Abstract
We report the sonographic features of an intracystic papillary carcinoma of the breast presenting as recurrent hemorrhagic cysts following trauma. A 56-year-old woman presented with palpable breast masses after a traumatic event; sonography showed multiple, well-defined, hemorrhagic cysts. Hemorrhagic fluid was evacuated by fine needle aspiration with no residual lesions. Cytology was negative for malignancy. Five months later, the mass reappeared; sonography demonstrated multiple cysts with solid nodules. US-guided core biopsy and surgery revealed invasive papillary carcinoma. We suggest close follow-up of cystic masses, even with negative cytology, and performance of surgical excisional biopsy in cases of rapid refilling after aspiration.
Keywords: Breast neoplasms, diagnosis, breast, ultrasonogram, breast, cysts
INTRODUCTION
It is well known that bloody cysts are suspicious and should be closely monitored for risk of intracystic neoplasm.1-4 However, in patients with history of trauma, the diagnosis of choice for cysts with bloody aspirate and negative cytology is trauma associated hemorrhagic cyst. In this report, we describe a case of intracystic papillary carcinoma that presented as recurrent hemorrhagic cysts after trauma.
CASE REPORT
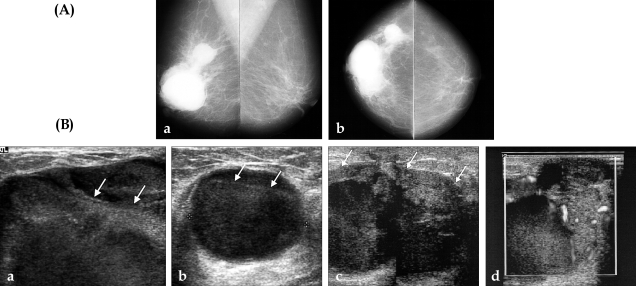
A 56-year-old woman presented with multiple, soft, palpable masses in her right breast. Three months earlier, heavy falling boxes injured her breast; soon after she noted gradually increasing multiple masses. Medical history was negative for any hematologic disease or bleeding tendency. Mammogram showed multiple, hyperdense masses with well-defined margins in the right subareolar and upper outer quadrant areas (Fig. 1A). Ultrasonogram (US) of the right breast showed multiple, round-shaped cysts with floating echogenic debris (Fig. 1B a,b); there were no suspicious echogenic lesions projecting from the wall. Fine needle aspiration biopsy was performed; aspirate was blood-stained, but there was no remaining residual mass and cytologic evaluation was negative for malignancy. Our impression was post-traumatic hemorrhagic cysts, and we recommended a three month follow-up US. The patient returned two months later for recurrence of the masses. The second US revealed recurrent cysts at the same site. Surgical excision and follow-up was recommended, however, the patient refused. After an additional three months, symptoms worsened and the patient complained of growing masses and circumferential, painful, nodular lesions. The third US revealed marked enlargement of the cystic masses with irregular, thickened, echogenic solid lesions along the cyst walls (Fig. 1B, c). Blood flow signals of the papillary solid portion were seen on color Doppler sonography (Fig. 1B, d). A biopsy of the solid portion was performed using a 14-gauge automated gun; the mass was diagnosed as papillary carcinoma. The patient was treated with modified radical mastectomy and axillary dissection. The gross sample was ill-defined with dark reddish colored cystic masses with polypoid projections from the wall. The confirmed final diagnosis was intracystic papillary carcinoma with invasion. Axillary dissection found metastasis at two lymph nodes among twenty resected nodes.
Fig. 1.
(A) Mammogram. Both mediolateral oblique (a) and craniocaudal mammogram (b) showed multiple round shape masses with well-defined margins in the subareolar area and upper outer quadrant of the right breast. (B) Ultrasonogram. The initial sonogram shows well-circumscribed cystic masses with echogenic floating debris (arrows) at the subareolar (a) and upper outer quadrant (b) of the right breast. Sonograms obtained at 5 month follow up. Cystic mass size was markedly increased, with irregular shaped soft tissue (arrows) along the cyst walls (c). The blood flow signals of the papillary solid portion are shown on color Doppler sonography (d).
DISCUSSION
Cystic carcinomas of the breast encompass a heterogeneous spectrum of tumors. These include intracystic papillary carcinoma with or without invasion, ductal carcinoma with cystic degeneration, such as comedo forms of ductal carcinoma in situ, medullary carcinoma, squamous carcinoma, and cystic hypersecretory ductal adenocarcinoma.1-3 The prognosis of high-grade ductal carcinomas with cystic degeneration is poor, while that of intracystic papillary carcinoma is excellent. The mammographic findings of intracystic papillary carcinomas are usually well-defined, high density masses, thought to be from hemosiderin hemorrhage deposits. Routine sonogram demonstrates solid or complex cystic and solid masses with posterior acoustic enhancement. By detecting vascularities or large feeding vessels, color Doppler sonogram is useful as a diagnostic method for solid portion differentiation of the cystic mass from echogenic debris.3,4 It is rare, but possible, that a cystic carcinoma has no solid portion. Liston et al. reported imaging of an intracystic breast carcinoma without solid component, masquerading as a simple cyst.5
Sonography and aspiration biopsy are the first steps in the diagnosis of cystic carcinomas. However, cytological examination alone has high false-negative rates due to sparse cellularity, abundant obscuring blood, necrotic debris, and degenerative changes in the diagnostic cells.6,7 The fluid appearance can give clues for the differential diagnosis and for the benign or malignant nature of the cyst. In malignant cysts, aspirate is usually bloody, however, the presence of blood is not pathognomic for cancer and conversely, its absence does not exclude malignancy.8,9 The combination of clinical criteria such as patient age, presence of residual mass after aspiration, and fluid character are valuable for accurate diagnosis.9 Core needle biopsy is a useful diagnostic tool for cystic carcinomas, however, it may be unable to distinguish between in situ and invasive lesions, as the center of the lesion is often targeted yet invasion is often identified at the periphery.10,11 In cases of complicated cysts, some authors have suggested measuring the CEA value of aspirated fluid, using anti-CEA monoclonal antibody, as this is easy, safe, and helpful for definitive diagnosis of intracystic carcinoma.12
Considering the patient's age, bloody nature of the cyst, and rapid refilling after aspiration, we should have performed an excisional biopsy at the second visit. Based on our experience, we suggest close follow-up of hemorrhagic cystic masses, regardless of trauma history, and consideration of surgical excisional biopsy for cysts that rapidly grow post-aspiration, even those with negative cytology.
References
- 1.Framarino dei Malatesta ML, Piccioni MG, Felici A, Paolucci A, Triglia A, Galati G, et al. Intracystic carcinoma of the breast. Our experience. Eur J Gynaecol Oncol. 1992;13(1) Suppl:40–44. [PubMed] [Google Scholar]
- 2.Hamed H, Coady A, Chaudary MA, Fentiman IS. Follow-up of patients with aspirated breast cysts is necessary. Arch Surg. 1989;124:253–255. doi: 10.1001/archsurg.1989.01410020127022. [DOI] [PubMed] [Google Scholar]
- 3.Liberman L, Feng TL, Susnik B. Case 35: Intracystic papillary carcinoma with invasion. Radiology. 2001;219:781–784. doi: 10.1148/radiology.219.3.r01jn10781. [DOI] [PubMed] [Google Scholar]
- 4.Soo MS, Williford ME, Walsh R, Bentley RC, Kornguth PJ. Papillary carcinoma of the breast: imaging findings. AJR. 1995;164:321–326. doi: 10.2214/ajr.164.2.7839962. [DOI] [PubMed] [Google Scholar]
- 5.Liston JC. Case report: an unusual interval breast cancer masquerading as a simple cyst. Clin Radiol. 1997;52:876–878. doi: 10.1016/s0009-9260(97)80089-x. [DOI] [PubMed] [Google Scholar]
- 6.Cowen PN, Benson EA. Cytological study of fluid from breast cysts. Br J Surg. 1979;66:209–211. doi: 10.1002/bjs.1800660323. [DOI] [PubMed] [Google Scholar]
- 7.Devitt JE, Barr JR. The clinical recognition of cystic carcinoma of the breast. Surg Gynecol Obstet. 1984;159:130–132. [PubMed] [Google Scholar]
- 8.Corkill ME, Sneige N, Fanning T, el-Naggar A. Fine-needle aspiration cytology and flow cytometry of intracystic papillary carcinoma of breast. Am J Clin Pathol. 1990;94:673–680. doi: 10.1093/ajcp/94.6.673. [DOI] [PubMed] [Google Scholar]
- 9.Markopoulos C, Kouskos E, Gogas H, Kakisis J, Kyriakou V, Gogas J, et al. Diagnosis and treatment of intracystic breast carcinomas. Am Surg. 2002;68:783–786. [PubMed] [Google Scholar]
- 10.Solorzano CC, Middleton LP, Hunt KK, Mirza N, Meric F, Kuerer HM, et al. Treatment and outcome of patients with intracystic papillary carcinoma of the breast. Am J Surg. 2002;184:364–368. doi: 10.1016/s0002-9610(02)00941-8. [DOI] [PubMed] [Google Scholar]
- 11.Dogan BE, Whitman GJ, Middleton LP, Phelps M. Intracystic papillary carcinoma of the breast. Am J Roentgenol. 2003;181:186. doi: 10.2214/ajr.181.1.1810186. [DOI] [PubMed] [Google Scholar]
- 12.Matsuo S, Eto T, Soejima H, Ohara O, Hidaka O, Miyazaki J, et al. A case of intracystic carcinoma of the breast: the importance of measuring carcinoembryonic antigen in aspirated cystic fluid. Breast Cancer Res Treat. 1993;28:41–44. doi: 10.1007/BF00666354. [DOI] [PubMed] [Google Scholar]