Abstract
The purpose of this study was to evaluate the effect and investigate the putative mechanism of botulinum toxin type A (BTA) applied to the treatment of benign prostatic hyperplasia (BPH). A total of 52 patients with symptomatic BPH were evaluated. Transperineal intraprostatic injection under transrectal ultrasonography was carried out. BTA dissolved in 4 to 9 mL of saline was used from 100 U to 300 U, according to prostate volume. Twenty-six patients received only BTA (BT group), and 26 received both BTA and one month of an α-adrenergic antagonist (BTα group). The therapeutic outcomes were evaluated by comparing parameters such as international prostate symptom score (IPSS), quality of life, prostate specific antigen, prostate volume, post-void residual urine, and peak urinary flow rate. At the one month follow-up, 18 patients in the BT group and 21 in the BTα group had subjective symptomatic relief (p = 0.337). Only IPSS5 (weak stream) was significantly different between the BT group and BTα groups (p = 0.034). At the three month follow-up, 39 patients had subjective symptomatic relief. The storage symptoms were improved more than the voiding symptoms. Additionally, about 50 percent of the patients whose voiding symptom improved expressed improved erectile function. BTA injection seems to be an alternative treatment for BPH. The differences after the one month evaluation between the BT and the BTα groups might suggest that the adrenergic influence could be relatively reinforced by the anticholinergic effect of BTA. Nitric oxide would thus be involved in a BTA action mechanism in BPH.
Keywords: Botulinum toxin type A, benign prostatic hyperplasia, neurotransmitter
INTRODUCTION
In benign prostatic hyperplasia (BPH), bladder outlet obstruction is considered to be due to a mechanical component caused by an enlarged gland and due to a dynamic component caused by the tone of prostatic stromal smooth muscle. The therapeutic goal of BPH has been to perfectly control both the mechanical and dynamic components. Several treatment options have been available. The treatments include watchful waiting, pharmacological therapy with an α-adrenergic antagonist and 5α-reductase inhibitor, and surgical therapies including simple prostatectomy and transurethral resection of the prostate (TURP).
Surgery and the 5α-reductase inhibitor have played an important role in the treatment of the mechanical component of BPH. The dynamic component of BPH is mediated mainly via α-adrenoceptor stimulation and has been one of the primary targets for therapeutic interventions with α-adrenergic antagonists.1 However, these traditional methods mainly charge one side between the mechanical and dynamic components. The traditional methods also have some adverse consequences. Although TURP has been the less invasive gold standard of treatment against the mechanical component of BPH, the procedure has some limitations. Up to 25% of patients who undergo surgery do not have satisfactory long-term outcomes.2 TURP also has some troublesome surgical complications. For these reasons, the goal for patients with BPH is to avoid surgery whenever possible and to seek a cost-effective alternative with at an acceptable level of risk for treatment-associated complications. While pharmacological therapies with α-adrenergic antagonists and 5α-reductase inhibitors have gained widespread acceptance as safe and effective treatments for BPH,3,4 they have adverse effects such as dizziness, asthenia, postural hypotension in patients taking α-adrenergic antagonists, and decreased libido and impotence in patients taking 5α-reductase inhibitors. Medical withdrawal is attributed to these adverse effects. Therefore, a number of minimally invasive alternatives to existing traditional treatment options of BPH have been introduced. The alternatives were devised to achieve symptomatic improvement without the surgery-associated morbidity, side effects, or compliance issues associated with medicine.
There has been much interest in botulinum toxin type A (BTA) as a powerful, versatile tool because of its safety, effectiveness, specificity, and reversibility. Urological applications of BTA have been associated with cases of detrusor external sphincter dyssynergia, dysfunctional voiding, detrusor over-activity, and chronic pain syndrome due to chronic prostatitis. Recent studies have suggested that BTA is able to be used as an alternative treatment for BPH, and it is effective enough to control both mechanical and dynamic components of BPH.5,6 These suggestions are based on studies which concluded that chemical denervation using BTA causes subsequent atrophy of the gland,5 that cholinergic stimulation causes prostatic stromal smooth muscle contraction,7 and that BTA blocks acetylcholine release at the neuromuscular junctions and in autonomic neurons. We evaluated the effect of BTA in treating patients with symptomatic BPH and deduced a putative intraprostatic mechanism of BTA. The conclusions were drawn from the analysis of BPH symptoms and the evaluation of values taken before and after BTA injection into the prostate.
MATERIALS AND METHODS
This study was approved by the institutional review board of our hospital and all patients provided informed consent.
Patients and baseline evaluation
Men between the ages of 45 and 84 years who had symptomatic BPH volunteered for the study. All patients were treated with an α-adrenergic antagonist (42 patients with 4 mg of doxazosin and 10 with 0.2 mg of tamsulosin) with or without a 5α reductase inhibitor at least one month before this study. They wanted to try a new alternative to improve BPH related symptoms. All refused surgical options such as TURP and open prostatectomy.
The inclusion criteria were as follows: urinary obstruction symptoms as determined by the IPSS and an enlarged prostate gland on digital rectal examination. Patients with neurogenic voiding disorders, prostate or bladder cancer, a serum PSA level of 15 ng/mL or more, or who had undergone surgery were excluded from the study.
The following evaluations were done at baseline. The symptoms were assessed with the International Prostate Symptom Score (IPSS), which assesses the occurrence of seven BPH symptoms and quality of life. Seven BPH symptoms of IPSS were divided into two groups. One group was determined to be storage symptoms (IPSS-SS) including IPSS 2 (frequency), IPSS 4 (urgency), and IPSS 7 (nocturia). The other group was determined to include those with voiding symptoms (IPSS-VS) including IPSS 1 (incomplete emptying), IPSS 3 (intermittency), IPSS 5 (weak stream), and IPSS 6 (straining). Uroflowmetry was performed to determine peak urinary flow rate. The postvoid residual urinary volume was determined ultrasonographically. Serum concentrations of prostate-specific antigen (PSA) were measured. Transrectal ultrasonography was performed to determine the prostate volume. Prostate biopsies were performed if clinically indicated.
Study design
Eligible applicants were enrolled from April 2004 to January 2005. Patients were divided into two groups under our hypothesis that BTA could induce change in neural balancing among adrenergic, cholinergic, and nonadrenergic-non-cholinergic (NANC) nerves in the prostate. We also hypothesized that a decreased cholinergic neural effect by BTA could increase adrenergic nerve activity after BTA application. This disruption of neural balancing would be modified by slowly increasing NANC neural activity, which is related with nitric oxide production. Under these hypotheses, one group received BPH related medical treatment of an α-adrenergic antagonist (Doxazosin, 4 mg) for one month just after BTA injection. The other group did not receive medical treatment after BTA injection. The study was designed to continue for at least 3 months. Randomization was performed after informed consent was obtained. The randomization data was available only to the doctors who performed medical treatment. The data was not available to the investigators who were in charge of the procedures of BTA injection and evaluation methods.
Each of the participants received BTA (Botox®), Allergan) dissolved in 4 to 9 mL of 0.9% sodium chloride solution via injection into the prostate. The BTA was divided into two equal injections into each transition zone of the gland. However, BTA was only injected into the bilateral lobe in the case of a small prostate. With the patient lying on the lithotomy position, a transperineal intra-prostatic injection was carried-out using a 22-gauge, 15-cm spinal needle. Transrectal ultrasonography was performed for appropriate localization of the injection site and confirmation of BTA diffusion. Neither sedation nor local anesthesia was used during the procedure. BTA from 100 to 300 U was given to each patient according to the prostate volume (below 30 mL, 100 U; 30 mL to 80 mL, 200 U; above 80 mL, 300 U). One hundred U, 200 U, and 300 U of BTA were dissolved in 4 mL, 6 mL, and 9 mL of normal saline, respectively.
A follow-up of the patients was done to evaluate their outcome at 1, 3, and 6 months. Regardless of their treatment and outcome, all patients underwent the same evaluation as performed at baseline. Evaluation of symptomatic improvement after treatment, as measured by the IPSS, peak urinary flow rates, postvoid residual urinary volume, evaluation of prostate volume, and serum PSA level, was performed. If patients complained that BTA had little effect and did not want to stay in the study, they were offered medical therapy or surgical resection at each follow-up visit.
Statistical analysis
All statistical analyses were obtained using the Statistical Package for the Social Science (SPSS, version 13.0, Chicago, IL, USA ) for Windows. The results are expressed as the mean ± standard deviation. Differences between data were compared using the Student t-test. The clinical outcomes of the two groups were compared using the Pearson Chi Square test. All p values were two tailed. A p < 0.05 was considered statistically significant.
RESULTS
Fifty-two outpatients were selected after confirmation of eligibility according to the inclusion criteria and were randomized. Among these patients, 10 were biopsy-proven benign conditions. A biopsy was performed if the serum PSA level was more than 4.0 ng/mL and/or a palpable nodule was present in the prostate. Twenty-six received only BT (BT group), and 26 received both BT and one month of an α-adrenergic antagonist (BTα group). A follow-up of 52 patients was done at one month and three months. At the six-month follow-up, 23 patients between the ages of 54 to 81 years stayed in the study and underwent the same evaluations as performed at baseline, except PSA. No complications during the procedure were observed in any patient. Urinary incontinence, urinary retention, and adverse events of BTA enough to stop the study were not reported during the follow-up period.
One-month evaluation
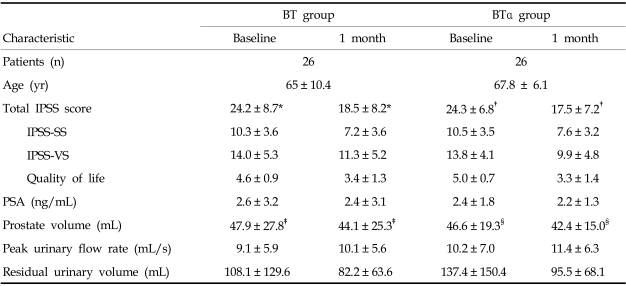
Eighteen patients in the BT group and 21 in the BTα group had subjective symptomatic relief with 23.8 percent and 27.8 percent reduction of IPSS, respectively (p = 0.337). No differences were found at baseline between the two groups or between the BT group and the total (52 patients) group (Table 1). In the BT patients, IPSS including the total IPSS score and 6 BPH symptoms (except IPSS 5), quality of life and prostate volume were significantly reduced compared with baseline values, by 23.8%, 25.8% and 8.1% for total IPSS score, quality of life and prostate volume, respectively. PSA and postvoid residual volume were reduced by 9.0% and 24.0%. Peak urinary flow rate was increased by 11.1%, while IPSS 5, PSA, peak urinary flow rate, and postvoid residual volume were not significantly different from baseline. IPSS-SS was reduced more than IPSS-VS (IPSS-SS, 30.0%; IPSS-VS, 19.3%). The number of improved patients was not significantly different between IPSS-SS (12/26) and IPSS-VS (18/26) (p = 0.092).
Table 1.
Evaluation Results at Baseline and One-month Follow-up
BT, Botulinum toxin A; BTα, botulinum toxin A plus a-adrenergic antagonist; PSA, prostate specific antigen; IPSS-SS, International prostate symptom score-storage symptom; IPSS-VS, International prostate symptom score-voiding symptom.
Data presented as the mean ± SD.
*p = 0.001, †p < 0.001, ‡p < 0.001, §p = 0.009.
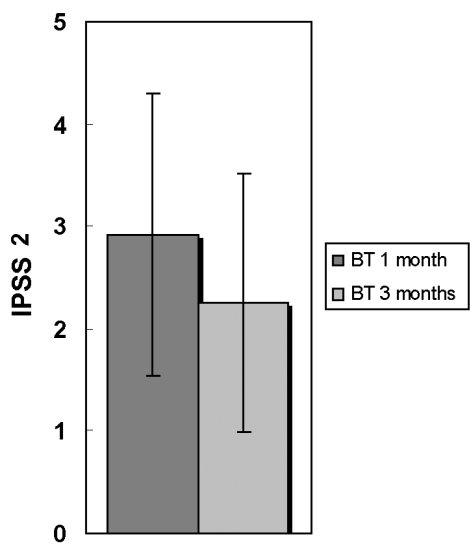
In the BTα patients, compared with baseline values, total IPSS (and all IPSS symptoms), quality of life and prostate volume were significantly reduced by 27.8%, 33.1% and 9.0%, respectively. PSA and postvoid residual volume were reduced by 8.7% and 30.5%, respectively. Peak urinary flow rate was increased by 12.0%, though this difference was not statistically significant. IPSS-SS was reduced less than IPSS-VS (IPSS-SS, 27.5%; IPSS-VS, 28.1%). The number of improved patients was not significantly different between IPSS-SS (19/26) and IPSS-VS (21/26) (p = 0.510). Only IPSS5 was significantly different between the BT group and the BTα groups (p = 0.034). IPSS5 showed weaker improvement after BTA injection in the BT group (Fig. 1). This means that the α-adrenergic antagonist might improve the urine stream in BTA treated conditions.
Fig. 1.
IPSS 5 changes between two groups after one month botulinum toxin A administration. p = 0.034; BT, Botulinum toxin A injection; BTα, botulinum toxin A plus a-adrenergic antagonist; IPSS, International prostate symptom score.
Three-month evaluation
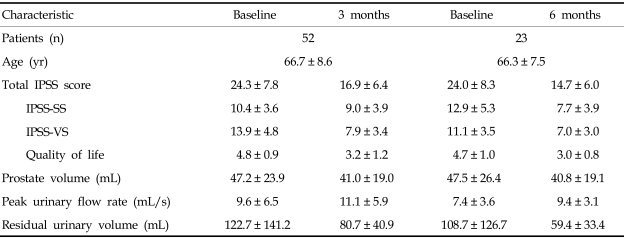
Thirty-nine patients had subjective symptomatic relief. The number of improved patients was not significantly different compared with the one-month BT group and the six-month follow-up group (p = 0.077). IPSS, quality of life, prostate volume, peak urinary flow rate, and postvoid residual volume were significantly different compared with baseline values of the total (52 patients) group. IPSS, quality of life, prostate volume, and residual urine were reduced by 30.3%, 34.4%, 13.1%, and 34.3%, respectively. Peak urinary flow rate was increased by 15.5% (Table 2).
Table 2.
Evaluation Results at Three and Six-months Follow-up
IPSS-SS, International prostate symptom score-storage symptom; IPSS-VS, International prostate symptom score-voiding symptom.
IPSS-SS was not significantly different between the one-month BT group (12/26) and the three-month follow-up group (45/52) (p = 0.293). IPSS-VS was not significantly different between the BT group of the one-month follow-up (18/26) and three-month follow-up groups (38/52) (p = 0.722). However, both the IPSS-SS and IPSS-VS were improved more than those of the BT group at the one-month follow-up. IPSS-SS was still reduced more than IPSS-VS (IPSS-SS, 39.1%; IPSS-VS, 23.8%).
Compared with the BT group at one-month follow-up, IPSS (except IPSS 2), quality of life, prostate volume, peak urinary flow rate, and residual urine were not significantly different. Only IPSS2 was significantly different (p = 0.039) (Fig. 2). PSA was not checked at the three-month follow-up.
Fig. 2.
IPSS 2 changes between one month and three months after botulinum toxin A administration. p = 0.039; IPSS, International prostate symptom score. BT, botulinum toxin.
Six-month evaluation
Twenty-one patients had subjective symptomatic relief. IPSS, quality of life, prostate volume, peak urinary flow rate, and postvoid residual volume of the six-month follow-up group were significantly different compared with the baseline values. IPSS, quality of life, prostate volume, and postvoid residual volume were reduced by 38.8%, 35.5%, 14.2%, and 45.3%, respectively. Peak urinary flow rate was increased by 27.6%.
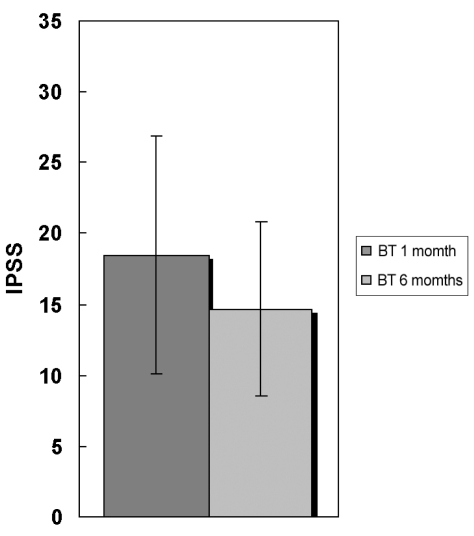
The number of patients who were improved in IPSS was significantly different between the BT group (12/26) of the one-month follow-up and the six month follow-up groups (21/23) (p = 0.015) (Fig. 3). The number of patients who were improved in IPSS-VS was not significantly different between the BT group of the one-month follow-up (18/26) and the three-month follow-up group (21/23) (p = 0.056). However, both IPSS-SS and IPSS-VS were improved more than those of the BT group of the one-month follow-up and the three-month follow-up groups. IPSS-SS was still reduced more than IPSS-VS (IPSS-SS, 39.4%; IPSS-VS, 38.5%).
Fig. 3.
IPSS changes between one month and six months after botulinum toxin A administration. p = 0.015; IPSS, International prostate symptom score. BT, botulinum toxin.
No differences were found between the total (52 patients) group at baseline and the six-month follow-up. Compared with the three-month follow-up values, IPSS, quality of life, prostate volume, peak urinary flow rate, and residual urine were not significantly different.
DISCUSSION
Neural activity has been thought to be involved in normal growth and functioning of the prostate.8 Dysregulation of this neuronal activity may alter the morphology and behavior of the prostate. These pathological events are caused by significant alteration of neurotransmitter/growth factor influence on the prostate and may result in BPH. Therefore, normalization or favorable correction of altered neural activity could significantly reverse pathological processes and improve stressful BPH related symptoms.
The prostate is a heterogeneous tissue composed of epithelial cells, smooth muscle cells, fibroblasts, and connective tissue element. Bartsch and colleagues, using quantitative morphometry, demonstrated that the ratio of stroma to epithelium in the normal prostate is two to one. Bartsch also demonstrated that benign prostatic hyperplasia is primarily a stromal process, as the ratio of stroma to epithelium is five to one.9 Fibromuscular stroma, especially smooth muscle element, which has the principal to cause and aggravate BPH symptoms, has been the primary target for BPH therapy. Human prostate contains α-1 adrenergic, cholinergic, and non-adrenergic non-cholinergic (NANC) neuroreceptors under the influence of various differentiated autonomous innervation. 10-12 It is widely believed that the prostatic epithelium receives a cholinergic innervation while the stroma receives a predominantly noradrenergic innervation. The fact has been known that nerve fibers innervating the smooth muscle may be both noradrenergic and cholinergic or separate. The fibers can cause an increase of smooth muscle tone.13 Nitric oxide (NO) plays an important role in relaxation of the prostate smooth muscle.14 Additionally, It has been suggested that cholinergic nerves in the prostatic stroma could suppress the release of noradrenaline from adrenergic terminals.15 The α-adrenergic innervation may be antagonized by NANC-induced relaxation mediated by NO.16,17 The adrenergic, cholinergic, and nitrinergic innervations and their interaction may be related with prostate smooth muscle tone. Thus, they may play an important role in the dynamic component of benign prostatic hyperplasia. The cholinergic and nitrinergic innervation are reduced in BPH as compared to that in normal prostate tissue. However, adrenergic activity is increased.16
Recent studies have suggested that BTA is able to be a prominent medical tool for BPH. BTA acts as a favorable controller of various neural activities that innervate the prostate. BTA has been known to reduce peripheral sensitization by inhibiting the release of acetylcholine at the presynaptic cholinergic junction. Though BTA exhibits prevalent cholinergic specificity in its action on nerve terminals, release of many neurotransmitters can be blocked when adequate concentrations of toxin are used.18
In our study, only subjective values like IPSS were improved. Objective values such as peak urinary flow rate and postvoid residual volume were not improved at the one-month follow-up. Most patients reported improvement of subjective values starting approximately one to three weeks, if improved. Improvement of objective values started after one month, reached a plateau within three months, and lasted for six months. IPSS 4, IPSS 6, and residual urine were more improved than other values. IPSS-SS improved more than IPSS-VS. On the contrary, the BT group patients complained of little effect of BTA on weak stream.
IPSS 5, PSA, peak urinary flow rate, and postvoid residual volume were not significantly different in the BT patients at one-month follow-up compared with baseline values. IPSS (except IPSS 5), quality of life, PSA, prostate volume, peak urinary flow rate, and postvoid residual volume urine were not significantly different between the BT and BTα groups at one-month follow-up. These facts mean that adrenergic nerve terminals were not affected by BTA. BTA has been suggested to be able to affect other neurotransmitters. If they were affected, peak urinary flow rate and postvoid residual volume should be improved in the BT patients. Additionally, the reinforcement of adrenergic activity may develop. These results could easily be deduced from the fact that little difference was found between the BT and BTα groups at the one-month follow-up. This means that the combined α-adrenergic antagonist in the BTα group did little work. Loss of acetylcholine-mediated suppression of adrenergic transmission from adrenergic terminals in the prostate may be one of the various putative BTA mechanisms to increase smooth muscle tone.
Prostate volume was reduced compared with baseline values at the one-month follow-up, but the change of prostate volume did not alter the PSA level to a lower level. Prostate volume was reduced only by 14.2% at the six-month follow-up. This is thought to result from the fact that BTA might induce atrophy and apoptosis of glandular epithelium. There was lesser effect on the stromal component of the transition zone, while the stromal component is dominant in BPH. However, pathological confirmation was not performed. It is easy to presume these findings from the fact that the rat prostate used in a recent study showed profound atrophy of the prostate gland which was mainly glandular.7 Also, BPH in human is primarily a stromal process as previously described.
Peak urinary flow rate was increased by 15.5% and 27.6% at the three and six-months follow-up, respectively. Residual urine was reduced by 34.3% and 45.3% at the three and six-months follow-up, respectively. This may result from the improvement of the mechanical component of BPH by glandular atrophy. It may also result from an improvement of the dynamic component by a disclosed effect of BTA related with the inhibition of acetylcholine release on the smooth muscle tone. The improvement of the dynamic component may be hidden at the one-month follow-up because of the transient contraction effect by stronger adrenergic activity. This may mask the relaxation effect of smooth muscle by inhibition of acetylcholine release by BTA. The good results in the parameters of the IPSS score and residual urine in patients at the six-months follow-up may be influenced by reduced objectives. Therefore, this study needs to be placebo-controlled in the future.
We found two interesting NO related events which were not expected in the study, and that it can induce an additional putative BTA mechanism. It has been reported that an NO donor, such as isosorbide dinitrate or glyceryl trinitrate medication, in ischemic heart disease patients improves micturition parameters in symptomatic BPH patients.19 Five patients who took oral isosorbide dinitrate for an ischemic heart disease and persisted stressful BPH symptoms received BTA injection. All patients showed no improvement of BPH related evaluation values during the follow-up period. Another unexpected effect was the improvement of erectile function. Nearly half of the improved patients (19/39; data not shown) at the three-month follow-up showed an improvement of erectile frequency [International Index of Erection Function (IIEF)]. This is likely caused by the diffusion of BTA to the adjacent nerve plexus outside the prostate. The prostate is surrounded by a dense nerve plexus derived from both hypogastric and pelvic nerves. These nerves project to other regions of the male genital tract including the corpus cavernosum. Acetylcholinesterase reactivity has been reported to be closely related to NO synthase immunoreactivity in the prostate.20 This suggests that there may be functional interactions between NO and acetylcholine in the prostate. Based on these facts, an additional mechanism of BTA, one that nitrinergic activity in the prostate is increased under inhibition of acetylcholine release by BTA, could be derived from the improvement of erectile dysfunction. The improvement of erectile function may be caused by accessory nerve sprouting or increased release of NO at the nitrinergic nerve endings.21 This increased nitrinergic activity does not occur when NO is sufficiently supplied to the prostate by oral nitrate.
In our study, there are several shortcomings of the baseline evaluation and study model. Firstly, the patients did not undergo urodynamic study at baseline evaluation. A second shortcoming was the lack of a placebo. These made it difficult to assess the true efficacy of BTA. Although a level of caution must be present in interpreting the results of the study, we believe that they may be a kind of guideline for more profound, generalized studies on the combination effect of BTA and existing BPH medicines.
In summary, we were able to deduce the intraprostatic mechanism of BTA from the difference between various evaluation values related to BPH before and after BTA injection. It is mainly related with the alteration of interactions between adrenergic, cholinergic, and nitrinergic activity. However, it is possible that more neurotransmitters than the three neurotransmitters mentioned are involved in making BPH. Additional histopathologic and pharmacophysiological investigations of BTA treatment are necessary before the effects of BTA seen in our results can be generalized in BPH treatment.
References
- 1.Caine M. The present role of alpha-adrenergic blockers in the treatment of benign prostatic hypertrophy. J Urol. 1986;136:1–4. doi: 10.1016/s0022-5347(17)44709-4. [DOI] [PubMed] [Google Scholar]
- 2.Lu-Yao GL, Barry MJ, Chang CH, Wasson JH, Wennberg JE. Transurethral resection of the prostate among Medicare beneficiaries in the United States: time trends and outcomes. Prostate Patient Outcomes Research Team (PORT) Urology. 1994;44:692–699. doi: 10.1016/s0090-4295(94)80208-4. [DOI] [PubMed] [Google Scholar]
- 3.McConnell JD, Bruskewitz R, Walsh P, Andriloe G, Lieber M, Holtgrewe HL, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med. 1998;338:557–563. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 4.Lepor H, Williford WO, Barry MJ, Brawer MK, Dixon CM, Gormley G, et al. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. Veterans Affairs Cooperative Studies Benign Prostatic Hyperplasia Study Group. N Engl J Med. 1996;335:533–539. doi: 10.1056/NEJM199608223350801. [DOI] [PubMed] [Google Scholar]
- 5.Doggweiler R, Zermann DH, Ishigooka M, Schmidt RA. Botox-induced prostatic involution. Prostate. 1998;37:44–50. doi: 10.1002/(sici)1097-0045(19980915)37:1<44::aid-pros7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Maria G, Brisinda G, Civello IM, Bentivoglio AR, Sganga G, Albanese A. Relief by botulinum toxin of voiding dysfunction due to benign prostatic hyperplasia: results of a randomized, placebo-controlled study. Urology. 2003;62:259–265. doi: 10.1016/s0090-4295(03)00477-1. [DOI] [PubMed] [Google Scholar]
- 7.Dail WG. Autonomic innervation of male reproductive genitalia. In: Maggi CA, editor. Nervous Control of the Urogenital System. Switzerland: Harwood Academic Publishers; 1993. pp. 69–101. [Google Scholar]
- 8.Pennefather JN, Lau WA, Mitchelson F, Ventura S. The autonomic and sensory innervation of the smooth muscle of the prostate gland: a review of pharmacological and histological studies. J Auton Pharmacol. 2000;20:193–206. doi: 10.1046/j.1365-2680.2000.00195.x. [DOI] [PubMed] [Google Scholar]
- 9.Bartsch G, Frick J, Ruegg I, Bucher M, Holliger O, Oberholzer M, et al. Electron microscopic stereological analysis of the normal human prostate and of benign prostatic hyperplasia. J Urol. 1979;122:481–486. doi: 10.1016/s0022-5347(17)56475-7. [DOI] [PubMed] [Google Scholar]
- 10.Lepor H. Role of long-acting selective alpha-1 blockers in the treatment of benign prostatic hyperplasia. Urol Clin North Am. 1990;17:651–659. [PubMed] [Google Scholar]
- 11.Ventura S, Pennefather J, Mitchelson F. Cholinergic innervation and function in the prostate gland. Pharmacol Ther. 2002;94:93–112. doi: 10.1016/s0163-7258(02)00174-2. [DOI] [PubMed] [Google Scholar]
- 12.Lange W, Unger J. Peptidergic innervation within the prostate gland and seminal vesicle. Urol Res. 1990;18:337–340. doi: 10.1007/BF00300783. [DOI] [PubMed] [Google Scholar]
- 13.Kester RR, Mooppan UM, Gousse AE, Alver JE, Gintautas J, Gulmi FA, et al. Pharmacological characterization of isolated human prostate. J Urol. 2003;170:1032–1038. doi: 10.1097/01.ju.0000080440.74266.b1. [DOI] [PubMed] [Google Scholar]
- 14.Takeda M, Tang R, Shapiro E, Burnett AL, Lepor H. Effects of nitric oxide on human and canine prostates. Urology. 1995;45:440–446. doi: 10.1016/S0090-4295(99)80013-2. [DOI] [PubMed] [Google Scholar]
- 15.Hedlund H, Andersson KE, Larsson B. Alpha-adrenoceptors and muscarinic receptors in the isolated human prostate. J Urol. 1985;134:1291–1298. doi: 10.1016/s0022-5347(17)47714-7. [DOI] [PubMed] [Google Scholar]
- 16.Bloch W, Klotz T, Loch C, Schmidt G, Engelmann U, Addicks K. Distribution of nitric oxide synthase implies a regulation of circulation, smooth muscle tone, and secretory function in the human prostate by nitric oxide. Prostate. 1997;33:1–8. doi: 10.1002/(sici)1097-0045(19970915)33:1<1::aid-pros1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Addicks K, Bloch W, Feelisch M. Nitric oxide modulates sympathetic neurotransmission at the prejunctional level. Microsc Res Tech. 1994;29:161–168. doi: 10.1002/jemt.1070290214. [DOI] [PubMed] [Google Scholar]
- 18.Coffield JA, Considine RV, Simpson LL. The site and mechanism of action of Botulinum neurotoxin. In: Jankovic J, Hallet M, editors. Therapy with Botulinum Toxin. New York: Marcel Dekker; 1994. pp. 3–13. [Google Scholar]
- 19.Klotz T, Mathers MJ, Bloch W, Nayal W, Engelmann U. Nitric oxide based influence of nitrates on micturition in patients with benign prostatic hyperplasia. Int Urol Nephrol. 1999;31:335–341. doi: 10.1023/a:1007174102953. [DOI] [PubMed] [Google Scholar]
- 20.Hedlund P, Ekstrom P, Larsson B, Alm P, Andersson KE. Heme oxygenase and NO-synthase in the human prostate-relation to adrenergic, cholinergic and peptidecontaining nerves. J Auton Nerv Syst. 1997;63:115–126. doi: 10.1016/s0165-1838(96)00139-7. [DOI] [PubMed] [Google Scholar]
- 21.Hedlund P, Ny L, Alm P, Andersson KE. Cholinergic nerves in human corpus cavernosum and spongiosum contain nitric oxide synthase and heme oxygenase. J Urol. 2000;164:868–875. doi: 10.1097/00005392-200009010-00064. [DOI] [PubMed] [Google Scholar]