Abstract
Although most epidemic human infectious diseases are caused by recently introduced pathogens, cospeciation of parasite and host is commonplace for endemic infections. Occasional host infidelity, however, provides the endemic parasite with an opportunity to survive the potential extinction of its host. Such infidelity may account for the survival of certain types of human lice, and it is currently exemplified by viruses such as HIV.
Hans Zinsser's Rats, Lice and History [1] is a classic in microbiology. Written in 1934 and subtitled The biography of a bacillus, it tells the tale of that dreaded disease typhus, its reservoir in rats and its transmission among humans by lice. Here, I discuss how we may in the course of prehistory have acquired the lice, and how other infections may, like the typhus bacillus, come to be shared by us and the animal species with which we are in close contact. It is a tale of infidelity that I shall begin with the recent research on lice of David Reed and colleagues [2,3] and of Mark Stoneking's group [4] who, on the basis of phylogenetic analysis, have speculated that we may have acquired a clade of head lice from another hominid species and pubic lice from gorillas; they have also suggested that lice might help determine the date when humans adopted clothing. I shall examine this unfolding story in the context of what we know about microbial infections, and will look at the promiscuity of viruses through the lens of modern molecular technology; and I will add my own speculation on why naked apes have pubic hair.
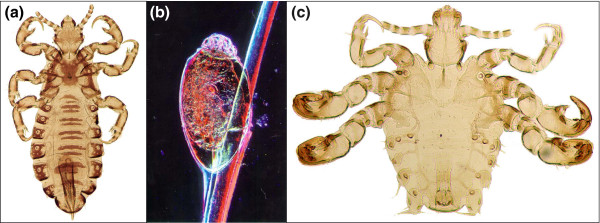
Lice are small, wingless insects that cannot live independently from their hosts (Figure 1). They are frequent parasites of birds and mammals, each host species having its own type of louse. Humans harbor three kinds of these ectoparasites: head lice, body lice and pubic lice. For much of human history and prehistory blood-sucking lice have been so prevalent that they became part of our everyday language. We speak about feeling lousy and admonish our friends for nit-picking. Nits are the eggs of lice, expertly cemented onto hair shafts, as many parents know from painstakingly combing them out of their children's hair. Grooming among monkeys and apes is not only a means of social bonding but also a useful way of controlling nits and lice (Figure 2).
Figure 1.
Human lice. (a) Head louse (Pediculus humanus). (b) Nit (egg) of head louse. With permission from http://www.headlice.org. (c) Pubic louse or 'crab' (Pthirus pubis). (a) and (c) are by Vince Smith and are reproduced with permission.
Figure 2.
Nit-picking is an ancient habit, as seen in (a) apes (photograph by Eric C Matthews, reproduced with permission) and in humans as shown in (b) a painting by Jan Siberechts, Cour de ferme, 1662. Musée des Beaux-Arts, Brussels, Belgium.
Family heirlooms and new acquisitions
Writing on infections of humankind, Tony McMichael and I [5] have called those that cospeciated with their hosts 'family heirlooms' and those that crossed over from other hosts in recent evolutionary time 'new acquisitions'. The new acquisitions were initially derived from zoonotic infections but have flourished as self-sustaining infections in the human population. They have diverged from their progenitors in the original host: for example, measles is now distinct from rindepest. The majority of zoonoses, however, remain in their animal reservoirs and, so far as their sojourn in humans goes – even with limited human-to-human transfer (as with Ebola or severe acute respiratory syndrome (SARS)) – we can regard them as 'temporary exhibits'.
Human DNA might show 98% similarity to that of chimps, but we share less than 50% of our microbes and parasites with them. Ashford [6] argues that the great apes became more specialized forest dwellers at the same time that early hominids explored the savannah, and that human gut parasites resemble those of omnivorous baboons more than those of chimps because humans, like baboons but unlike chimps, are omnivorous. Further opportunities for horizontal crossover of microbes and parasites from animals to humans arose when humans spread out of Africa. When we domesticated ruminants, and animals such as dogs, cats and rats 'domesticated' us for the rich pickings around human habitation, we acquired many infections from our new neighbors [5,7]. Thus a shared habitat, rather than a shared ancestry, is important for the acquisition of many infections.
Most human pandemic infections were acquired horizontally very recently on the evolutionary timescale, even though diseases such as typhus, measles and smallpox first occurred in prehistoric times. These new acquisitions originate from evolutionarily distant host species. The influenza pandemic of 1918–1919 came from birds, and the 1968 influenza strain could be an avian-porcine recombinant. Today's novel viral infections are more likely to originate from exotic species than from animals that were domesticated long ago [5]. The market for 'bushmeat' has led to Ebola virus outbreaks in Africa from butchering primates, and to the SARS outbreak in China from eating small carnivores, such as civet cats. The primary reservoirs of the Ebola filovirus, the SARS coronavirus and the Nipah paramyxovirus, however, seem to be in fruit bats (flying foxes).
Lice and nits have been found in textiles, hair and combs excavated from archaeological sites [1,8]. Given that the closest relative of the human head and body louse, Pediculus humanus, is P. schaeffi, which infests chimpanzees, one might assume that human and chimp lice have cospeciated with their hosts as family heirlooms ever since they diverged from a common ancestor. This requires, however, that the divergence among hosts and parasites approximates to the same timescale. After all, the closest relative to human immunodeficiency virus type 1 (HIV-1) is the simian immunodeficiency virus of chimpanzees (SIVcpz), but it would be facile to suggest that HIV-1 co-evolved with humans, because molecular clock estimates place the most recent common ancestor of the pandemic form of HIV-1 at 75–100 years ago [9,10], and this is likely to be close to the time of the species crossover event. HIV-1 has invaded humans at least three times (groups M, N and O), and such is the power of modern forensic DNA virology that the precise location in Cameroon has been mapped for the chimpanzees carrying the SIVcpz most closely related to group M [11,12].
Origins of head and body lice
Using nuclear and mitochondrial DNA markers, Reed et al. [2] estimated the divergence of chimp and human Pediculus lice at 5.5 million years ago (MYA) and provided evidence of cospeciation with their hosts. A recent revision to 4.1 MYA for the most recent common ancestor of chimps and humans [13] may require a similar adjustment of the louse molecular clock. What is more remarkable, however, is that Reed et al. [2] found that human lice split into two quite distinct clades, A and B, about 1.18 MYA. There is a worldwide clade (which includes both head and body lice) and a New World clade (exclusively head lice). So how can humans harbor two clades of louse that diverged from each other over one million years ago, when that separation is tenfold older than the emergence of Homo sapiens?
The answer, Reed et al. [2] suggested, is that the separation took place around the time of divergence of the ancestors of modern humans from Homo erectus. These two hominid lineages then co-existed for about one million years until the demise of H. erectus. When modern humans radiated across Asia they might have had contact with H. erectus, just as in more recent millennia H. sapiens met H. neanderthalensis in Europe, as dramatized by the Nobel laureate William Golding [14]. There is no evidence that different human species interbred, but they may well have exchanged ectoparasites. Thus, the New World clade of head louse may have crossed horizontally from H. erectus to H. sapiens within the last 100,000 years.
Zinsser [1] noted that the hair of ancient Peruvian mummies and the scalps of pre-Columbian Native Americans contained nits or lice. Recent DNA analysis of lice from similar remains indicates that they belong to the worldwide clade A, so this clade must have been present in pre-Columbian American populations [15]. A third clade of head lice has been delineated in Ethiopia and Nepal and this clade, C, diverged from clades A and B about 2 MYA [16]. If Reed et al. [2] were correct to postulate that clade B came from H. erectus, one must wonder in which hominid population might clade C lice have maintained their separate identity.
Lice and clothing
Head and body lice used to be designated Pediculus capitis and P. corporis but they are now known to belong to the same species, P. humanus x[16,17]. Fifty years ago Levene and Dobzhansky [18] showed that head lice could be trained or adapted to become the rather larger body lice by attaching them to the body in small pill boxes. As we celebrate the 150th anniversary of Darwin's Origin of Species we might recall that it was Theodosius Dobzhansky, an eminent evolutionary biologist and a Russian Orthodox Christian, who in 1973 famously challenged creationists by declaring that "Nothing in biology makes sense except in the light of evolution." His research on lice was no exception.
Kittler et al. [4] initially reckoned that body lice diverged from head lice approximately 70,000 years ago, but they later increased this estimate to 107,000 years ago by correcting an error concerning the original outlier sequence. They postulated that this date of about 100,000 years ago coincided with or followed soon after the origin of clothing, because the naked human body is an inhospitable place for lice to breed. Head lice feed on the scalp and breed in hair, whereas body lice feed on the skin but breed in clothing.
More extensive phylogenetic analyses [16,17] indicate that body lice evolved from head lice several times within the worldwide clade A, as they are found in many branches of the cladisitic tree. Multiple derivations of body lice from head lice had already been considered by Zinsser [1], and it makes good sense if one considers that clothing was not a single invention. Wearing animal pelts fur-side next to the skin would have provided a suitable place for lice to breed before fabrics were developed with the inventions of spinning and weaving.
In 17th and 18th century Europe, most of the aristocracy and gentry shaved their hair and wore wigs. Had this custom arisen to protect them from lice as Zinsser [1] suggests? Not according to Samuel Pepys' diary, as he complained more than once about his wig being infested: "Thence to my barbers, to have my periwig cleared of its nits." I wonder if they were head or body lice – is a wig hair or clothing?
Lice and nudity
Why naked apes are naked and when we 'lost' our hair has long been disputed, as discussed by Desmond Morris in The Naked Ape [19]. Rantala [20] suggested that nakedness could have had a selective advantage to rid the body of lice and other ectoparasites, a view also championed by Pagel and Bodmer [21], who added that being seen to be free of lice would be a fitness indicator and a good mating strategy. I am rather drawn to the theory first postulated by Alister Hardy [22] that humans evolved through an aquatic stage, although most anthropologists disparage this hypothesis. Yet Ashford [6] points out that several parasites specific to humans, such as three Schistosoma species, depend for their transmission on our entering water, which again distinguishes us from the great apes.
Pubic lice
Pubic lice are commonly called crabs because of their appearance (Figure 1c). They belong to a different genus, Pthirus (a misspelling of Phthirus dating back to Linnaeus), from head and body lice. On the basis of morphology, human Pthirus pubis is closely related to the gorilla louse, Pthirus gorillae. In a recent paper David Reed [3] chose a punning Miltonian title, "Pair of lice lost or parasites regained", because it poses the conundrum of whether all the great apes had variants of both Pediculus and Pthirus and lost one or the other, or whether humans gained Pthirus in addition to Pediculus.
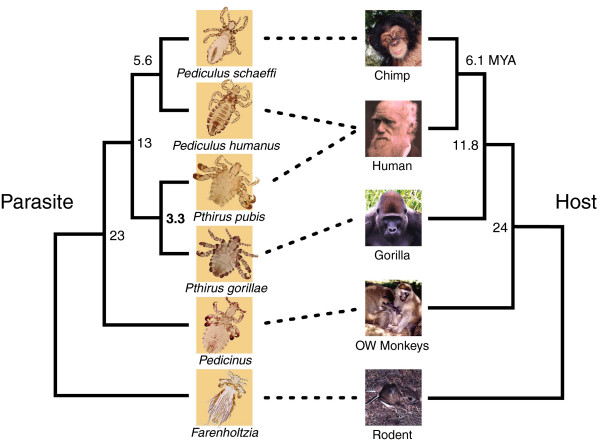
Molecular phylogeny indicates that human pubic lice diverged from gorilla lice as recently as 3.3 MYA [3], whereas the chimp and human host lineage split from the gorilla lineage at least 7 MYA (Figure 3). Thus, it seems clear that humans acquired pubic lice horizontally, possibly at the time of the Pthirus species' split and probably directly from gorillas. Because they were already adapted to the coarse body hair of the gorilla, crabs would have found a suitable niche in human pubic hair. Indeed, the diameter of the hair is most likely the key rather than the pubic region, because pediatric infestation of P. pubis is well documented: the crab is then found on the eyelashes of the infant.
Figure 3.
Host and louse phylogenies. Dotted lines indicate which lice parasitize which host. MYA, million years ago; OW, Old World. Adapted from Reed et al. [3].
Origin of pubic hair
Reed et al. [3] suggest that the most recent common ancestor of the genera Pediculus and Pthirus was about 12.5 MYA, which is earlier than the estimated divergence of gorillas and the chimpanzee-human lineage. So was there duplication and separation of lice in the African anthropoid ape lineage, where they could have occupied separate ecological niches, rather as human head lice and pubic lice do today? Although this is an intriguing hypothesis, I was having difficulty envisioning a clear separation of habitats between the groin and other parts of our ancient common ancestor. My 'eureka moment' came, appropriately enough, in the shower: although naked apes have pubic hair, surely our hairy cousins don't?
How could I test my hypothesis? I knew that there was a stuffed chimpanzee in the Grant Zoological Museum at University College London and I called in on the way to my laboratory. Alas, he was a juvenile, which left the question open. A brisk walk across Regent's Park to inspect the adult gorillas in their splendid new pavilion at London Zoo strengthened my suspicion, and this was later confirmed by a visit to the chimpanzees at Whipsnade Zoo north of London. Indeed, as I noted previously [23], all the species of apes, Old World monkeys and New World monkeys seem to be less hairy in the pubic region than elsewhere; fur is present but it is short and fine.
Why do adult humans sport a thick bush of wiry pubic hair, uniquely among primates? It must surely be because we are otherwise naked. It probably serves both a visual and an odorous function, because hair aids the distribution of apocrine scent secretion, like our less visually stunning axillary hair. Unlike a beard, pubic hair is not sexually dimorphic, yet it is a feature of sexual maturation. No wonder that pubic lice are said to be the most contagious of all sexually acquired infections. Which came first, nakedness or pubic hair? I would postulate that the development of pubic hair was a consequence of the visible nakedness elsewhere on the body. Perhaps the acquisition of P. pubis 3.3 MYA provides the clue to when hominids developed thick pubic hair, rather as the evolution of body lice is thought to be broadly contemporaneous to the development of clothing.
It is noteworthy that the prevalence of infestation by pubic lice seems to be decreasing among women and men who remove their pubic hair using 'bikini wax', rather as the Renaissance painters discreetly depilated classical female nudes. A study from The General Infirmary, Leeds, UK, records the increasing predilection among attendants at a clinic for sexually transmitted infections to undertake extensive pubic hair waxing known as the 'Brazilian', leaving only a thin strip of hair. Armstrong and Wilson [24] noted a significant fall in the incidence of pubic lice among patients, although gonorrhea and chlamydia increased over the same period. Thus, there may be a health benefit to this emerging sexual lifestyle, and this finding would also lend support to the notion that nakedness protects humans from ectoparasites.
Humans as repositories of ancient infections
Given that humans are nouveaux riches regarding our collection of infections [5], can we infer further examples of infections that could have come from other hominid species or from more distantly related primates? The infections for which we have the most accurate evolutionary record are the endogenous retroviruses that have invaded host DNA. Some 8% of the human genome represents 'fossil' integrated proviruses. The human lineage accumulated several thousand retroviral genomes after the split between New World and Old World primates, but only a handful since we diverged from chimpanzees.
Parasites that have tightly cospeciated with their hosts are, of course, in grave danger of extinction when that host becomes an endangered species. But the 'smart' parasite would gain a whole new lease of evolutionary opportunity if it engaged in occasional 'infidelity', analogous to mutation in DNA replication. A DNA lineage that does not mutate would have an extraordinarily slow rate of evolution, whereas a super-mutator without repair would provide little opportunity for natural selection before the genotype changed further. Thus, a low mutation rate within broad fidelity of DNA replication allows for both inheritance and evolution. Likewise, total cospeciation dooms the parasite to the fate of the host, whereas the ability to move horizontally to closely related hosts would provide flexibility. Parasite jumping between related hosts might occur more frequently than realized because it might easily be overlooked.
By this reckoning, the New World clade of head lice formerly faithful to the H. erectus lineage [2] adopted modern humans in the nick of time. HIV-1 might well be another successful case of jumping off a sinking ship, because its former host is not likely to survive for many generations longer in the wild. Now that HIV-1 has adopted a new host species, it is enjoying a most successful adaptive radiation and has already colonized around 60 million humans (25 million of whom have died from AIDS). Such crossover events, however, are relatively rare, and only one of the three ape-to-human transfers of HIV-1 has taken off to cause the AIDS pandemic [11]. It pays the host to place barriers known as restriction factors in the path of potential pathogens. If a species barrier is not recognized by the new invader or is successfully circumvented, the infection can be more virulent in the new host.
Regarding the intestinal parasites and ectoparasites that specialize in human infestation, Ashford [6] pointed out that we not only house two kinds of closely related lice (he meant Pediculus head and body lice), but also two species of Cimex bedbugs, two of Demodex mites and two of Taenia tapeworms. He therefore asked if we were once two separate populations that rejoined after a long separation, but Ashford did not have DNA sequences and molecular clock estimates available to him. Can we now view this phenomenon in the same way as the lice, as one of the pair of parasites cospeciating with H. sapiens and the other jumping from non-ancestral archaic humans to the modern human lineage? It would be intriguing to conduct similar molecular phylogenies of Ashford's other pairs.
Ashford [6] finished by stating: "Over to the microbiologists: What do the bacteria, viruses and fungi tell us?" There are ample examples both of cospeciation and horizontal transmission. The malaria parasite Plasmodium falciparum exemplifies a complex cospeciation between the parasite, its human host and its mosquito vector. Anopheles gambiae has also coevolved to be a specialist feeder on humans; by contrast, the other human malaria parasite, P. vivax, has an origin in South East Asian monkeys and is transmitted by the more promiscuous Culex species. Might vivax malaria have first adapted to H. erectus as an intermediate between monkeys and humans?
As a virologist, I have felt stimulated [23] to take up Ashford's challenge to consider pairs of related human viruses. HIV-1 and HIV-2 are very recent 20th century arrivals, from chimpanzees and sooty mangabey monkeys, respectively. With human T-cell lymphotropic viruses types 1 and 2 (HTLV-1 and HTLV-2), there have been multiple introductions of HTLV-1 from African and Asian monkeys and apes [25]. The provenance of HTLV-2 is puzzling, as its reservoir is in indigenous American populations and in African pygmies, which are as far apart in H. sapiens as one can find. Humans have five distinct polyoma viruses and multiple genital papilloma virus types. The different clades of hepatitis C virus (HCV) have deep roots but there are no known animal relatives to provide an anchor or time calibration. It would be fascinating to learn whether archaic humans harbored HCV variants, but as HCV has an RNA genome there is not much hope of gaining direct evidence by sequence amplification from ancient specimens.
All members of the herpesvirus family are thought to have strictly cospeciated with their hosts, though I have my doubts. The closest relatives to human herpes simplex virus (an α herpesvirus), cytomegalovirus (β) and Epstein-Barr virus (γ), seem to be those in the chimpanzee. Several simian species have a pair of distinct rhadinoviruses, whereas so far humans are only known to harbor one, Kaposi's sarcoma herpesvirus. On the other hand, humans have two herpes simplex viruses (HSVs), types 1 and 2. Phylogenetic analysis [26] indicates that HSV-1 and HSV-2 are further apart from each other than HSV-1 is from its chimp ortholog [27]. So where did HSV-2 come from? From its estimated age of divergence from the chimp-human HSV-1 lineage, I would place a bet on horizontal transmission from gorillas or possibly from orang-utans [23].
Is it a coincidence that three human parasites acquired horizontally from great apes, namely pubic lice, HIV-1 and speculatively HSV-2, are sexually transmitted? Now, before one conjures up a King Kong scenario, it should be noted that predators can pick up parasites from their prey. The close contact involved in human ancestors butchering gorillas could have enabled Pthirus to jump hosts, rather as bushmeat slaughter practices probably led SIVcpz and other retroviruses to invade humans from chimpanzees in modern times [11,25].
Humans as a source of infections
With the advent of globalization, previously isolated human populations lost ninetenths of their number to the infections introduced by intrepid migrants and invaders [7]. Hernan Cortes conquered the mighty Aztec empire thanks to smallpox and measles, which the invaders inadvertently introduced to the disease-naive New World peoples [5]. The subsequent population crash was severe; so few indigenous people survived that the lucrative African slave trade was established in order to provide labor in the plantations. This pattern of export of Old World diseases was repeated in South America, Australia and Oceania. As Charles Darwin remarked in his diary on the voyage of the Beagle, "Wherever the European has trod death seems to pursue the Aboriginal."
It is plausible, then, that modern humans could have transmitted lethal infectious diseases to archaic human species. H. erectus might have given us a clade of head lice, but H. sapiens may have conveyed more deadly infections to H. erectus and later to H. neanderthalensis. Hard evidence is lacking, and prehistoric legends seldom tell the tale from the point of view of the vanquished, although Golding did through the eyes of the Neanderthals [14]. To paraphrase Darwin, wherever modern humans trod during the Pleistocene era, death seemed to pursue archaic humans. There is also a danger today that the surviving great apes may be subjected to a coup de grace from human infections transmitted through jungle safaris and ecotourism.
Cross-species virulence is well known. Myxamotosis resident in American cotton-tail rabbits devastated the European rabbit, and the disappearance of red squirrels in Britain wherever American gray squirrels occur is probably due to a pox virus rather than direct competition for habitat. Similarly, the α-herpesviruses that have cospeciated with Indian and African elephants cause nothing more severe than cold sores in their natural host but each seems to be lethal to the other when the two species are unnaturally housed together in zoos [28]. SIVcpz has little effect on chimpanzees but HIV-1 causes AIDS in humans. Thus, it might pay the host to carry a fairly harmless parasite if that infection is lethal to the host's competitors.
Epilog
Body lice, and occasionally head and pubic lice, transmit bacterial diseases: typhus (Rickettsia prowazekii) [29], trench fever (Bartonella quintana) and relapsing fever (Borellia recurrentis). The lice themselves succumb to typhus infection but pass the Rickettsia in their feces, which the human then scratches into the skin. Typhus is known as 'war fever' and 'jail fever' because it appears in conditions where lice thrive. At the end of Rats, Lice and History Zinsser [1] wrote "Typhus is not dead. It will live on for centuries and will break into the open whenever human stupidity and brutality give it a chance." Sadly, Zinsser's words were prophetic. Within a few years, typhus became the major slayer in the Nazi concentration camps; typhus broke out among Rwandan refugees in Burundi in 1994, in Bosnia in 1995 and most recently in Goma.
Human brutality is another feature shared with chimpanzees that has survived in the human lineage [30]. It is too bad that we are not closer to the pygmy chimp (bonobo), which evolved a means of conflict resolution between troupes through alpha females engaging in lesbian sex. But that is another story.
Acknowledgments
Acknowledgements
I am grateful to Tim Harrison and David Reed for commenting on a draft of this paper.
References
- Zinsser H. Rats, Lice and History. London: Macmillan; 1934. [Google Scholar]
- Reed DL, Smith VS, Hammond SL, Rogers AR, Clayton DH. Genetic analysis of lice supports direct contact between modern and archaic humans. PLoS Biol. 2004;2:e340. doi: 10.1371/journal.pbio.0020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DL, Light JE, Allen JM, Kirchman JJ. Pair of lice lost or parasites regained: the evolutionary history of anthropoid primate lice. BMC Biol. 2007;5:7. doi: 10.1186/1741-7007-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler R, Kayser M, Stoneking M. Molecular evolution of Pediculus humanus and the origin of clothing. Curr Biol. 2003;13:1414–1417. doi: 10.1016/S0960-9822(03)00507-4. Erratum: Curr Biol 2004, 14:2309. [DOI] [PubMed] [Google Scholar]
- Weiss RA, McMichael AJ. Social and environmental risk factors in the emergence of infectious diseases. Nat Med. 2004;10:S70–S76. doi: 10.1038/nm1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford RW. Parasites as indicators of human biology and evolution. J Med Microbiol. 2000;49:771–772. doi: 10.1099/0022-1317-49-9-771. [DOI] [PubMed] [Google Scholar]
- Diamond J. Guns, Germs and Steel: a Short History of Everybody for the Last 13,000 Years. London: Jonathan Cape; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumcuoglu KY, Zias JE, Tarshis M, Lavi M, Stiebel CD. Body louse remains found in textiles excavated at Masada, Israel. J Med Entomol. 2004;40:585–587. doi: 10.1603/0022-2585-40.4.585. [DOI] [PubMed] [Google Scholar]
- Korber B, Muldoon M, Theiler J, Gao F, Gupta R, Lapedes A, Hahn BH, Wolinsky S, Bhattacharya T. Timing the ancestor of the HIV-1 pandemic strains. Science. 2000;288:1789–1796. doi: 10.1126/science.288.5472.1789. [DOI] [PubMed] [Google Scholar]
- Worobey M, Gemmel M, Teuwen DE, Haselkorn T, Kunstman K, Bunce M, Muyembe JJ, Kabongo JM, Kalengayi RM, Van Marck E, Gilbert MTP, Wolinsky SM. Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960. Nature. 2008;455:661–664. doi: 10.1038/nature07390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Heuverswyn FV, Li Y, Bailes E, Takehisa J, Santiago ML, Bibollet-Ruche F, Chen Y, Wain LV, Liegeois F, Loul S, Ngole EM, Bienvenue Y, Delaporte E, Brookfield JFY, Sharp PM, Shaw GM, Peeters M, Hahn BH. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313:523–526. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heuverswyn F, Li Y, Bailes E, Neel C, Lafay B, Keele BF, Shaw KS, Takehisa J, Kraus MH, Loul S, Butel C, Liegeois F, Yangda B, Sharp PM, Mpoudi-Ngole E, Delaporte E, Hahn BH, Peeters M. Genetic diversity and phylogeographic clustering of SIVcpzPtt in wild chimpanzees in Cameroon. Virology. 2007;368:155–171. doi: 10.1016/j.virol.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Hobolth A, Christensen OF, Mailund T, Schierup MH. Genomic relationships and speciation times of human, chimpanzee, and gorilla inferred from a coalescent hidden Markov model. PLoS Genet. 2007;3:e7. doi: 10.1371/journal.pgen.0030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding W. The Inheritors. London: Faber and Faber; 1955. [Google Scholar]
- Raoult D, Reed DL, Dittmar K, Kirchman JJ, Rolain JM, Guillen S, Light JE. Molecular identification of lice from pre-Columbian mummies. J Infect Dis. 2008;197:535–543. doi: 10.1086/526520. [DOI] [PubMed] [Google Scholar]
- Light JE, Toups MA, Reed DL. What's in a name: the taxonomic status of human head and body lice. Mol Phylogenet Evol. 2008;47:1203–1216. doi: 10.1016/j.ympev.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Leo NP, Barker SC. Unravelling the evolution of the head lice and body lice of humans. Parasitol Res. 2005;98:44–47. doi: 10.1007/s00436-005-0013-y. [DOI] [PubMed] [Google Scholar]
- Levene H, Dobzhansky T. Possible genetic difference between the head louse and the body louse. Am Nat. 1959;873:347–353. doi: 10.1086/282094. [DOI] [Google Scholar]
- Morris D. The Naked Ape: A Zoologist's Study of the Human Animal. London: Jonathan Cape; 1967. [Google Scholar]
- Rantala MJ. Human nakedness: adaptation against ectoparasites? Int J Parasitol. 1999;29:1987–1989. doi: 10.1016/S0020-7519(99)00133-2. [DOI] [PubMed] [Google Scholar]
- Pagel M, Bodmer W. A naked ape would have fewer parasites. Proc R Soc Lond B Biol Sci. 2003;270(Suppl 1):S117–S119. doi: 10.1098/rsbl.2003.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy MG. Sir Alister Hardy and the aquatic ape theory: a brief account of his life. Nutr Health. 1993;9:161–164. doi: 10.1177/026010609300900303. [DOI] [PubMed] [Google Scholar]
- Weiss RA. Lessons from naked apes and their infections. J Med Primatol. 2007;36:172–179. doi: 10.1111/j.1600-0684.2007.00235.x. [DOI] [PubMed] [Google Scholar]
- Armstrong NR, Wilson JD. Did the "Brazilian" kill the pubic louse? Sex Transm Inf. 2006;82:265–266. doi: 10.1136/sti.2005.018671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch DJ, Rixon FJ, Davison AJ. Topics in herpesvirus genomics and evolution. Virus Res. 2006;117:90–104. doi: 10.1016/j.virusres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Luebcke E, Dubovi E, Black D, Ohsawa K, Eberle R. Isolation and characterization of a chimpanzee alphaherpesvirus. J Gen Virol. 2006;87:11–19. doi: 10.1099/vir.0.81606-0. [DOI] [PubMed] [Google Scholar]
- Wolfe ND, Heneine W, Carr JK, Garcia AD, Shanmugam V, Tamoufe U, Torimiro JN, Prosser AT, Lebreton M, Mpoudi-Ngole E, McCutchan FE, Birx DL, Folks TM, Burke DS, Switzer WM. Emergence of unique primate T-lymphotropic viruses among central African bushmeat hunters. Proc Natl Acad Sci USA. 2005;102:7994–7999. doi: 10.1073/pnas.0501734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman LK, Montali RJ, Garber RL, Kennedy MA, Lehnhardt J, Hildebrandt T, Schmitt D, Hardy D, Alcendor DJ, Hayward GS. Novel endotheliotropic herpesviruses fatal for Asian and African elephants. Science. 1999;283:1171–1176. doi: 10.1126/science.283.5405.1171. [DOI] [PubMed] [Google Scholar]
- Walker DH, Ismail N. Emerging and re-emerging rickettsioses: endothelial cell infection and early disease events. Nat Rev Microbiol. 2008;6:375–386. doi: 10.1038/nrmicro1866. [DOI] [PubMed] [Google Scholar]
- Wrangham R, Peterson D. Demonic Males: Apes and the Origins of Human Violence. London: Bloomsbury; 1997. [Google Scholar]