Abstract
[6]-Gingerol, a major phenolic compound derived from ginger, has anti-bacterial, anti-inflammatory and anti-tumor activities. While several molecular mechanisms have been described to underlie its effects on cells in vitro and in vivo, the underlying mechanisms by which [6]-gingerol exerts anti-tumorigenic effects are largely unknown. The purpose of this study was to investigate the action of [6]-gingerol on two human pancreatic cancer cell lines, HPAC expressing wild-type (wt) p53 and BxPC-3 expressing mutated p53. We found that [6]-gingerol inhibited the cell growth through cell cycle arrest at G1 phase in both cell lines. Western blot analyses indicated that [6]-gingerol decreased both Cyclin A and Cyclin-dependent kinase (Cdk) expression. These events led to reduction in Rb phosphorylation followed by blocking of S phase entry. p53 expression was decreased by [6]-gingerol treatment in both cell lines suggesting that the induction of Cyclin-dependent kinase inhibitor, p21cip1, was p53-independent. [6]-Gingerol induced mostly apoptotic death in the mutant p53-expressing cells, while no signs of early apoptosis were detected in wild type p53-expressing cells and this was related to the increased phosphorylation of AKT. These results suggest that [6]-gingerol can circumvent the resistance of mutant p53-expressing cells towards chemotherapy by inducing apoptotic cell death while it exerts cytostatic effect on wild type p53-expressing cells by inducing temporal growth arrest.
Keywords: [6]-gingerol, pancreatic cancer, G1 phase, apoptosis, AKT
INTRODUCTION
Various plant-derived compounds or phytochemicals are known to have ability to interfere with carcinogenesis and tumorigenesis. There are many evidences that many phytochemicals, such as epigallocatechin-3-gallate (EGCG), genestine, tangeretin, silymarin, silibinin, and quercetin, can inhibit the proliferation or survival of various cancer cells and also induce cell cycle arrest.1-5 Plant of ginger (Zingeiber officinale Roscoe, Zingiberaceae) family is one of the most highly consumed dietary substances in the world. In China and Malaysia, the rhizome of ginger has been used in traditional oriental herbal medicine for the management of common cold, digestive disorders, rheumatism, neuralgia, colic and motion-sickness.6 The oleoresin from rhizome of ginger contains pungent ingredients including gingerol, shoagol, and zingerone.7 Recently, these phenolic substances have been found to possess many interesting pharmacological and physiological activities. Of these, [6]-gingerol (1-[4'-hydroxy-3'-methoxyphenyl]-5-hydroxy-3-decanone), the major pungent principle of ginger, has anti-oxidant, anti-inflammation and anti-tumor promoting activities.7
Anti-cancer and/or chemopreventive activities of [6]-gingerol have been reported. For instance, [6]-gingerol inhibited pulmonary metastasis in mice bearing B16F10 melanoma cells through the activation of CD8+ T cells.8 It also inhibited tumor promotion of ICR mice induced skin tumor by tumor promoter (TPA),9 and blocked the azoxymethane-induced intestinal carcinogenesis in rodents.10 [6]-Gingerol interfered with EGF-induced transformation of mouse epidermal JB6 cell line, and reduced the activation of Activator Protein-1 (AP-1), which plays a critical role in tumor promotion.11 Moreover, [6]-gingerol exerted inhibitory effects on the cell viability and DNA synthesis, also induced apoptosis of human promyelocytic leukemia HL-60 cells.12 Recently, it has been reported that ginger root extracts and gingerol inhibit the growth of Helicobacter pylori CagA+ strains, which has a specific gene linked to the development of gastric premalignant and malignant lesions.13 It suggests that ginger and gingerol have effects of chemoprevention to the gastric-intestinal cancers.
Pancreatic ductal adenocarcinoma (pancreatic cancer) is the fifth leading cause of cancer death in Korea. Pancreatic cancer is a fatal disease, with a 5-year survival rate of less than 5%.14 In the majority of cases (> 80%), at first diagnosis, pancreatic cancer has already become metastatic so that conventional treatment regimens provide minimal, if any, clinical benefit in prolonging life or ameliorating the negative prognosis of this disease.15 Resistance of recurrent disease to cytotoxic drugs is the principal factor limiting long-term treatment success against pancreatic cancer. The oncogenesis of pancreatic cancer in particular appears to favor the development and subsequent expansion of cell clones that are resistant to apoptotic triggers. The basis for failed apoptosis and more specifically the cause of chemotherapy resistance in pancreatic cancer is multifactorial. Molecular mechanisms implicated to date include expression of P-glycoproteins and other multidrug resistance proeins, mutations of K-ras and p53, and high-level expression of Bcl-2 and other inhibitors of apoptosis.16-18 Therefore, an important research objective is the identification of lead compounds that circumvent the resistance mechanisms that limit the success of conventional drugs.
Although anticancer activities of ginger extract and constituents have been examined, the underlying mechanism has not been clarified yet. The purpose of this work was to develop an understanding of [6]-gingerol's effects on pancreatic cancer cells to begin to determine its therapeutic value in preventing or treating this disease. Therefore we examined the anticancer activities of [6]-gingerol, and investigated its mechanism in two different pancreatic cancer cell lines, HPAC with wt p53 or BxPC-3 with mutant p53. [6]-Gingerol's antiproliferative effects are associated with changes in the expression or phosphorylation of Cyclin A, Cdks, p21 and Rb, and most commonly, causes cell cycle arrest at the G1 phase. We found that [6]-gingerol is capable of inducing cell death in the mutant p53-expressing cells, while arresting mutant p53-expressing cells at G1 phase. Thus, [6]-gingerol can circumvent drug resistance induced by p53 mutations.
MATERIALS AND METHODS
Chemicals and cell culture
A purified preparation of [6]-gingerol (> 98.0% pure) was purchased from Wako Pure Chemicals (Osaka, Japan). It was dissolved in sterile DMSO at a stock concentration of 50 mM and stored at -20℃. Human pancreatic cancer cells, BxPC-3 and HPAC, were obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in RPMI1640 and DMEM/F12 medium respectively, containing 10% fetal bovine serum. Both cell lines were incubated at 37℃ in a humidified atmosphere with 5% CO2.
Assessment of cell viability
The viability of the cells was measured by the MTT [3,(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] (Sigma, St. Louis, MO, USA) assay. The cells (4 × 103 cells/well) were seeded into 96-well plates, after 24 hr they were treated with various concentration of [6]-gingerol or vehicle alone (0.1% DMSO) in serum containing media. After incubation for indicated times, MTT solution was added to the plate at a final concentration of 0.5 mg/mL. The cells were incubated for 4 hr in dark at 37℃. The resulting MTT-products were dissolved by DMSO and determined by measuring the absorbance at 570 nm with ELISA reader (Molecular Devices, Sunnyvale, CA, USA). Each point represents the mean of triplicated experiments.
Cell cycle analysis
Flow cytometry was performed as previously described.19 Briefly, cells were seeded into 100 mm dishes, after 24 hr treatment with the indicated concentrations of [6]-gingerol for indicated hours. Following treatment, cells were trypsinized, and washed twice with cold PBS (pH 7.4). The pellet was fixed with cold ethanol (70%) for 12 hr at 4℃ and washed with cold PBS. Then, they were incubated with RNase (200 µg/mL final concentration) and stained with Propidium Iodide (100 µg/mL final concentration) for 1 hr and analyzed by flow cytometry. Flow cytometry was performed on a FACSCalibur system equipped with argonion laser (Becton Dickison Immunocytometry system, San Jose, CA, USA). Percentages of cells in each phase were calculated using Cell Modfit software programs (Becton Dickinson).
Assessment of apoptosis
To identify cells undergoing apoptosis, Annexin V-FITC Apoptosis Detection Kit (BD Pharmingen, Franklin Lakes, NJ, USA) was used. Cells were harvested at different intervals after [6]-gingerol treatment, containing floating and adherent cells. After washing with cold PBS (pH 7.4), the cells were stained and analyze by the flow cytometry. For each tube, 20,000 cells were immediately measured on a FACSCalibur flow cytometer. The quantitative analysis was performed with Win MDI program version 2.8 (provided by the Flow Cytometry Core Facility, The Scripps Research Institute, La Jolla, CA, USA).
Protein extraction and Western blot analysis
Whole cell lysates, used to determine the levels of various proteins, were prepared by following method. In brief, the pancreatic cancer cells were seeded onto culture-dishes, after 24 hr incubation with [6]-gingerol for the indicated times. The cells were washed twice with cold PBS (pH 7.4) and added cell lysis buffer (70 mM beta-glycerol-phosphate (pH 7.2), 0.6 mM sodium vanadate, 2 M MgCl2, 1 mM Ethylene glycol-bis (2-aminoethylether-) N,N,N',N'-tetraacetic acid (EGTA), 1 mM dithiothreitol (DTT), 0.5% Triton X-100, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), 1X Protease Inhibitor; Leupeptin, Pepstatin, Aprotinin, and Antipain each 5 µg/mL). Cell lysates were then centrifuged at 13,200 rpm for 30 min at 4℃. The protein concentrations were measured by the Bradford dye-binding protein assay, using bovine serum albumin (Sigma) as a standard. For Western blot analysis, protein samples (30 µg) were solubilized by boiling in sample buffer and subjected to SDS-PAGE followed by electrotransfer onto nitrocellulose membrane (Amersham Life Science, Buckinghamshire, UK). Blots were blocked for 2 hr at rom temperature with 5% nonfat dry milk and incubated at 4℃ overnight with the following antibodies: rabbit polyclonal anti-Cyclin A, rabbit polyclonal anti-Cyclin D1, rabbit polyclonal anti-Cyclin E, rabbit polyclonal anti-Cdk 2 and rabbit polyclonal anti-Cdk 4 (Delta Biolabs, Campbell, CA, USA), goat or rabbit polyclonal anti-pRb (Ser 780) (BioSource, Camarillo, CA, USA), mouse monoclonal anti-Rb, rabbit polyclonal anti-Cdk 6, rabbit polyclonal anti-p21, mouse monoclonal anti-p53, rabbit polyclonal anti-phosphatidylinositol-3 kinase (PI3K) p85α subunit, goat polyclonal anti-ERK1, mouse monoclonal anti-pERK, mouse monoclonal anti-phospho-c-jun-N-terminal kinase (pJNK), goat or chicken polyclonal anti-AKT1, rabbit polyclonal anti-pAKT1/2/3 (Ser 473), and mouse monoclonal anti-K-Ras (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). The membranes were washed three times and incubated with horseradish peroxidase-conjugated species-appropriate secondary antibodies (Santa Cruz). After additional washing, they were developed with enhanced chemiluminescence reagents (Amersham Life Science), and exposed to films in a dark room.
Statistical analysis
When appropriate, statistical significance was tested using a two tailed Student's t-test; p ≤ 0.05 was considered significant. All values shown are means with the corresponding standard error.
RESULTS
[6]-Gingerol inhibits cell growth on both BxPC-3 and HPAC cells
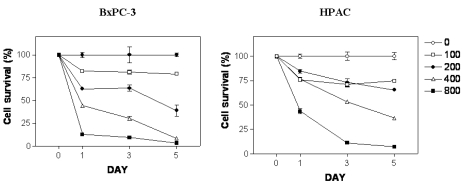
To determine antiproliferative effects of [6]-gingerol on two pancreatic cancer cells expressing either wt (HPAC) or mutant p53 protein (BxPC-3), we treated cells with [6]-gingerol ranging from 50 to 800 µM. As shown in Fig. 1, cells treated with different concentrations of [6]-gingerol for 0, 24, 72, and 120 h resulted in the growth inhibition in a dose- and time-dependent manner. [6]-Gingerol at the highest concentration (800 µM) had similar mean cytotoxicity over the 72 h of 89.4% and 91.2% on BxPC-3 and HPAC cells, respectively. There was no significant difference in IC50 values between BxPC-3 cells and HPAC cells (387.4 µM and 405.3 µM at day 3, respectively, p < 0.006). IC25 values were 186.1 and 146.3 µM for BxPC-3 and HPAC, respectively.
Fig. 1.
Effect of [6]-gingerol on survival of BxPC-3 and HPAC cells. [6]-Gingerol inhibited growth of both BxPC-3 and HPAC cells in a dose-dependent manner. Cells were treated with different concentrations of [6]-gingerol. Control cells (empty circle) were treated with vehicle alone (0.1% DMSO). Cells were harvested at day 1, 3 and day 5 and cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described in Materials and methods. Percent viability was calculated by comparison with controls. Values shown as the mean ± SEM obtained from four independent experiments.
[6]-Gingerol induces cell cycle arrest
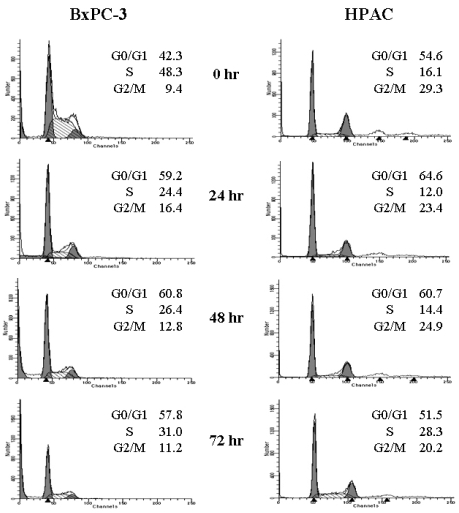
Although it was apparent that [6]-gingerol was growth inhibitory to the pancreatic cancer cell lines, the sustained effects on culture growth raised suspicion that it might also affect the proliferation of surviving cells. To test this possibility, we examined whether [6]-gingerol affects cell cycle progression. BxPC-3 and HPAC cells were treated with 400 µM of [6]-gingerol (approximate IC50 at day3). As shown in Fig. 2, [6]-gingerol caused BxPC-3 cells to accumulate in G1-phase (59.2% in [6]-gingerol-treated vs. 42.3% in control) within 24 hr at the expense of cells mainly in S phase. Furthermore, this was associated with the induction of apoptosis as represented by the large sub-G1 peak. On the other hand, cell cycle arrest of HPAC by [6]-Gingerol was less dramatic in HPAC cells where cellular accumulation in G0/G1 phase was slightly higher in [6]-gingerol treated group (64.6%) compared to the control (54.6%). In contrast to BxPC-3 cells, we did not detect an increase in HPAC cells with hypodiploid DNA content, suggesting that apoptotic cell death was not induced. At 72 hr, HPAC cells partially recovered from [6]-gingerol-induced growth arrest suggesting the operation of compensatory mechanism(s).
Fig. 2.
[6]-Gingerol induces cell cycle arrest in both BxPC-3 and HPAC cell lines. Exponentially growing cells were exposed to either 0.1% DMSO (control) or [6]-gingerol (400 µM) for 24, 48 or 72 hrs. Cells were then harvested, washed in PBS, and fixed in 70% ethanol. DNA content was evaluated with propidium iodide staining and fluorescence measured and analyzed as described in Materials and Methods. Data are representative of three independent experiments.
[6]-Gingerol caused changes in various cell cycle related protein expressions
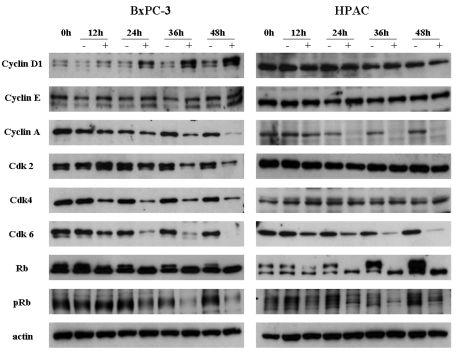
We examined the effect of [6]-gingerol on the levels of G1 phase-regulatory proteins by Western blot analyses (Fig. 3). Both BxPC-3 and HPAC cells were treated with 400 µM of [6]-gingerol and harvested in every 12 hr. In BxPC-3 and HPAC cells, expression levels of various proteins were changed by [6]-gingerol. In BxPC-3, [6]-gingerol induced decrease in Cyclin A, Cdk 2, Cdk 4 and Cdk 6 expression and increase in Cyclin D1 expression. [6]-Gingerol decreased the Cyclin A and Cdk 6 expression, but not Cdk 2 and Cdk 4, in HPAC cells. Phosphorylated Rb protein was decreased in both BxPC-3 and HPAC cells by [6]-gingerol.
Fig. 3.
Western blot analyses of the cell cycle regulatory proteins. Both BxPC-3 and HPAC were treated with 400 µM of [6]-gingerol, and harvested every 12 hr, and levels of Cyclins, Cdks, Rb and pRb were determined by Western blotting. The upper band of Rb indicates phosphorylated form. pRb antibody detects specifically on Ser-780 phophorylated Rb. Data are representative of three independent experiments. Actin was used as a standard for each sample.
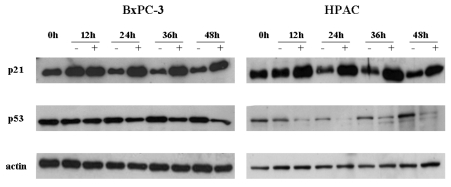
Next, we studied the difference in basal and [6]-gingerol-induced levels of p53 and p21cip1, between wt and mutant p53-expressing cells. In line with previous report,20 the basal levels of p53 were elevated in mutant p53-expressing cells. [6]-Gingerol reduced the p53 levels of wt, but less significantly of mutant, p53-expressing cells (Fig. 4). Expression of p21cip1 was increased by [6]-gingerol in both BxPC-3 and HPAC cells. It is well known that induction of p21cip1 is associated with the expression of p53.21 The treatment of [6]-gingerol reduced the p53 level in the both cells implying that the overexpression of p21cip1 by [6]-gingerol might be caused by p53-independent events in both cell lines.
Fig. 4.
Effects of [6]-gingerol on the expression of p21cip and p53. Both BxPC-3 and HPAC were treated with 400 µM of [6]-ingerol, and harvested every 12 hr. The levels of p21cip1, a Cyclin dependent kinase inhibitor, and p53 changed by [6]-Gingerol in BxPC-3 and HPAC cells. Three replicate experiments were done with similar results.
[6]-Gingerol induces apoptosis in BxPC-3 cell
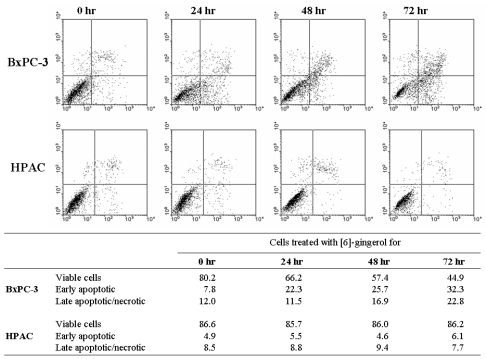
Next, we assessed potential differences between the [6]-gingerol-induced cell death in wt and mutant p53-expressing cells. Since mutations in p53 often compromise the ability of cells to undergo apoptosis, we analyzed the mode of cell death involved. As can be seen in Fig. 5, early apoptosis (Annexin-V+/propidium iodide-) could be detected in the mutant p53-expressing BxPC-3 cells 24 hr after treatment with [6]-gingerol. The percentage of early-apoptotic cells and late apoptotic/necrotic cells (Annexin-V+/propidium iodide+) continued to rise, reaching a peak at 72 hr. On the other hand, [6]-gingerol did not induce death in wild type p53 expressing HPAC cells. HPAC cells displayed few early apoptosis and late apoptotic/necrotic fractions until 72 hr suggesting that HPAC cells are highly resistance to [6]-gingerol induced apoptosis. This observation corresponds to the data of cell cycle analysis where HPAC cells had minimal sub-G1 population upon [6]-gingerol treatment even after 72 hr.
Fig. 5.
Differential mode of death induced by [6]-Gingerol in pancreatic cancer cells expressing wt versus mutant p53. Cells were treated with 400 µM of [6]-gingerol for 24, 28 and 72 hrs. Annexin-V and PI staining revealed increasing percentage of Annexin-positive and PI-positive cells with increasing time of [6]-gingerol in BxPC-3 cells. On the other hand, [6]-gingerol treatment on HPAC cells showed no changes in cell viability under the identical culture condition. The X and Y axis represents annexin V-FITC and Propidium Iodide (PI) fluorescence respectively. Population in lower-left part (Annexin V-FITC and PI negative) is viable, and lower-right part (Annexin V-FITC positive and PI negative) is undergoing apoptosis. Cells observed in Annexin V-FITC and PI positive (upper-right and upper-left parts) indicates either late stage of apoptosis or dead cells. The percentage of each part is calculated in the bottom table. The figure is representative of three independent experiments.
[6]-Gingerol affects AKT activation
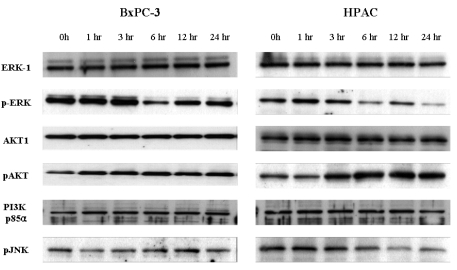
Based on these findings, we sought to examine whether [6]-gingerol-induced apoptotic cell death was accompanied by the activation of common pro-apoptotic signaling pathways. The difference in [6]-gingerol response of these two cell lines may be caused by the altered PI3K/AKT pathways which play important roles in cell cycle progression and cell survival.22 [6]-Gingerol was found to inhibit nuclear factor kappa B (NF-kB), AP-1, cyclooxygenase-2 (COX-2) and p38 mitogen activated protein kinase (MAPK).23 Thus, we examined the levels of pERK, pJNK and PI3K/AKT pathway proteins by Western blot analysis (Fig. 6). The down regulation of pJNK and pERK MAPK was time-dependently induced by [6]-gingerol in both cell types. In both cell lines, there was no change of PI3K regulatory subunit, p85α, levels by [6]-gingerol. While [6]-gingerol did not change phosphorylation of AKT protein in BxPC-3 cells, active phosphorylated AKT (pAKT) started to appear 3 hr after incubation and increased further up to 24 hr in [6]-gingerol-treated HPAC cells. These results imply that [6]-gingerol-induced apoptosis in HPAC cells (p53 wild type) is suppressed via the PI3K/AKT pathway.
Fig. 6.
Western bolt analysis for MPAK and PI3K/AKT pathways in [6]-gingerol-treated pancreatic cancer cells. The BxPC-3 (A) and HPAC (B) were incubated with 400 µM of gingerol for indicated times. Cells were lysed and the cell lysates (30 µg) were resolved by SDS-PAGE. AKT, ERK and JNK activation was analyzed by Western blotting with anti-phospho-AKT, anti-phosph-ERK, anti-phosph-JNK or anti-p85-specific antibodies as indicated. Equal protein loading was confirmed by probing the membranes with antibodies detecting the respective unphosphorylated proteins (ERK and AKT). Three replicate experiments were done with similar results.
DISCUSSION
Phenolic compounds comprise one of the largest and most ubiquitous groups of plant metabolites. They are formed to protect the plant from photosynthetic stress, reactive oxygen species (ROS), wounds, and herbivores.24 The most commonly contained ones in foods are flavonoids and phenolic substances. Hence, phenolic compounds take important parts of the human diet. In addition, current interest is raised up by many observations that dietary phenolic compounds have various activities such as antioxidant, anti-inflammation and anti-carcinogenesis.
In this present study, we first investigated the effects of [6]-gingerol, a phenolic substance derived from ginger roots, on two pancreatic cancer cell lines. We found that [6]-gingerol inhibited the cell growth, disrupted the cell cycle progression in both HPac cells (wild type p53) and BXPC-3 cells (mutant p53 protein), and also induced apoptosis in BxPC-3 cells. Interestingly, it is noticeable that normal cell showed highly resistance to the cytotoxic effects of [6]-gingerol. RIE (rat intestinal epithelial cell) showed 50 percents growth inhibition at over 900 µM (data not shown) of [6]-gingerol for 3 days treatment. This selectivity may be the great advantage of [6]-gingerol for the therapeutic or preventative use. While there were some reports that various phenolic substances induce cell cycle arrest in some phases,1-5 this is the first report that reveals on [6]-gingerol effect upon cell cycle in cancer cell lines. Western blot analyses indicated that [6]-gingerol decreased the expression of Cyclin A and Cdks including Cdk2, Cdk4, and Cdk6 in BxPC-3. Also, Cyclin A and Cdk 6 expression levels were decreased in HPAC. Thus, the reduction of Cyclin or Cdk expressions results the blocking of Cyclin-Cdk complexes formation and that lowers the level of phospho-Rb. Since Rb proteins remain in unphosphorylated form, E2F cannot be activated and the cells fail to enter the S phase. Cyclin D1 might be the cause of apoptotic cell death when overexpressed.25,26 Alternatively, increase of Cyclin D1 in BxPC-3 cells may be a feedback response to drug-induced cell cycle arrest.
p53 is a tumor suppressor gene encoding a transcription factor. Its tumor-suppressive activity involves inhibition of cell proliferation through cell cycle arrest and/or apoptosis. Mutation in p53 occurs in more than half of human cancers.27 Cells harboring mutated p53 lose the ability to elicit the enzymatic DNA repair cascade, to inhibit cell proliferation and to induce programmed cell death, resulting in induction of uncontrolled proliferation and malignancy.28,29 Tumors consisting of mutant p53-expressing cells exhibit high resistance to radiation and chemotherapeutic drugs. Circumventing this abnormal resistance is a major challenge in cancer therapy.20,30 In the present study, we showed that [6]-gingerol exerted its cytotoxic activy toward cancer cells harboring mutant p53. Based on in vitro and in vivo studies, the Cip/Kip family including p21Cip1 were initially thought to interfere with the activation of G1/S phase related Cyclin/Cdk complexes. The Cyclin-dependent kinase inhibitor p21cip1 is a major transcriptional target of the tumor-suppressor p53.21 BxPC-3 cells have point mutated p53 proteins and the HPAC cells have the wild type p53 proteins. While the basal levels of p53 were elevated in mutant p53-expressing cells as previously reported,20 the level of p53 is decreased by [6]-gingerol in both cell lines. Thus, p21cip1 induction by [6]-gingerol was not necessarily dependent on p53 wild-type status since we detected p21 induction in mutated p53 cells.
It has been reported Ras signaling through the Raf/MAPK pathway also elevates levels of p21cip1 in some cell types.31-33 However it is still unclear whether the over-expression of p21cip1 by [6]-gingerol is related with Ras signaling activations. A number of phytochemicals, including EGCG,34 tangeretin,3 genestein and silymarin4 have been shown to induce cell cycle arrest accompanied by increased p21cip1 expression, an important Cdk inhibitor in G1 and S phase. [6]-Gingerol also increased the level of p21cip1 in both cell lines. The overexpression of p21cip1 facilitated cell cycle arrest effects of [6]-gingerol. Unlike other Cyclins, Cyclin D1 level was increased in BxPC-3. It is probably as a feedback response to G1 arrest. More recent study, however, has altered this view and revealed that even p21cip1 proteins are specific inhibitors of Cyclin E- and A-dependent Cdk2, they act as positive regulators of Cyclin D-dependent kinases.31 Thus, the increase of Cyclin D1 level in BxPC-3 cells may be the consequence of the overexpression of p21cip1. However, it is unclear why there was no change of Cyclin D1 level in spite of G1 phase arrest and overexpression of p21cip1 in HPAC cells. The fact that [6]-gingerol has different effects on the cell cycle in different cell lines is intriguing and suggests that the ability of [6]-gingerol to affect the cell cycle may be dependent on other genetic alterations that the tumor harbors. For example, the levels of Cyclins, Cyclin-dependent kinases, their inhibitors, or the status of tumor suppressor genes such as p53 and Rb, that are all involved in cell cycle regulation, may determine whether a chemical inhibitor (drug) results in cell cycle arrest or not.
Exposure of mammalian cells to growth factors or genotoxic stress elicits a variety of cellular responses, including the activation of protein kinase cascades involving ERKs, stress-activated protein kinases (SAPK/JNK) and p38 MAPK.35 Therefore, we determined the effect of [6]-gingerol on the activation of extracellular signal-regulatted protein kinase-1/2 (ERK1/2), p38 MAPK, and JNK, which are representative MAPKs involved in a wide array of cellular signaling cascade. PI3K/AKT pathway has an important role in preventing cells from undergoing apoptosis and contributing to the pathogenesis of malignancy.36 More recently evidences have suggested that this pathway is also associated with the regulation of cell cycle progression.22 The anti-cancer activities of [6] gingerol could be associated with a control of signal transduction of PI3K/AKT pathway and this led us to investigate the change of the survival pathway-associated proteins. In the both cells [6]-gingerol could not affect the expression of the regulatory subunit of PI3K, p85α. However, [6]-gingerol increased phosphorylation of AKT, which is regulated by PI3K, in only the HPAC cells. Activated AKT is known to promote the cell survival by anti-apoptoic mechanism.37 Additionally, activated AKT also phosphorylates and inactivates the proapoptotic protein, BAD.22,36,38 In HPAC cells, the increase of phospholated AKT might protect apoptosis, despite of cell cycle arrest by [6]-gingerol. On the other hand, there was no change in phosphorylation of AKT by [6]-gingerol-treated BxPC-3 cells and thus failed to counteract the gingerol-induced apoptotic cell death.
In conclusion, we describe experiments that show [6]-gingerol induces apoptotic cell death in p53-mutant cancer cells. The death mechanism was characterized, revealing that [6]-gingerol not only initiated cell cycle arrest but ultimately caused cell death through apoptosis. Thus, [6]-gingerol, is capable of killing cancer cells expressing mutant p53, overcoming the phenotypic resistance to chemotherapy- and irradiation-induced cell death. These findings support the importance of studying [6]-gingerol and gingerol-related compounds as anticancer agents that can potentially eradicate tumors resistant to radiation and to currently available chemotherapy.
Footnotes
This work was supported in part by the Brain Korea 21 Project for Medical Science.
References
- 1.Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst. 1997;89:1881–1886. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 2.Liu XJ, Yang L, Mao YQ, Wang Q, Huang MH, Wang YP, et al. Effects of the tyrosine protein kinase inhibitor genistein on the proliferation, activation of cultured rat hepatic stellate cells. World J Gastroenterol. 2002;8:739–745. doi: 10.3748/wjg.v8.i4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan MH, Chen WJ, Lin-Shiau SY, Ho CT, Lin JK. Tangeretin induces cell-cycle G1 arrest through inhibiting Cyclin-dependent kinases 2 and 4 activities as well as elevating Cdk inhibitors p21 and p27 in human colorectal carcinoma cells. Carcinogenesis. 2002;23:1677–1684. doi: 10.1093/carcin/23.10.1677. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal R. Cell signaling and regulators of cell cycle as molecular targets for prostate cancer prevention by dietary agents. Biochem Pharmacol. 2000;60:1051–1059. doi: 10.1016/s0006-2952(00)00385-3. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia N, Agarwal C, Agarwal R. Differential responses of skin cancer-chemopreventive agents silibinin, quercetin, and epigallocatechin 3-gallate on mitogenic signaling and cell cycle regulators in human epidermoid carcinoma A431 cells. Nutr Cancer. 2001;39:292–299. doi: 10.1207/S15327914nc392_20. [DOI] [PubMed] [Google Scholar]
- 6.Surh Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res. 1999;428:305–327. doi: 10.1016/s1383-5742(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 7.Surh YJ, Lee E, Lee JM. Chemoprotective properties of some pungent ingredients present in red pepper and ginger. Mutat Res. 1998;402:259–267. doi: 10.1016/s0027-5107(97)00305-9. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki F, Kobayashi M, Komatsu Y, Kato A, Pollard RB. Keishi-ka-kei-to, a traditional Chinese herbal medicine, inhibits pulmonary metastasis of B16 melanoma. Anticancer Res. 1997;17:873–878. [PubMed] [Google Scholar]
- 9.Park KK, Chun KS, Lee JM, Lee SS, Surh YJ. Inhibitory effects of [6]-gingerol, a major pungent principle of ginger, on phorbol ester-induced inflammation, epidermal ornithine decarboxylase activity and skin tumor promotion in ICR mice. Cancer Lett. 1998;129:139–144. doi: 10.1016/s0304-3835(98)00081-0. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimi N, Wang A, Morishita Y, Tanaka T, Sugie S, Kawai K, et al. Modifying effects of fungal and herb metabolites on azoxymethane-induced intestinal carcinogenesis in rats. Jpn J Cancer Res. 1992;83:1273–1278. doi: 10.1111/j.1349-7006.1992.tb02758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bode AM, Ma WY, Surh YJ, Dong Z. Inhibition of epidermal growth factor-induced cell transformation and activator protein 1 activation by [6]-gingerol. Cancer Res. 2001;61:850–853. [PubMed] [Google Scholar]
- 12.Lee E, Surh YJ. Induction of apoptosis in HL-60 cells by pungent vanilloids, [6]-gingerol and [6]-paradol. Cancer Lett. 1998;134:163–168. doi: 10.1016/s0304-3835(98)00253-5. [DOI] [PubMed] [Google Scholar]
- 13.Mahady GB, Pendland SL, Yun GS, Lu ZZ, Stoia A. Ginger (Zingiber officinale Roscoe) and the gingerols inhibit the growth of Cag A+ strains of Helicobacter pylori. Anticancer Res. 2003;23:3699–3702. [PMC free article] [PubMed] [Google Scholar]
- 14.Oya N. Chemoradiotherapy for pancreatic cancer: current status and perspectives. Int J Clin Oncol. 2004;9:451–457. doi: 10.1007/s10147-004-0449-6. [DOI] [PubMed] [Google Scholar]
- 15.Ridwelski K, Meyer F. Current options for palliative treatment in patients with pancreatic cancer. Dig Dis. 2001;19:63–75. doi: 10.1159/000050655. [DOI] [PubMed] [Google Scholar]
- 16.Nio Y, Dong M, Uegaki K, Hirahara N, Minari Y, Sasaki S, et al. p53 expression affects the efficacy of adjuvant chemotherapy after resection of invasive ductal carcinoma of the pancreas. Anticancer Res. 1998;18:3773–3779. [PubMed] [Google Scholar]
- 17.King TC, Estalilla OC, Safran H. Role of p53 and p16 gene alterations in determining response to concurrent paclitaxel and radiation in solid tumor. Semin Radiat Oncol. 1999;9(2) Suppl 1:4–11. [PubMed] [Google Scholar]
- 18.Xu ZW, Friess H, Buchler MW, Solioz M. Overexpression of Bax sensitizes human pancreatic cancer cells to apoptosis induced by chemotherapeutic agents. Cancer Chemother Pharmacol. 2002;49:504–510. doi: 10.1007/s00280-002-0435-5. [DOI] [PubMed] [Google Scholar]
- 19.Song SY, Meszoely IM, Coffey RJ, Pietenpol JA, Leach SD. K-Ras-independent effects of the farnesyl transferase inhibitor L-744,832 on Cyclin B1/Cdc2 kinase activity, G2/M cell cycle progression and apoptosis in human pancreatic ductal adenocarcinoma cells. Neoplasia. 2000;2:261–272. doi: 10.1038/sj.neo.7900088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace-Brodeur RR, Lowe SW. Clinical implications of p53 mutations. Cell Mol Life Sci. 1999;55:64–75. doi: 10.1007/s000180050270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natsugoe S, Nakashima S, Matsumoto M, Xiangming C, Okumura H, Kijima F, et al. Expression of p21 WAF1/Cip1 in the p53-dependent pathway is related to prognosis in patients with advanced esophageal carcinoma. Clin Cancer Res. 1999;5:2445–2449. [PubMed] [Google Scholar]
- 22.Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 23.Kim SO, Kundu JK, Shin YK, Park JH, Cho MH, Kim TY, et al. [6]-Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-kappaB in phorbol ester-stimulated mouse skin. Oncogene. 2005;24:2558–2567. doi: 10.1038/sj.onc.1208446. [DOI] [PubMed] [Google Scholar]
- 24.Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 25.Quelle DE, Ashmun RA, Shurtleff SA, Kato JY, Bar-Sagi D, Roussel MF, et al. Overexpression of mouse D-type Cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 26.Kranenburg O, van der Eb AJ, Zantema A. Cyclin D1 is an essential mediator of apoptotic neuronal cell death. EMBO J. 1996;15:46–54. [PMC free article] [PubMed] [Google Scholar]
- 27.Willis AC, Chen X. The promise and obstacle of p53 as a cancer therapeutic agent. Curr Mol Med. 2002;2:329–345. doi: 10.2174/1566524023362474. [DOI] [PubMed] [Google Scholar]
- 28.Nelson WG, Kastan MB. DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol Cell Biol. 1994;14:1815–1823. doi: 10.1128/mcb.14.3.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 30.Seeman S, Maurici D, Oliver M, de Formentel CC, Hainaut P. The tumor suppressor gene TP53: implications for cancer management and therpay. Crit Rev Clin Lab Sci. 2004;41:551–583. doi: 10.1080/10408360490504952. [DOI] [PubMed] [Google Scholar]
- 31.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 32.Coleman ML, Marshall CJ, Olson MF. Ras promotes p21(Waf1/Cip1) protein stability via a Cyclin D1-imposed block in proteasome-mediated degradation. EMBO J. 2003;22:2036–2046. doi: 10.1093/emboj/cdg189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olson MF, Paterson HF, Marshall CJ. Signals from Ras and Rho GTPases interact to regulate expression of p21Waf1/Cip1. Nature. 1998;394:295–299. doi: 10.1038/28425. [DOI] [PubMed] [Google Scholar]
- 34.Liberto M, Cobrinik D. Growth factor-dependent induction of p21(CIP1) by the green tea polyphenol, epigallocatechin gallate. Cancer Lett. 2000;154:151–161. doi: 10.1016/s0304-3835(00)00378-5. [DOI] [PubMed] [Google Scholar]
- 35.Cho SG, Choi EJ. Apoptotic signaling pathways: caspases and stress-activated protein kinases. J Biochem Mol Biol. 2002;35:24–27. doi: 10.5483/bmbrep.2002.35.1.024. [DOI] [PubMed] [Google Scholar]
- 36.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Zhu GD. Targeting serine/threonine protein kinase B/Akt and cell-cycle checkpoint kinases for treating cancer. Curr Top Med Chem. 2002;2:939–971. doi: 10.2174/1568026023393318. [DOI] [PubMed] [Google Scholar]
- 38.Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci USA. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]