Abstract
In an effort to investigate the molecular basis of growth discordance in embryos that experience the same uterine environment, we compared telomerase activity and apoptosis in placental trophoblasts obtained from growth discordant twins. Between January 2003 and February 2005, placental tissue from twenty pairs of twins was obtained within thirty minutes of delivery. Eleven cases were classified as growth discordant, with birth weight discordance greater than 20%. Nine cases comprised the control group, with less than 20% discordance. Telomerase and apoptotic activities in placental trophoblasts were analyzed by ELISA and immunoblot. Statistical significance was analyzed by a paired t-test, chisquared test, and ANOVA (SPSS ver 11.0). The average growth discordance was 26.8% in the growth discordant group and 14.4% in the control group. There were no significant differences in maternal age, week of gestation at delivery, parity, or chorionisity between the two groups. In the growth discordant group, the larger twin showed significantly higher telomerase activity (p < 0.01), whereas no significant difference was observed in the control group (p = 0.36). In addition, there was no definitive correlation between telomerase activity and the degree of growth discordance in the larger or smaller twins (R = -0.521 and -0.399, p = 0.15 and 0.25, respectively). The apoptosis proteins Bax and Bcl 2 were detected in both the larger and smaller twins in the growth discordant and control groups. There was no statistically significant difference in Bax expression between the larger and smaller twins (p = 0.25 and 0.92, respectively) for either the growth discordant or the control groups. Bcl 2 expression also showed no significant difference between groups. In conclusion, a tendency toward reduced telomerase activity and increased apoptosis was discovered in placental trophoblasts of the smaller growth-discordant twin, possibility resulting in delayed fetal growth.
Keywords: Telomerase, apoptosis, trophoblast, twins
INTRODUCTION
Growth discordance is a phenomenon specific to twins. By definition, a 20 to 25% difference in twin birth weights is considered growth discordance. Growth discordant twins show significant differences in their growth rate and sizes even though they develop in the same intrauterine environment. It has also been reported that, in growth discordant twins, fetal and neonatal deaths are approximately three to six times more common than in normal twins.1,2
Telomeres at the distal ends of chromosomes are known to be involved in chromosome stabilization by preventing degradation, fusion, or rearrangement of chromosome ends. In most human somatic cells, telomeres gradually shorten with repeated cell divisions, until they make proper cell division difficult. This leads to senescence and, eventually, cell death.3-6 In placental trophoblasts, telomerase activity is relatively high during the early stages of pregnancy and decreases as the pregnancy progresses. This change is purported to be due to enzymatic activity related to cellular proliferation and regeneration.7,8 Previous reports state that telomerase activity is low in spontaneous abortion and conditions of restricted fetal growth, such as preeclampsia. This evidence suggests a possible relationship between telomerase activity and the maintenance of pregnancy and fetus growth.9-11
Apoptosis is another important variable with a critical role in development, growth, and senescence. Disturbing apoptosis can cause a variety of diseases, such as cancer, AIDS, and autoimmune disease. Bax and Bcl 2 are two important apoptotic protein; the former promotes apoptosis, and the latter suppresses it.12-14 Studies of Bax and Bcl 2 have shown that preeclampsia and fetal growth restriction also relate to apoptosis.9,15
Previous studies, undertaken to explain growth discordance, attempted to use macroscopic factors, such as chorionisity, the number of placentas, and umbilical cord insertion. The number of studies at the molecular level is limited. In this study, we presumed that molecular factors in the placental trophoblasts are involved in the differences in fetal sizes. Therefore, we compared telomerase activity and level of apoptotic proteins, such as Bax and Bcl 2, in the placental trophoblast and assessed their effects on growth discordance.
MATERIALS AND METHODS
Sample collection
From January 2003 to February 2005, placental tissues were collected from twenty pairs of twins born at Gil Medical Center, Gachon Medical School. Among them, eleven pairs with weight differences over 20% were classified as the growth discordant group, and nine cases with less than 20% weight difference comprised the control group. About 2 g of tissue was taken from the area below the umbilical cord insertion within thirty minutes of each baby's delivery. These tissues were stored at -70℃ until used.
Protein extraction
About 50-100 mg from each of the twenty placental samples was added to a tube containing 300 µL protein lysis reagent (50 mM Tris-HCl, pH 7.5, 0.2 M NaCl, 5 mM CaCl2, 1% Triton X-100) in a pre-cooled glass tube and homogenized (Biospec Product, Inc, Bartlesville, USA). Each homogenate was aliquoted into a 1.5 mL test tube, incubated on ice for 30 min, and centrifuged at 16,000 rpm for 20 min at 4℃ (Micro-17R Plus, Micro High Speed Centrifuge, Hanil Co., Bucheon, Korea). Each supernatant was collected and frozen at -70℃ for at least 1 hour and then thawed at room temperature and centrifuged at 15,000 rpm for 15 min at 4℃. The supernatants were stored at -70℃ after adding EDTA-free protease inhibitor. Protein concentrations were quantified using the Bradford method.
Polymerase chain reaction
For each sample, the volume of PCR reagents, polymerase, and template was brought increased to 30 µL with distilled water. This mixture was aliquoted into PCR tubes, heated at 85℃ for 10 minutes, and allowed to react at 25℃ for 30 min for primer elongation. The tubes were then incubated at 94℃ for 5 min, followed by 30 reaction cycles. Each cycle consisted of 30 sec at 94℃, 30 sec at 50℃, and 90 sec at 72℃, in that order, with a final 72℃ incubation for 10 min. The tubes were stored at 4℃. As a control, a telomerase assay kit was used by mixing 1 µL each of solution 4 and solution 1 in one tube and 1 µL each of solution 5 and solution 1 in another tube.
ELISA assay
In each of two test tubes, 2.5 µL of the PCR amplified sample and 10 µL of denaturation reagent were mixed and incubated at room temperature for 10 min. 100 µL hybridization buffer T was added to the first tube, and 100 µL hybridization buffer IS was added to the second tube. Buffer T was added to the lysis buffer and denaturation reagent mixture as a control. For the two PCR controls, buffer T and buffer IS were added. The tubes were mixed well, and 100 µL aliquots were planted to MTP. There were 3 aliquots per sample (heated sample + buffer T/sample + buffer T/sample + buffer IS). Subsequently, samples were shaken at 37℃ for 2 hours, after which the supernatant was removed and washed 3 times with 250 µL washing buffer. 100 µL Anti-DIG-HRP working solution was added at room temperature for 30 min with shaking. After removing the supernatant, the samples were washed 5 times with 250 µL washing buffer. 100 µL pre-warmed TMB substrate solution was added with shaking at room temperature for 20 min. After adding 100 µL stop reagent, it was read at 450 nm, using an ELISA reader.
Bax and Bcl 2 immunoblots
5 mg of total protein was separated on a 10% polyacrylamide gel. The protein was then transferred onto a PVDF membrane (Roche, Mannheim, Germany), using an electrode transfer kit (Bio-Rad, Hercules, CA, USA). The membrane was incubated for 8 hours in a shaker with blocking solution (5% nonfat dry milk in PBS-T with 0.1% Tween 20). The blocking solution was removed completely, and the primary antibodies against Bax and Bcl 2 (rabbit polyclonal IgG, Santa Cruz Biotechnology, CA, USA) were added. The samples were shaken for 16 hours at 4℃. Next, the membrane was rinsed 4 times with PBS, and the secondary antibody (Horseradish peroxidase conjugate anti-rabbit Ig, Amersham Corp, Arlington Heights, IL, USA) was added. The blot was shaken at room temperature for 1 hour, rinsed 3 times with PBS, and treated with Western detection solution (Intron Biotechnology, Suwon, Korea). A film (Eastman Kodak, Rochester, NY, USA) was exposed to the membrane and developed in a dark room. The Bax and Bcl 2 band intensities were then measured.
Statistical analysis
The difference in telomerase activity in each placental tissue was analyzed by the paired Student's t-test. The degree of discordance and correlation to chorionisity were analyzed by Pearson's correlation and ANOVA regression (SPSS ver 11.0, Chicago, IL, USA). The difference in Bax and Bcl 2 concentrations was analyzed by a chi-squared test. A p < 0.05 was considered statistically significant.
RESULTS
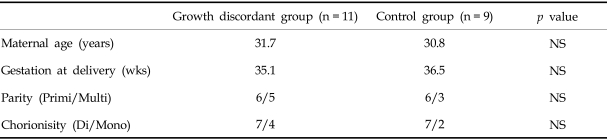
The average maternal age for the growth discordant and control groups was 31.7 and 30.8 yrs, respectively. The weeks of gestation at delivery were 35.1 and 36.5 weeks, respectively. The distributions were similar between the two groups for both parameters. The ratio of primiparity to multiparity was 6:5 and 6:3, respectively, and the ratio of monochorions to dichorions was 4:7 and 2:7, respectively. The differences between the two groups in the above parameters are not significant (Table 1).
Table 1.
Clinical Characteristics of the Growth Discordant Twin Fetuses and the Control Group
Primi, primiparity; Multi, multiparity; Di, dichorion; Mono, monochorion; NS, not significant.
Telomerase activity
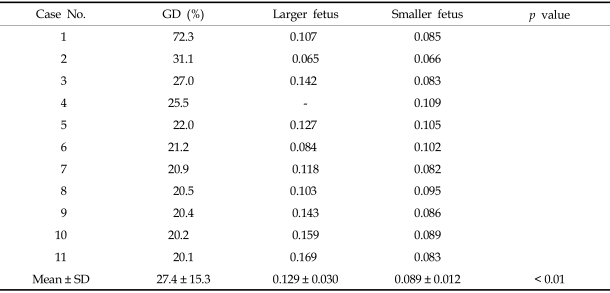
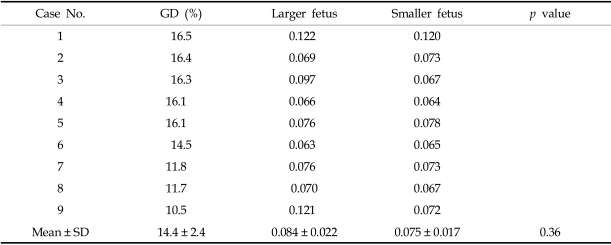
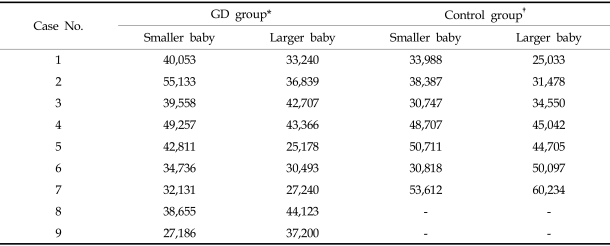
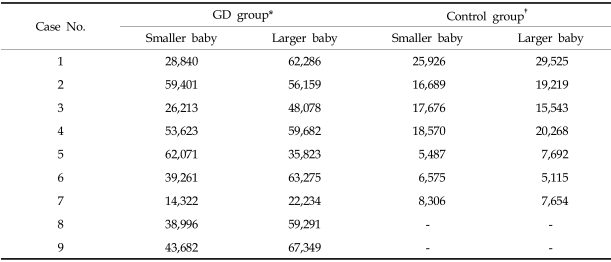
The telomerase activity, assayed by ELISA, of the larger twins in the growth discordant group was found to be 0.156 ± 0.082, which is significantly higher than the activity of the smaller twins (0.090 ± 0.012, p < 0.02, Table 2). In the control group, the enzyme activities were 0.084 ± 0.022 and 0.075 ± 0.017 for the larger and smaller twins, respectively. These numbers are not significantly different (p = 0.36, Table 3).
Table 2.
Telomerase Activities of the Growth Discordant Group (n = 11) by ELISA (at 450 nm)
GD, growth discordance.
Table 3.
Telomerase Activities of the Control Group (n = 9) by ELISA (at 450 nm)
GD, growth discordance.
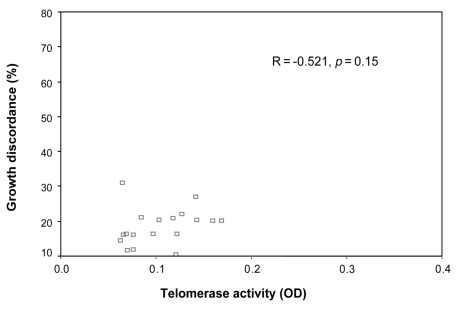
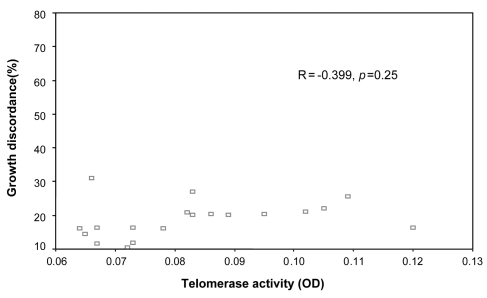
There was no clear correlation between the degree of growth discordance and the difference in telomerase activity (R = -0.521 and -0.399, p = 0.15 and 0.25, for large and small twins, respectively, Fig. 1 and 2).
Fig. 1.
The correlation between the telomerase activities of larger fetuses and growth discordance.
Fig. 2.
The correlation between the telomerase activities of smaller fetuses and growth discordance.
Apoptosis protein expression
Bax and Bcl 2 expression was measured in the large and small twins in both the growth discordant and control groups. The Bax expression levels for the large and small twins in either group were not significantly different (p = 0.25 in the growth discordant group and 0.92 in the control group, Table 4).
Table 4.
The Intensities of Bax in Trophoblasts of the Growth Discordant Group and the Control Group
*p = 0.25, †p = 0.92.
Not significant between smaller and larger fetuses of the growth discordant group and the control group.
GD, growth discordance.
Bcl 2 expression was also not significantly different between the large and the small twins in either group (p = 0.12 in the growth discordant group and 0.85 in the control group, Table 5).
Table 5.
The Intensities of Bcl 2 in Trophoblasts of the Growth Discordant Group and the Control Group
*p = 0.12, †p = 0.85.
Not significant between smaller and larger fetuses of the growth discordant group and the control group.
GD, growth discordance.
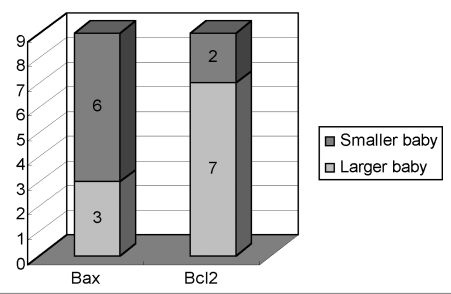
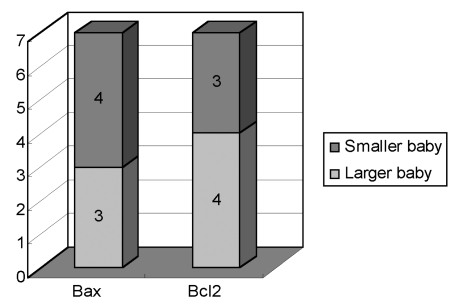
In the growth discordant group, the Bax expression was higher in 6 of 9 smaller twins. Bcl 2 expression was lower in seven of nine smaller twins (Fig. 3). Bax and Bcl 2 were expressed similarly in all of the larger and smaller twins in the control group (Fig. 4).
Fig. 3.
Comparison of the apoptotic findings of trophoblasts between the larger fetus and the smaller fetus in the growth discordant group.
Fig. 4.
Comparison of the apoptotic findings of trophoblasts between the larger fetus and the smaller fetus in the control group.
DISCUSSION
It has been reported that fetal and prenatal deaths are approximately three to six times higher in growth discordant twins than normal twins.1 Historically, growth discordance has been attributed to the type of placental chorionisity. In fact, dichorionic twins are often heavier than monochorionic twins.16-18 This is due to the fact that monochorionic twins share one placenta, while each dichorionic twin has its own placenta. Hence, dichorionic twins have a more stable supply of nutrition, resulting in better growth and development.19,20 In this study, the growth discordant group included two more pairs of monochorionic twins than the control group, but the overall distributions were the same for each group.
Telomerase, a specialized reverse transcriptase enzyme that elongates telomeres and synthesizes telomeric DNA, is present in most human somatic cells.3,4 Telomere shortening at each cell division is thought to contribute to senescence.5,6 The telomeric repeat amplification protocol (TRAP) assay, based on highly sensitive polymerase chain reaction (PCR), has been used to identify telomerase activity in chorionic villi and placental trophoblasts.21 It has been reported that telomerase is closely associated with cell proliferation and regeneration in placental trophoblasts. In situations of high proliferation, such as early pregnancy and molar pregnancy, telomerase activity is high. Advanced pregnancy, fetal growth restriction, or pregnancy-induced hypertension can decrease telomerase activity.7,8 These findings suggest a close relationship between telomerase activity and pregnancy maintenance and fetal development. In this study, higher telomerase activity was observed in the larger twins than in the smaller twins in the growth discordant group. These data are similar to previous results. There was no clear correlation between the telomerase activity and the degree of growth discordance.
This lack of a correlation may be due to the continuously changing supply of nutrients and metabolites necessary for fetal growth in the placenta. According to Kodo et al., the human placenta exists for a limited time period during pregnancy, is involved in various biological processes, and has a large, complex structure. The trophoblast, comprising the base of the placenta, undergoes various biochemical changes to maintain fetal homeostasis.22 Benirschke et al. have described various pathological placental factors that may influence fetal growth, such as persisting placental immaturity, placental infarction, terminal villous deficiency, and fetoplacental vasculopathy.23 Macara et al. reported that various histological changes, such as the pyknotic nuclei or syncytial knotting, correlate with the normal placental aging phenomenon.24 In this study, however, we did not observe any pathohistological changes in the growth discordant trophoblasts.
In the human placenta Bax is expressed in cytotrophoblasts to promote apoptosis in circumstances, such as fetal growth restriction, pregnancy-induced hypertension, and fetal hypoxia.22,25 Bcl 2, on the other hand, is expressed in syncytotrophoblast under suppressed apoptosis conditions in placenta without growth restriction.22,25,26 Apoptosis is energy-dependent and detected by terminal transferase-mediated in situ end labeling (TUNEL) or immunohistochemical studies, using monoclonal antibodies. Bcl 2 expression decreases as the trophoblast matures and pregnancy advances.27 Decreased Bcl 2 expression was reported when pyknotic nuclei or syncytial knotting were observed in the trophoblast, which is characteristic of the normal aging process.22 Izutsu et al. examined the correlation between telomerase activity and apoptosis using an in situ TRAP assay and TUNEL. They found that telomerase activity was lower in growth restricted placentas than normal placentas. Furthermore, the number of TUNEL positive cells was higher in the growth restricted placentas than the normal placentas.22,28 Levy et al. could not find any significant differences in the expression of genes within the Bcl 2 gene family in normal and pathologic placentas.29 In this study, Bax and Bcl 2 expression was observed in the larger and the smaller twins in both the growth discordant and control groups, but differences in expression levels were not significant. Nevertheless, Bcl 2 expression was higher in the larger twin, and Bax expression was higher in the smaller twin. Therefore, some relationship between apoptosis and fetal growth is suggested.
A limitation of this study is the relatively small number of samples. We also could not further classify the trophoblasts as cytotrophoblast, syncytotrophoblast, or decidual cells. Various pathological apoptotic phenomena were not examined. We could not correlate apoptosis to the umbilical cord insertion, placental volume, or chorionisity, all of which are considered to be important factors in growth discordance. The molecular changes in the placentas of twins were examined directly, and the differences were assessed. These data may be useful basic information for further investigation of fetal growth.
In conclusion, the assessment of the two molecular factors in placental trophoblasts of twins has shown a tendency toward reduced telomerase activity and increased apoptosis exists in placental trophoblasts of the smaller twin. These two factors delayed fetal growth, resulting in growth-discordant twins.
References
- 1.O'Brien WF, Knuppel RA, Scerbo JC, Rattan PK. Birth weight in twins: an analysis of discordancy and growth retardation. Obstet Gynecol. 1986;67:483–486. [PubMed] [Google Scholar]
- 2.Spellacy WN, Handler A, Ferre CD. A case-control study of 1253 twin pregnancies from a 1982-1987 perinatal data base. Obstet Gynecol. 1990;75:168–171. [PubMed] [Google Scholar]
- 3.Counter CM. The role of telomeres and telomerase in cell life span. Mutat Res. 1996;366:45–63. doi: 10.1016/s0165-1110(96)90006-8. [DOI] [PubMed] [Google Scholar]
- 4.Harley CB. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991;256:271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- 5.Blackburn EH. Structure and function of telomere. Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 6.Collins K. Structure and function of telomerase. Curr Opin Cell Biol. 1996;8:374–380. doi: 10.1016/s0955-0674(96)80013-5. [DOI] [PubMed] [Google Scholar]
- 7.Izutsu T, Kudo T, Sato T, Nishiya I, Ohyashiki K, Nakagawara K. Telomerase and proliferative activity in placenta from women with and without fetal growth restriction. Obstet Gynecol. 1999;93:124–129. doi: 10.1016/s0029-7844(98)00383-4. [DOI] [PubMed] [Google Scholar]
- 8.Nishi H, Yahata N, Ohyashiki K, Isaka K, Shiraishi K, Ohyashiki JH, et al. Comparison of telomerase activity in normal chorionic villi to trophoblastic diseases. Int J Oncol. 1998;12:81–85. doi: 10.3892/ijo.12.1.81. [DOI] [PubMed] [Google Scholar]
- 9.Jung YN, Kim SK, Chung HC, Park KH, Kim CH, Cho HJ, et al. Telomerase activity in human placenta tissues: Comparison between preeclampsia with and without fetal growth restriction. Korean J Obstet Gynecol. 2000;43:172–178. [Google Scholar]
- 10.Kyo S, Takakura M, Tanaka M, Kanaya T, Sagawa T, Kohama T, et al. Expression of telomerase activity in human chorion. Biochem Biophys Res Commun. 1997;241:498–503. doi: 10.1006/bbrc.1997.7767. [DOI] [PubMed] [Google Scholar]
- 11.Kim SG, Jung YN, Jung HC, Park KH, Kim CH, Park JH, et al. Comparison of Telomerase Activity in Human Chorionic Villi between Eutopic and Ectopic Pregnancies. Korean J Obstet Gynecol. 2000;43:1431–1436. [Google Scholar]
- 12.Sagol S, Sagol O, Ozkal S, Asena U. Role of apoptosis, bcl-2 and bax protein expression in premature rupture of fetal membranes. J Reprod Med. 2002;47:809–815. [PubMed] [Google Scholar]
- 13.Fortunato SJ, Menon R, Bryant C, Lombardi SJ. Programmed cell death (apoptosis) as a possible pathway to metalloproteinase activation and fetal membrane degradation in premature rupture of membranes. Am J Obstet Gynecol. 2000;182:1468–1476. doi: 10.1067/mob.2000.107330. [DOI] [PubMed] [Google Scholar]
- 14.Cotran RS, Kumar V, Collins T. Robbins Pathologic Basis of Disease. Philadelphia: WB Saunders; 1999. pp. 1–31. [Google Scholar]
- 15.Smith SC, Baker PN, Symonds EM. Increased placental apoptosis in intrauterine growth restriction. Am J Obstet Gynecol. 1997;177:1395–1401. doi: 10.1016/s0002-9378(97)70081-4. [DOI] [PubMed] [Google Scholar]
- 16.Gruenwald P. Environmental influences on twins apparent at birth. A preliminary study. Biol Neonate. 1970;15:79–93. doi: 10.1159/000240213. [DOI] [PubMed] [Google Scholar]
- 17.Corney G, Robson EB, Strong SJ. The effect of zygosity on the birth weight of twins. Ann Hum Genet. 1972;36:45–59. doi: 10.1111/j.1469-1809.1972.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 18.Ramos-Arroyo MA, Ulbright TM, Yu PL, Christian JC. Twin study: relationship between birth weight, zygosity, placentation, and pathologic placental changes. Acta Genet Med Gemellol. 1988;37:229–238. doi: 10.1017/s0001566000003834. [DOI] [PubMed] [Google Scholar]
- 19.Bleker OP, Breur W, Huidekoper BL. A study of birth weight, placental weight and mortality of twins as compared to singletons. Br J Obstet Gynaecol. 1979;86:111–118. doi: 10.1111/j.1471-0528.1979.tb10577.x. [DOI] [PubMed] [Google Scholar]
- 20.Machin GA. Velamentous cord insertion in monochorionic twin gestation. An added risk factor. J Reprod Med. 1997;42:785–789. [PubMed] [Google Scholar]
- 21.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 22.Kudo T, Izutsu T, Sato T. Telomerase activity and apoptosis as indicators of aging in placenta with and without intrauterine growth retardation. Placenta. 2000;21:493–500. doi: 10.1053/plac.2000.0538. [DOI] [PubMed] [Google Scholar]
- 23.Benirschke K, Kaufmann P. Pathology of the human placenta. 3rd ed. New York: Springer-Verlag; 1995. pp. 64–80. [Google Scholar]
- 24.Macara L, Kingdom JC, Kaufmann P, Kohnen G, Hair J, More IA, et al. Structural analysis of placental terminal villi from growth-restricted pregnancies with abnormal umbilical artery Doppler waveforms. Placenta. 1996;17:37–48. doi: 10.1016/s0143-4004(05)80642-3. [DOI] [PubMed] [Google Scholar]
- 25.Cotran RS, Kumar V, Collins T. Robbins Pathologic Basis of Disease. Philadelphia: WB Saunders; 1999. pp. 1–31. [Google Scholar]
- 26.Levy R, Smith SD, Chandler K, Sadovsky Y, Nelson DM. Apoptosis in human cultured trophoblasts is enhanced by hypoxia and diminished by epidermal growth factor. Am J Physiol Cell Physiol. 2000;278:C982–C988. doi: 10.1152/ajpcell.2000.278.5.C982. [DOI] [PubMed] [Google Scholar]
- 27.Kim CJ, Choe YJ, Yoon BH, Kim CW, Chi JG. Patterns of bcl-2 expression in placenta. Pathol Res Pract. 1995;191:1239–1244. doi: 10.1016/S0344-0338(11)81132-5. [DOI] [PubMed] [Google Scholar]
- 28.Izutsu T, Kudo T, Sato T, Nishiya I, Ohyashiki K, Mori M, et al. Telomerase activity in human chorionic villi and placenta determined by TRAP and in situ TRAP assay. Placenta. 1998;19:613–618. doi: 10.1016/s0143-4004(98)90022-4. [DOI] [PubMed] [Google Scholar]
- 29.Levy R, Smith SD, Yusuf K, Huettner PC, Kraus FT, Sadovsky Y, et al. Trophoblast apoptosis from pregnancies complicated by fetal growth restriction is associated with enhanced p53 expression. Am J Obstet Gynecol. 2002;186:1056–1061. doi: 10.1067/mob.2002.122250. [DOI] [PubMed] [Google Scholar]