Abstract
A recent report in BMC Biology indicates that modern humans first arrived in southern East Asia 60,000 years ago and settled the rest of East Asia from there. This early date and migration route has significant implications for our understanding of the origins of present-day human populations.
Studies of human origins have witnessed a radical transition from studies based on morphological comparisons to a reliance on molecular genetics. One of the first molecular comparisons estimated human divergence from the African apes at a now generally accepted timescale of 5 million years ago, when most paleontologists then placed it between 15 and 30 million years ago. Later, measurement of genetic diversity revealed the relatively recent origin in Africa of modern humans, who then spread over the entire globe (see [1] and citations therein). Much current research in anthropology centers on understanding the timing and migration routes of modern humans. An analysis of Y-chromosome genetic diversity published by Shi et al. [2] in BMC Biology has now clarified migration routes and times of settlement for East Asia, with wide-ranging implications.
Previously, it seemed equally possible that the modern humans who settled East Asia came either from Southeast Asia or, alternatively, migrated southward from northern Asia. Researchers have long noted a significant genetic difference between northern and southern East Asian populations that could be interpreted to support either scenario – or some mix of the two. Both archaeological and genetic evidence for settlement times were also ambiguous [2,3]. Timings for southern East Asia ranged from an earliest date of 30,000 years ago to 50,000 years ago at the latest, while dates for the settlement of Siberia ranged from 40,000 to 45,000 years ago, or even earlier. However, the new data from Shi et al. [2] suggest that our species reached southern East Asia 60,000 years ago, twice as long ago as most previous estimates, and then spread rapidly northward.
The amount of genetic diversity in present-day populations is a useful variable for inferring geographic origins and migration routes. Africa was pinpointed as the homeland of Homo sapiens because of the higher genetic diversity among Africans compared with populations elsewhere in the world, while the last geographic regions to be settled, South America and the Pacific Islands, show the lowest genetic diversity. Greater variation has been noted among Africans not only in their genes but in variables such as craniometrics, dental traits and even skin color [4,5].
The original 'out of Africa' hypothesis of modern human origins and subsequent pattern of global migration was based on mitochondrial DNA (mtDNA) evidence, which revealed a series of population bottlenecks and a progressive loss of diversity moving away from East Africa. mtDNA, because it is located outside of the cell nucleus, is inherited only through the female line. In contrast, the Y chromosome is passed only from father to son, and so can be similarly used to follow the male line. Both mtDNA and most Y-chromosomal DNA are non-recombinant and analysis of their inheritance is therefore more straightforward than for other parts of the genome, which are mostly scrambled samples of DNA from both parents. Human populations on the branches of the mtDNA or Y-chromosome 'tree' can be distinguished by sets of accumulated mutational differences – or haplotypes – in stretches of DNA. Because these mutations accumulate at a fairly regular rate over time, they can be used as a 'clock' to estimate the time of human population splits.
Reconstruction of human origins and migration now mainly relies on the Y chromosome because it is much larger than the mitochondrial genome, consisting of tens of millions of nucleotides compared to the 16,000 of human mtDNA. Thousands of differences can be found in Y-chromosomal DNA from different human populations. The different Y-chromosome haplotypes function as signatures for different human lineages and are often highly associated with different geographic regions, making them extremely useful for tracing human origins and migration.
Out of Africa and into Asia
Shi et al. [2] thoroughly tested settlement hypotheses by collecting the largest East Asian sample to date, more than 5,000 males from 73 populations. Many new samples came from underrepresented south and southwestern China and they also incorporated data from many published reports.
The D-M174 lineage was already known from previous studies, and, even if thought to be up to 50,000 years old, the prevalent hypothesis viewed it just as one of the various lineages moving northward with the predominant O-M175 lineage around 25,000 to 30,000 years ago. Recently, it was found that D-M174 has a high frequency in Andamanese (considered the earliest settlers of Southeast Asia). Shi et al. [2] then cogently argued that the disjunct distribution of lineage D-M174 today found primarily in the Andaman Islands, Tibet and Japan indicated that it is the oldest lineage in East Asia. Its sporadic presence in other populations, they concluded, was due to recent gene flow that previously went unappreciated.
A more detailed subtyping within the D-M174 lineage also allowed Shi et al. to identify the deep, hidden structure of various male lineages. Dates in excess of 60,000 years were needed to account for the differences and distributions of these subtypes. This date is older than those previously reported based on both Y-chromosomal DNA and mtDNA. It seems an inescapable conclusion that the lineage is indeed ancient, southern in origin and preceded others found in contemporary East Asian populations. Recent archaeological evidence of Tibetan settlement between 30,000 to 40,000 years ago also supported this conclusion.
This genetic signature of early migration has been masked in much of East Asia by a later, overriding migration due to the population explosion in Neolithic times of the Han ethnic group, which is characterized by a different Y-chromosome lineage. Today, both D-M174 and the Han Y-chromosome lineages are found in Tibet and Japan, showing that both these populations are the result of two distinct migrations. Shi et al. [2] also find from their new data that previous impressions that northern Asian genetic diversity is greater than southern diversity are probably incorrect and due to incomplete sampling, thus removing one important motive for proposing a northern Asian origin.
The authors also estimate the age of the unique Japanese haplotype D2-M57 as 37,000 years, which suggests that this first wave of migration brought people to Japan before the date of the earliest archeological evidence at 30,000 years ago. Tibetan settlement apparently predates that of Japan. The origin of the Tibetan D-M174 sublineage is older (52,000 years ago) and the Tibetan population also has a higher genetic diversity, indicative of earlier settlement.
Implications for the global pattern of human migration
Pushing back the date of migration into East Asia to 60,000 years ago has wide implications for global scenarios of human dispersion. It makes it necessary to opt for the earliest possible dates for the initial migration out of Africa and for settlement in the Indian subcontinent. Estimates of the time of origin of modern humans in Africa fall between 150,000 and 200,000 years ago (Figure 1), as supported by paleontological finds in Ethiopia [4]. Most dates for the coastal migration out of Africa range from 50,000 to 77,000 years ago, but these now seem to be too late. There is paleontological evidence for modern humans in the Middle East around 92,000 years ago [4] and, if populations were as small as predicted, then we should seriously consider an even earlier out-of-Africa date, perhaps even before 100,000 years ago. Better sampling of African populations maybe needed to answer the question of whether there was more than one migration and route out of Africa. However, the limited worldwide genetic variability among present-day humans has led many researchers to exclude such a possibility and suggests that only a handful of people were involved, perhaps as few as a total of 600 founding females [6].
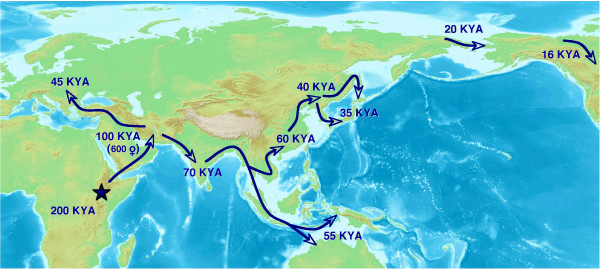
Figure 1.
A simplified scenario of early human migration routes and dates. Modern humans originated in Africa, probably around 200,000 years ago (200 KYA). One or more routes out of Africa are possible, but the number of individuals involved was very limited, with perhaps only 600 females. Migration probably followed a coastal route, with humans arriving in the Indian subcontinent about 70,000 years ago. The analysis by Shi et al. [2] suggests that humans arrived in southern East Asia around 60,000 years ago and then proceeded north to occupy northern East Asia and Japan.
The migration route to East Asia must pass through the Indian subcontinent. Genetic diversity in present-day India is second only to that in Africa and implies settlement soon after humans left Africa. Dispersal across Eurasia from Africa to India was often previously estimated to have occurred between 45,000 and 59,000 years ago, but a recent proposal of 66,000 to 70,000 years ago [7] would be more congruent with the estimate of Shi et al. Genetic markers and an early date for East Asian settlement also support the hypothesis that independent migrations populated East Asia and Australia, as current estimates for human settlement in Australia and New Guinea are around 55,000 years ago. The estimate by Shi et al. [2] of human settlement in Northeast Asia earlier than 30,000 years ago could also have implications for the time of entry of humans into North America; this is currently put at 16,000 years ago [3], but could be an underestimate.
Cautionary tales and future directions
We should caution, however, that dates inferred from present-day genetic diversity can vary greatly, as a result of unknown differences, in variables such as population size, rates of genetic drift, gene flow, and the presence of selection. For example, Y chromosome and mtDNA reconstructions of human origins can differ, in part because Y chromosomes reflect the activities of males whereas mtDNA reflects those of females. It is often the case that dates from mtDNA are up to twice as old as those for the Y chromosome. The widespread practice of polygyny means that the number of males contributing to the next generation is always smaller than that of females [8]. Migration patterns of men and women may also differ due to patterns of marriage exchange and post-marital residence. In brief, it is clear that cultural differences can strongly affect levels of genetic diversity and a correct interpretation of human diversity requires that the biases introduced by migration and admixture patterns must be disentangled from the effects of selection, drift and demography on the human genome.
The work of Shi et al. [2] also indicates that human founder populations were small and isolated, and that the initial migration signal may be difficult to detect, especially when it is hidden beneath layers of subsequent migrations. In part this problem can be resolved by sampling peripheral, more isolated populations. Analysis of ancient DNA can also cut through the layers of time and is an important test of conclusions based on DNA from living populations. Ancient DNA served as an important test of the out-of-Africa theory, which predicted that modern humans replaced regional archaic hominids. Indeed, analysis of mtDNA extracted from Neanderthal specimens and from early modern human remains strongly suggests that Neanderthals did not contribute to our genome [9].
Although accurate characterization of geographic ancestry is possible using a small number of markers, a detailed understanding of human diversity will require more extensive sampling. Together, mtDNA and the Y chromosome constitute only a small fraction of the human genome and we must take more of the genome into consideration for a full understanding of human evolution. A more complete survey of populations and sophisticated statistical analysis of thousands of additional markers is needed [10]. The results will not be trivial and will permit a better understanding of human adaptation, susceptibility to diseases, and even success in pharmacological therapy [6]. High-throughput genotyping and massively parallel sequencing technologies hold great promise that we will soon achieve much more than the tracking of human global migration and settlement. The golden age of human evolutionary genetics is just beginning to dawn.
Contributor Information
Roscoe Stanyon, Email: roscoe.stanyon@unifi.it.
Marco Sazzini, Email: Not@valid.com.
Donata Luiselli, Email: Not@valid.com.
References
- Stoneking M. Human origins. The molecular perspective. EMBO Rep. 2008;9(Suppl 1):S46–S50. doi: 10.1038/embor.2008.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Zhong H, Peng Y, Dong YL, Qi XB, Zhang F, Liu LF, Tan SJ, Ma RZ, Xiao CJ, Wells RS, Jin L, Su B. Y chromosome evidence of earliest modern human settlement in East Asia and multiple origins of Tibetan and Japanese populations. BMC Biol. 2008;6:45. doi: 10.1186/1741-7007-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel T, Waters MR, O'Rourke DH. The late Pleistocene dispersal of modern humans in the Americas. Science. 2008;319:1497–1502. doi: 10.1126/science.1153569. [DOI] [PubMed] [Google Scholar]
- Relethford JH. Genetic evidence and the modern human origins debate. Heredity. 2008;100:555–563. doi: 10.1038/hdy.2008.14. [DOI] [PubMed] [Google Scholar]
- Roseman CC, Weaver TD. Molecules versus morphology? Not for the human cranium. BioEssays. 2007;29:1185–1188. doi: 10.1002/bies.20678. [DOI] [PubMed] [Google Scholar]
- Campbell MC, Tishkoff SA. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu Rev Genomics Hum Genet. 2008;9:403–433. doi: 10.1146/annurev.genom.9.081307.164258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Padmanabham PB, Ravuri RR, Uttaravalli K, Koneru P, Mukherjee PA, Das B, Kotal M, Xaviour D, Saheb SY, Rao VR. The earliest settlers' antiquity and evolutionary history of Indian populations: evidence from M2 mtDNA lineage. BMC Evol Biol. 2008;8:230. doi: 10.1186/1471-2148-8-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer MF, Mendez FL, Cox MP, Woerner AE, Wall JD. Sex-biased evolutionary forces shape genomic patterns of human diversity. PLoS Genet. 2008;4:e1000202. doi: 10.1371/journal.pgen.1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramelli D, Lalueza-Fox C, Vernesi C, Lari M, Casoli A, Mallegni F, Chiarelli B, Dupanloup I, Bertranpetit J, Barbujani G, Bertorelle G. Evidence for a genetic discontinuity between Neandertals and 24,000-year-old anatomically modern Europeans. Proc Natl Acad Sci USA. 2003;100:6593–6597. doi: 10.1073/pnas.1130343100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JZ, Absher DM, Tang H, Southwick AM, Casto AM, Ramachandran S, Cann HM, Barsh GS, Feldman M, Cavalli-Sforza LL, Myers RM. Worldwide human relationships inferred from genome-wide patterns of variation. Science. 2008;319:1100–1104. doi: 10.1126/science.1153717. [DOI] [PubMed] [Google Scholar]