27 As a metalloprotease, it can cleave versican or aggrecan, resulting in impaired ovulation.28 In mouse uterus, versican has been reported to localize in the decidualized region.29 Since ADAMTS-1 depends on a progesterone receptor in response to luteinizing hormone in the periovulatory follicles of mice,30 it seems to play a role in the uterus, a major target of progesterone, by acting on ECM molecules and/or growth factors.
While our studies suggest that all of these ADAM genes might play a role in the tissue remodeling of mouse uterus during the periimplantation period, birth and/or normal development of knockout mice of ADAM-8,31 -9,32 -12,33 -15,34 -17,34 or ADAMTS-128 have shown that these genes do not play a critical role in uterine function. One possible explanation for the discrepancy is that other related genes could overcome the lack of one gene's function. Many studies have suggested the compensation for loss of function in knockout mice. For example, desmoglein-1 could compensate for the function of desmoglein-3 I telogen in hair.35 Epidermal fatty acid binding protein (E-FABP)-deficient mice appeared normal by the compensation of heart-type FABP for a E-FABP deficiency.36 A three- to seven-fold increase in nidogen-2 was observed in the heart and muscle of mice with nidogen-1 deficiency.37 In closer relation to ADAMs, expressions of MMP-3/stromelysin-1 and MMP-10/stromelysin-2 were dramatically upregulated in MMP-7/matrilysin-deficient mice, while expressions of MMP-7 and MMP-10 were also upregulated in MMP-3-deficient mice.38 In these contexts, normality reported in knockout mice of the ADAM-9, -12, -15, -17, and ADAMTS-1 genes could be due to compensation by other ADAM genes or related genes.
Based on these results, we suggest that ADAM-8, -9, -10, -12, -15, -17, and ADAMTS-1 might play roles in uterus remodeling during the periimplantation period. However, the normality of ADAM gene knockout mice, except for ADAM-10, indicates that they might be not critical for uterine function. Further studies will clarify whether the compensation by other genes might indeed take place in ADAM-deficient mice.
Footnotes
This work was supported by a grant from Korea Research Foundation, Republic of Korea (KRF-2003-041-C00304).
References
- 1.Zhou M, Graham R, Russell G, Croucher PI. MDC-9 (ADAM-9/Meltrin gamma) functions as an adhesion molecule by binding the alpha(v)beta(5) integrin. Biochem Biophys Res Commun. 2001;280:574–580. doi: 10.1006/bbrc.2000.4155. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Z, Gruszczynska-Biegala J, Cheuvront T, Yi H, von der Mark H, von der Mark K, et al. Interaction of the disintegrin and cysteine-rich domains of ADAM12 with integrin alpha7beta1. Exp Cell Res. 2004;298:28–37. doi: 10.1016/j.yexcr.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Eto K, Puzon-McLaughlin W, Sheppard DJ, Sehara-Fujisawa A, Zhang XP, Takada Y. RGD-independent binding of integrin alpha9beta1 to the ADAM-12 and -15 disintegrin domains mediates cell-cell interaction. J Biol Chem. 2000;275:34922–34930. doi: 10.1074/jbc.M001953200. [DOI] [PubMed] [Google Scholar]
- 4.Shindo T, Kurihara H, Kuno K, Yokoyama H, Wada T, Kurihara Y, et al. ADAMTS-1, a metalloproteinase-disintegrin essential for normal growth, fertility, and organ morphology and function. J Clin Invest. 2000;105:1345–1352. doi: 10.1172/JCI8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurskainen TL, Hirohata S, Seldin MF, Apte SS. ADAM-TS5, ADAM-TS6, and ADAM-TS7, novel members of a new family of zinc metalloproteases. General features and genomic distribution of the ADAM-TS family. J Biol Chem. 1999;274:25555–25563. doi: 10.1074/jbc.274.36.25555. [DOI] [PubMed] [Google Scholar]
- 6.Blankenship TN, Given RL. Loss of laminin and type IV collagen in uterine luminal epithelial basement membranes during blastocyst implantation in the mouse. Anat Rec. 1995;243:27–36. doi: 10.1002/ar.1092430105. [DOI] [PubMed] [Google Scholar]
- 7.Greca CD, Nader HB, Dietrich CP, Abrahamsohn PA, Zorn TM. Ultrastructural cytochemical characterization of collagen-associated proteoglycans in the endometrium of mice. Anat Rec. 2000;259:413–423. doi: 10.1002/1097-0185(20000801)259:4<413::AID-AR50>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 8.San Martin S, Soto-Suazo M, Zorn TM. Perlecan and syndecan-4 in uterine tissues during the early pregnancy in mice. Am J Reprod Immunol. 2004;52:53–59. doi: 10.1111/j.1600-0897.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 9.Curry TE, Jr, Osteen KG. The matrix metalloproteinase system, changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24:428–465. doi: 10.1210/er.2002-0005. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Kim H, Lee SJ, Chio YM, Lee SJ, Lee JY. Abundance of ADAM-8, -9, -10, -12, -15, -17 and ADAMTS-1 in mouse uterus during the oestrous cycle. Reprod Fertil Dev. 2005;17:543–555. doi: 10.1071/rd04110. [DOI] [PubMed] [Google Scholar]
- 11.Fourie AM, Coles F, Moreno V, Karlsson L. Catalytic activity of ADAM8, ADAM15, and MDC-L (ADAM28) on synthetic peptide substrates and in ectodomain cleavage of CD23. J Biol Chem. 2003;278:30469–30477. doi: 10.1074/jbc.M213157200. [DOI] [PubMed] [Google Scholar]
- 12.Schlomann U, Rathke-Hartlieb S, Yamamoto S, Jockusch H, Bartsch JW. Tumor necrosis factor alpha induces a metalloprotease-disintegrin, ADAM8 (CD 156), implications for neuron-glia interactions during neurodegeneration. J Neurosci. 2000;20:7964–7971. doi: 10.1523/JNEUROSCI.20-21-07964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi SJ, Han JH, Roodman GD. ADAM8, a novel osteoclast stimulating factor. J Bone Miner Res. 2001;16:814–822. doi: 10.1359/jbmr.2001.16.5.814. [DOI] [PubMed] [Google Scholar]
- 14.Schlomann U, Wildeboer D, Webster A, Antropova O, Zeuschner D, Knight CG, et al. The metalloprotease disintegrin ADAM8. Processing by autocatalysis is required for proteolytic activity and cell adhesion. J Biol Chem. 2002;277:48210–48219. doi: 10.1074/jbc.M203355200. [DOI] [PubMed] [Google Scholar]
- 15.Kadota N, Suzuki A, Nakagami Y, Izumi T, Endo T. Endogenous meltrin alpha is ubiquitously expressed and associated with the plasma membrane but exogenous meltrin alpha is retained in the endoplasmic reticulum. J Biochem (Tokyo) 2000;128:941–949. doi: 10.1093/oxfordjournals.jbchem.a022845. [DOI] [PubMed] [Google Scholar]
- 16.Kawaguchi N, Sundberg C, Kveiborg M, Moghadaszadeh B, Asmar M, Dietrich N, et al. ADAM12 induces actin cytoskeleton and extracellular matrix reorganization during early adipocyte differentiation by regulating beta1 integrin function. J Cell Sci. 2003;116:3893–3904. doi: 10.1242/jcs.00699. [DOI] [PubMed] [Google Scholar]
- 17.Loechel F, Fox JW, Murphy G, Albrechtsen R, Wewer UM. ADAM 12-S cleaves IGFBP-3 and IGFBP-5 and is inhibited by TIMP-3. Biochem Biophys Res Commun. 2000;278:511–515. doi: 10.1006/bbrc.2000.3835. [DOI] [PubMed] [Google Scholar]
- 18.Lum L, Reid MS, Blobel CP. Intracellular maturation of the mouse metalloprotease disintegrin MDC15. J Biol Chem. 1998;273:26236–26247. doi: 10.1074/jbc.273.40.26236. [DOI] [PubMed] [Google Scholar]
- 19.Martin J, Eyestone LV, Davies M, Williams JD, Steadman R. The role of ADAM 15 in glomerular mesangial cell migration. J Biol Chem. 2002;277:33683–33689. doi: 10.1074/jbc.M200988200. [DOI] [PubMed] [Google Scholar]
- 20.Olson GE, Winfrey VP, Matrisian PE, NagDas SK, Hoffman LH. Blastocyst-dependent upregulation of metalloproteinase/disintegrin MDC9 expression in rabbit endometrium. Cell Tissue Res. 1998;293:489–498. doi: 10.1007/s004410051141. [DOI] [PubMed] [Google Scholar]
- 21.Thathiah A, Blobel CP, Carson DD. Tumor necrosis factor-alpha converting enzyme/ADAM 17 mediates MUC1 shedding. J Biol Chem. 2003;278:3386–3394. doi: 10.1074/jbc.M208326200. [DOI] [PubMed] [Google Scholar]
- 22.Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, Julian J, et al. Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology. 1995;136:3639–3647. doi: 10.1210/endo.136.8.7628404. [DOI] [PubMed] [Google Scholar]
- 23.Schwettmann L, Tschesche H. Cloning and expression in Pichia pastoris of metalloprotease domain of ADAM 9 catalytically active against fibronectin. Protein Expr Purif. 2001;21:65–70. doi: 10.1006/prep.2000.1374. [DOI] [PubMed] [Google Scholar]
- 24.Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srour N, Lebel A, McMahon S, Fournier I, Fugere M, Day R, et al. TACE/ADAM-17 maturation and activation of sheddase activity require proprotein convertase activity. FEBS Lett. 2003;554:275–283. doi: 10.1016/s0014-5793(03)01159-1. [DOI] [PubMed] [Google Scholar]
- 26.Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17:7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- 27.Luque A, Carpizo DR, Iruela-Arispe ML. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J Biol Chem. 2003;278:23656–23665. doi: 10.1074/jbc.M212964200. [DOI] [PubMed] [Google Scholar]
- 28.Mittaz L, Russell DL, Wilson T, Brasted M, Tkalcevic J, Salamonsen LA, et al. ADAMTS-1 is essential for the development and function of the urogenital system. Biol Reprod. 2004;70:1096–1105. doi: 10.1095/biolreprod.103.023911. [DOI] [PubMed] [Google Scholar]
- 29.San Martin S, Soto-Suazo M, Zorn TM. Distribution of versican and hyaluronan in the mouse uterus during decidualization. Braz J Med Biol Res. 2003;36:1067–1071. doi: 10.1590/s0100-879x2003000800013. [DOI] [PubMed] [Google Scholar]
- 30.Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, et al. Progesterone-regulated genes in the ovulation process, ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci USA. 2000;97:4689–4694. doi: 10.1073/pnas.080073497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly K, Hutchinson G, Nebenius-Oosthuizen D, Smith AJ, Bartsch JW, Horiuchi K, et al. Metalloprotease-disintegrin ADAM8: expression analysis and targeted deletion in mice. Dev Dyn. 2005;232:221–231. doi: 10.1002/dvdy.20221. [DOI] [PubMed] [Google Scholar]
- 32.Weskamp G, Cai H, Brodie TA, Higashyama S, Manova K, Ludwig T, et al. Mice lacking the metalloprotease-disintegrin MDC9 (ADAM9) have no evident major abnormalities during development or adult life. Mol Cell Biol. 2002;22:1537–1544. doi: 10.1128/mcb.22.5.1537-1544.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurisaki T, Masuda A, Sudo K, Sakagami J, Higashiyama S, Matsuda Y, et al. Phenotypic analysis of Meltrin alpha (ADAM12)-deficient mice, involvement of Meltrin alpha in adipogenesis and myogenesis. Mol Cell Biol. 2003;23:55–61. doi: 10.1128/MCB.23.1.55-61.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horiuchi K, Weskamp G, Lum L, Hammes HP, Cai H, Brodie T, et al. Potential role for ADAM15 in pathological neovascularization in mice. Mol Cell Biol. 2003;23:5614–5624. doi: 10.1128/MCB.23.16.5614-5624.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanakawa Y, Matsuyoshi N, Stanley JR. Expression of desmoglein 1 compensates for genetic loss of desmoglein 3 in keratinocyte adhesion. J Invest Dermatol. 2002;119:27–31. doi: 10.1046/j.1523-1747.2002.01780.x. [DOI] [PubMed] [Google Scholar]
- 36.Owada Y, Suzuk I, Noda T, Kondo H. Analysis on the phenotype of E-FABP-gene knockout mice. Mol Cell Biochem. 2002;239:83–86. [PubMed] [Google Scholar]
- 37.Miosge N, Sasaki T, Timpl R. Evidence of nidogen-2 compensation for nidogen-1 deficiency in transgenic mice. Matrix Biol. 2002;21:611–621. doi: 10.1016/s0945-053x(02)00070-7. [DOI] [PubMed] [Google Scholar]
- 38.Rudolph-Owen LA, Hulboy DL, Wilson CL, Mudgett J, Matrisian LM. Coordinate expression of matrix metalloproteinase family members in the uterus of normal, matrilysin-deficient, and stromelysin-1-deficient mice. Endocrinology. 1997;138:4902–4911. doi: 10.1210/endo.138.11.5478. [DOI] [PubMed] [Google Scholar]