Abstract
The contextual interference (CI) effect affirms that learning is enhanced when interference during practice is high, such as when participants practice multiple tasks in a random order. Previous research showed a distinct response in the cortical motor (CM) regions of participants performing under high CI practice conditions compared with low CI conditions. Specifically, there was increased corticomotor activity in a high CI condition when participants practiced 3 arm tasks, each with specific spatial and temporal requirements. Using disruptive transcranial magnetic stimulation (TMS), the authors' purpose was to determine whether CM is preferentially processing the spatial, temporal, or both parameters of the task during high CI practice. Participants were randomized to 1 of 6 practice conditions derived from 3 stimulation conditions (no TMS, TMS, sham TMS) and 2 CI conditions (blocked [low CI] and random [high CI]). The authors measured performance accuracy in movement timing (temporal) and amplitude (spatial) across practice and no-stimulation recall phases. TMS perturbation deterred learning of movement timing under random, but not blocked, practice order; the authors did not observe this in spatial parameter learning. The authors' data suggest that increased corticomotor activity during high CI practice may reflect preferential processing of the temporal parameter of the task.
Keywords: contextual interference, kinematics, motor learning, transcranial magnetic stimulation
It is well established that interference during practice has substantial influence on skill learning (Battig, 1956, 1966, 1979; Guadagnoli & Lee, 2004). One way to manipulate interference during practice of multiple tasks is to change the order in which the tasks are practiced. For example, a random practice order in which tasks are practiced in a quasi-random order (i.e., C-A-B, A-B-C, B-C-A, where each letter represents a task) is thought to introduce more interference than a blocked practice order in which each task is practiced repeatedly prior to switching to the next task (i.e., A-A-A, B-B-B, C-C-C).
Results from studies investigating the effects of practice order on motor learning typically show that a random practice order enhances motor learning more when compared with that of a blocked practice order. This phenomenon is known as the contextual interference (CI) effect (Giuffrida, Shea, & Fairbrother, 2002; Meira & Tani, 2001; Perez, Meira, & Tani, 2005; Wright, Magnuson, & Black, 2005). The CI effect elicited under random-order conditions (i.e., high-interference condition) has been explained, in part, by additional planning and parameter specification that is required when different tasks are being practiced in the same session. The processing involved in repeated parameter specification and action planning is thought to result in a stronger memory representation of the practiced tasks (Hall & Magill, 1995; T. D. Lee & Magill, 1983, 1985; T. D. Lee, Wishart, Cunningham, & Carnahan, 1997; Shea & Zimny, 1983). This enhanced processing related to the CI effect may, in part, take the form of increased cortical motor activity.
Transcranial magnetic stimulation (TMS) is a technique that can introduce a transient perturbation to modulate cortical motor activity by means of noninvasive stimulation of the human brain (Hallett, 2000). Lin, Fisher, Winstein, Wu, and Gordon (2008) demonstrated a brain–behavior relation between the cortical motor regions and the CI effect in motor-skill learning. Lin et al. used single-pulse TMS as an identical external perturbation during blocked and random practice. The TMS perturbation centered over the primary motor cortex (M1) during practice selectively disrupted motor learning under the random, but not the blocked, practice condition, suggesting that TMS may have interfered with those essential brain processes evoked distinctly by random practice (Lin et al.). Increased cortical motor activity during random practice can be hypothesized as necessary neural processing to repeatedly specify, with every practice trial, kinematic parameters such as the amplitude or timing of the movement. Several neuroimaging studies support this hypothesis by demonstrating broad activation in primary and secondary cortical motor regions—such as M1, sensory motor cortex (S1), dorsal and ventral premotor cortex (PMd and PMv, respectively), supplementary motor area (SMA), and posterior parietal cortices—when participants attempt to perform tasks requiring skillful action or when participants are trained in high-interference practice conditions (Aoki et al., 2005; Chung, Min, Kim, & Cho, 2000; Demiralp, Karamürsel, Karakullukçu, & Gökhan, 1990; Gorbet, Staines, & Sergio, 2004; Hatfield, Haufler, Hung, & Spalding, 2004; Lewis, Wing, Pope, Praamstra, & Miall, 2004; Meister et al., 2005; Schubotz & von Cramon, 2002; Seidler, Noll, & Thiers, 2004; Sergio & Kalaska, 1998; Zhang, Riehle, & Requin, 1997).
The aforementioned studies have firmly established that high-interference practice conditions evoke extensive processing in cortical motor regions (Aoki et al., 2005; Chung et al., 2000; Demiralp et al., 1990; Gorbet et al., 2004; Hatfield et al., 2004; Lewis et al., 2004; Meister et al., 2005; Schubotz & von Cramon, 2002; Seidler et al., 2004; Sergio & Kalaska, 1998; Zhang et al., 1997). However, researchers have yet to determine what specific feature(s) of the task are associated with this increased cortical motor activity. Because motor-skill development requires learning kinematic features of the movement (Krakauer, Mazzoni, Ghazizadeh, Ravindran, & Shadmehr, 2006; Krakauer & Shadmehr, 2006; Sainburg, 2002), the increased cortical motor activity may reflect neural processing of spatial or temporal parameters of the movement. Therefore, the purpose of the present study was to determine whether cortical motor regions are preferentially processing the spatial, temporal, or both parameters of the task. We used three different arm movements that required specification of both amplitude (spatial) and timing (temporal) information. If the cortical motor regions were associated with processing kinematic information, learning of timing or amplitude would be deterred by TMS for participants who practiced under random-practice conditions. Because of the repetitive nature for blocked practice, the need for parameter specification is less in a blocked practice condition. We did not expect cortical motor perturbation during blocked practice to deter learning of kinematic parameters.
Method
Participants
We recruited 60 healthy, right-handed volunteers (ages 18–38 years) who were naive to the task. All participants gave written informed consent and participated in the study under a protocol approved by the Institutional Review Board of the University of Southern California Health Sciences Campus. Exclusion criteria were all contraindications to receiving TMS (Wassermann, 1998) and included the presence of a pacemaker, metal in the head, pregnancy, any neurological disorder, current use of stimulants or medications known to lower seizure threshold, and personal or family history of a seizure disorder. Participants were the same as those in Lin et al. (2008).
Instrumentation and Task
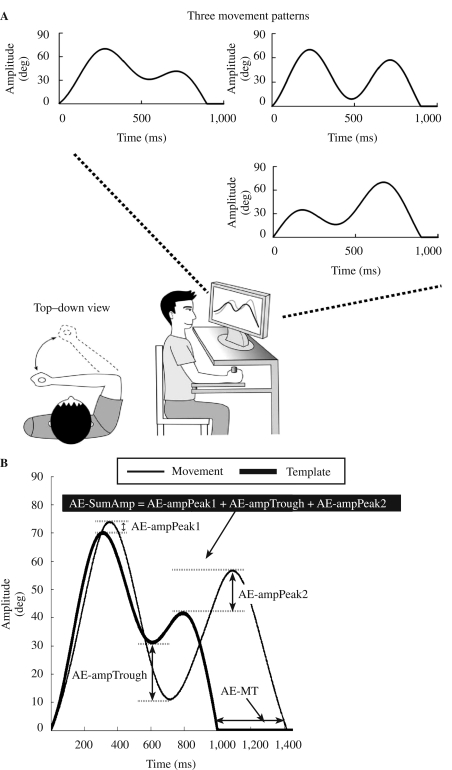
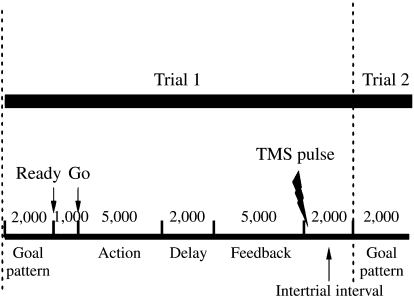
A lightweight lever affixed to a frictionless vertical axle was attached to a table and positioned parallel to the floor. We adjusted a handle at the end of the lever to accommodate the participant's forearm. We attached a linear potentiometer to the transducer at the base of the vertical axle and signals from the transducer were converted to a digital signal and sampled at 1,000 Hz. To manipulate movement trajectory, the timeline of each event, and data storage for each trial, we applied a LabView-based (Labview, National Instruments, Austin, TX) custom-made software program (Weekley, 2004). We applied a low-pass filter of 10 Hz to remove high-frequency noise and smooth out the signals. The motor task was to move the lever to replicate three template movement trajectories. One of the three template trajectories was displayed on the computer monitor before each trial. Each template trajectory comprised two elbow extension–flexion reversal actions (see Figure 1A). The three trajectories were differentiated by the timing and amplitude of the reversal actions. Participants saw a template trajectory for 2 s, and then the template disappeared. Participants were instructed to initiate movements on a “go” signal. The timeline of each event in a single trial is illustrated in Figure 2. Participants practiced the three arm tasks in a blocked or random order for 144 total trials. We divided the 144 trials into three 48-trial sets to prevent fatigue or tiredness. During blocked practice, there were 48 movement trials of each arm task, and we counterbalanced the order of tasks across participants. Because three arm trajectories were practiced, there were six different orders to arrange the three trajectories in blocked practice (i.e., A-B-C, A-C-B, B-A-C, B-C-A, C-A-B, and C-B-A, where each letter represents a task). We counterbalanced the three trajectories by using five of the six orders for practice phase (i.e., A-C-B, B-A-C, B-C-A, C-A-B, and C-B-A). These five orders can be counterbalanced evenly across each 10-participant group. During retention, we applied the other order that participants had not practiced before (i.e., A-B-C) as the testing order for blocked retention test. During random practice, the three tasks were practiced in a nonrepetitive manner in each 48-trial set; the randomization scheme was the same for all random-practice participants.
FIGURE 1.
(A) Experimental setup with lever arm, feedback display, and three template movement trajectories, with specific time and amplitude requirements. (B) Accuracy in movement timing was quantified using absolute error in movement time (AE-MT). Accuracy in movement amplitude was quantified using the sum of absolute error in movement amplitude derived from the three reversal points in each movement trajectory (AE-SumAmp). AE-ampPeak1 = absolute value of amplitude error at the Peak1; AE-ampTrough = absolute value of amplitude error at the trough; AE-ampPeak2 = absolute value of amplitude error at the Peak2.
FIGURE 2.
Timeline of each event in a single trial in milliseconds. For the TMS and sham-TMS groups, a single TMS pulse (sham or real) was synchronized to the onset of the intertrial interval. TMS = transcranial magnetic stimulation.
During the practice phase, the participants were presented with feedback after each movement trial. The feedback included (a) an overall error score, representing the difference between the template movement trajectory and the participants' response, and (b) a graphic representation of the participants' response superimposed on the template movement trajectory. All participants received written instructions and additional verbal information about the experiment. The timeline of each event in a single trial is illustrated in Figure 2. Participants were allowed to perform each movement up to 5 s. The time to complete the movement was variable and depended on how accurately the participant performed, whereas the other interval times (e.g., feedback delay) were fixed.
The participants linked the template trajectories to their own performance empirically. We did not provide any explicit instruction about movement amplitude and timing. Participants were instructed to match (as closely as possible) their movement trajectories with template trajectories.
Experimental Design
Participants were randomly assigned to one of six practice conditions derived from three stimulation conditions and two practice orders: no TMS (blocked–no TMS, random–no TMS), TMS (blocked–TMS, random–TMS), and sham TMS (blocked–sham TMS, random–sham TMS). TMS pulses were delivered so that they were centered over the M1 contralateral to the moving arm. We implemented the sham-TMS condition (i.e., coil applied to same location, normal clicking noise apparent, but no magnetic pulse applied) to control for the nonspecific effects of TMS, including coil noise and scalp sensation.
Experimental Procedure
Because the learning performance distinction is a well-recognized phenomenon in learning and memory research, we used a recall test paradigm to measure the relatively permanent changes on motor performance (Cahill, McGaugh, & Weinberger, 2001; Giuffrida et al., 2002; Goodwin & Meeuwsen, 1996; Hall & Magill, 1995; T. D. Lee et al., 1997; Meira & Tani, 2001; Perez et al., 2005; Schmidt & Lee, 2005; Shea & Morgan, 1979; Wright et al., 2005). Participants came for 2 consecutive days with the practice phase on Day 1 and recall phase on Day 2. Participants practiced three sets of 48 trials during the practice phase, resulting in a total of 144 practice trials. Participants performed 24 recall-accuracy trials in the recall phase. To ensure that the recall test was not biased toward either practice order, participants were given 12 recall trials, each under blocked- and random-order conditions, and the recall test order was counterbalanced across participants. We administered the trials in the recall phase without feedback and TMS, real or sham.
Magnetic Stimulation
We used single-pulse TMS as an identical external perturbation to cortical motor activity during blocked and random practice (Boroojerdi et al., 2001; Flitman et al., 1998; Hallett, 2000; Ziemann, 2004). On the basis of neuroimaging studies of motor learning and to maximize effects of a single TMS pulse, we used a circular coil in which the effect was centered over M1, but in which the influence would cover most of the contralateral primary and secondary cortical motor regions. Because the interval between trials (i.e., intertrial interval) is a critical time in which neural activity subserving the current action plan is distinct between blocked and random practice, single pulses of disruptive TMS were delivered to each intertrial interval during motor practice (Battig, 1979; Gabriele, Hall, & Buckolz, 1987; T. D. Lee & Magill, 1983, 1985; Limons & Shea, 1988; Shea & Zimny, 1983; Wright, Li, & Coady, 1997; Wright, Li, & Whitacre, 1992).
Magnetic pulses were delivered by a magnetoelectric stimulator (MES-10, Cadwell Laboratories, Kennewick, WA) through a circular magnetic coil (9.0-cm diameter). In considering how to stimulate most candidate cortical motor regions, we chose a circular coil rather than a focal figure of eight. We placed the coil tangentially to the scalp in a direction such that monophasic pulses induced a current flowing anteroposteriorly perpendicular to the central sulcus (Kaneko, Kawai, Fuchigama, Shiraishi, & Ito, 1996; Werhahn et al., 1994). To center our stimulation over the motor cortex (Butefisch, Khurana, Kopylev, & Cohen, 2004), we placed one edge of the coil on the participant's scalp and identified an optimal location where stimuli consistently elicited a maximal motor evoked potential (MEP) in the contralateral biceps brachii muscle (Kamen, 2004; Lin et al., 2008). We monitored electromyography (EMG) throughout data collection to ensure the specificity of magnetic pulses. Motor threshold was defined as the minimum TMS intensity to the nearest 1% of maximum stimulator output that evoked a MEP ≥ 50 μV in at least 5 of 10 trials in the target muscle (Rossini et al., 1994). Single pulses of TMS were applied only during the acquisition phase and synchronized to the onset of each intertrial interval (see Figure 2). We chose this interval because it is a critical period for cognitive processing during practice when information from the previous trial may be used in the preparation for the next trial (T. D. Lee & Magill, 1983; Lee et al., 1997; Shea & Zimny, 1983). We chose a suprathreshold TMS intensity (120% of resting motor threshold) to produce a disruptive influence on cortical activity centered on and around the motor cortex (Berardelli et al., 1994; Pascual-Leone, Houser, Grafman, & Hallett, 1992; Taylor, Wagener, & Colebatch, 1995; Ziemann, Ilic, Pauli, Meintzschel, & Ruge, 2004).
Dependent Measures
Because the motor tasks have specific amplitude and timing requirements, accuracy in movement timing and amplitude was quantified across practice and recall phases. Performance in movement timing was quantified using absolute error in movement time (AE-MT; see Figure 1B). Performance in movement amplitude was quantified using the sum of absolute error in movement amplitude derived from the three reversal points in each movement trajectory (AE-SumAmp; see Figure 1B). We determined absolute amplitude error at each reversal point by placing an event marker on each reversal point using a customized MATLAB-based program (Wu, 2004).
We also calculated the mean difference between the last practice block (Block 12) and the retention block on the second day (Block 13) for each practice group (this measure is described as Block 12–13 difference). The Block 12–13 difference is an index to demonstrate how participants' performance deteriorated from Day 1 to Day 2.
Statistical Analysis
Individual trial timing and amplitude data were grouped into 12 trial blocks for the practice phase (Blocks 1–12) and one 24 trial block for the recall phase (Block 13). Data from the recall phase were presented after collapsing across two recall test conditions (blocked and random conditions). Prior to collapsing the recall data, we performed an analysis to confirm that there was no testing condition effect. We used a 3 × 2 between-subject factorial design: 3 stimulation conditions (no TMS, TMS, sham TMS) and 2 practice orders (blocked and random). For the practice phase, average performance errors (timing and amplitude error) were submitted to a 3 (stimulation condition) × 2 (practice order) × 12 (practice block) analysis of variance (ANOVA) with repeated measures. We calculated a 3 (stimulation condition) × 2 (practice order) ANOVA for the average recall accuracy of the recall phase and for the average Block 12–13 difference. When appropriate, the ANOVAs were followed by a simple main effect test and a post hoc Tukey test to control for inflated Type 1 errors. For all statistical tests, significance level was set at p < .05. We used SPSS (Version 13.0) statistical software for all statistical analysis.
Results
Practice Phase
Timing Error (AE-MT)
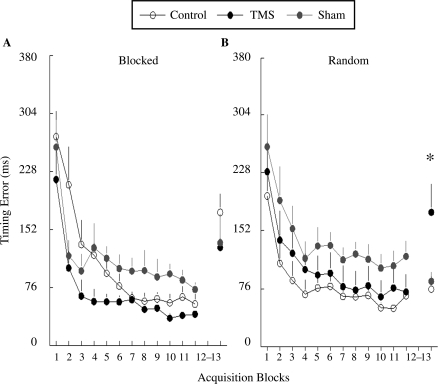
There were no group differences in baseline (Block 1), F(5, 59) = 0.59, p = .71. Each group showed reduction of timing error over the acquisition phase that resulted in a significant main effect for practice block (p < .001; see Figure 3). Participants in the blocked- and random-order groups performed similarly during practice, resulting in no significant effect of practice order, F(1, 54) = 0.94, p = .34, as is typical in experiments that studied the effect of practice order on motor learning. There was also no significant main effect of stimulation condition, F(2, 54) = 2.17, p = .12, or significant Stimulation Condition × Practice Order interaction, F(2, 54) = 1.57, p = .22.
FIGURE 3.
Timing error (y axis) of the six experimental groups across practice and recall phases. The practice phase includes Block 1–12; Block 13 is the 2nd-day recall phase. Data of the recall phase are collapsed across the two testing conditions. Error bars are standard errors of the mean. *indicates the group differences are statistically significant. TMS = transcranial magnetic stimulation.
During the practice phase, real TMS appeared to decrease timing errors for the blocked practice groups (see Figure 3A). However, sham TMS appeared to increase timing errors for the blocked practice groups. Statistical measures showed no significant difference between the blocked–no TMS group and the blocked–TMS group (p = .14) and between the blocked–no TMS group and the blocked–sham TMS group (p = .96). For the random practice groups, TMS and sham TMS appeared to increase timing errors (see Figure 3B). However, we found no significant difference between the random–no TMS and the random–TMS groups (p = .71) and between the random–no TMS and the random–sham TMS groups (p = .20).
Amplitude Error (AE-SumAmp)
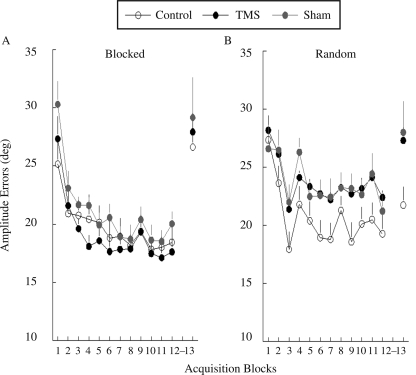
There were no group differences in baseline, F(5, 59) = 3.56, p = .47. Each group showed a reduction of amplitude error over the acquisition phase that resulted in a significant main effect for practice block (p < .001; see Figure 4). There was no significant main effect of stimulation condition, F(2, 54) = 2.30, p = .16, or Stimulation Condition × Practice Order interaction, F(2, 54) = 1.65, p = .28.
FIGURE 4.
Amplitude error in degrees (y axis) of the six experimental groups across practice and recall phases. The practice phase includes Block 1–12; Block 13 is the second-day recall phase. Data of the recall phase are collapsed across the two testing conditions. Error bars are standard errors of the mean. TMS = transcranial magnetic stimulation.
During the practice phase, TMS and sham TMS appeared to deteriorate amplitude performance for the random practice groups (see Figure 4B). However, the difference between the random–no TMS and the random–TMS groups (p = .08) and between the random–no TMS and the random–sham TMS groups (p = .09) did not reach significance.
Recall Phase
Timing error (AE-MT)
TMS during practice phase selectively deterred the recall of movement timing for participants who practiced under the random, but not blocked, condition. The differential effect of TMS and sham TMS to blocked- and random-order practices is associated with a significant Stimulation Condition × Practice Order interaction, F(2, 54) = 3.45, p = .04. Consistent with the literature, we demonstrated the benefit of random practice in the learning of movement timing (i.e., the CI effect). During retention, timing error of the random–no TMS group was significantly lower than that of the blocked–no TMS group (p = .01, see Figure 3, Block 13). This benefit of random practice was abolished by TMS perturbation during practice phase; practice with TMS did not make the random–TMS group better than the blocked–no TMS group (p = .79, see Figure 3, Block 13).
For the blocked practice groups, TMS and sham TMS appeared to lower timing error when compared to the control no–TMS condition, but the results did not yield significant group differences. Conversely, for the random practice groups, TMS deteriorated the recall performance (higher timing error; see Figure 3, Block 13). A post hoc test indicated that the timing error of the random–TMS group was higher than that of the random–control group (p = .03), and marginally higher than the random–sham TMS group (p = .07), suggesting that the disruptive effect of TMS on random practice was mediated through direct perturbation over the cortical motor region.
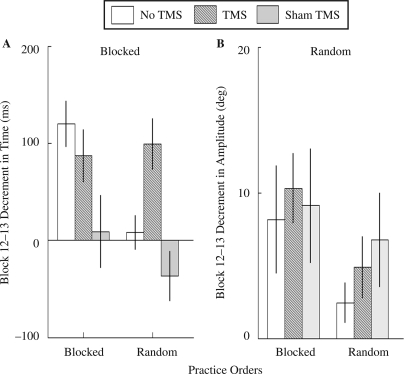
Similar to the results of the timing errors during recall phase, Block 12–13 difference in timing errors, an index of how participants' performance deteriorated from Day 1 to Day 2, also suggested a pattern that TMS and sham TMS differentially influenced timing learning for blocked and random practice groups (see Figure 5A). TMS and sham TMS enhanced timing learning for blocked groups with the most robust effect by sham TMS. In contrast, real TMS deterred timing learning for random groups. The Stimulation Condition × Practice Order interaction was marginally significant, F(2, 54) = 2.85, p = .07. A post hoc test indicated that Block 12–13 difference in timing errors was significantly higher for the random–TMS than for the random–no TMS groups (p = .01). The Block 12–13 difference of random–sham TMS and random–no TMS groups was not significant (p = .17), suggesting that the effect was from cortical perturbation. In blocked practice groups, we found that Block 12–13 difference in timing errors of the blocked–sham TMS group was low compared with the blocked–no TMS group (p = .02). But the difference between blocked–no TMS group and blocked–TMS group was not significant (p = .37).
FIGURE 5.
(A) Block 12–13 difference in time (ms) of the six experimental groups, a positive value indicates increasing decrement from Day 1 to Day 2. (B) Block 12–13 difference in amplitude (deg) of the six experimental groups. Error bars are standard errors of the mean. TMS = transcranial magnetic stimulation.
Amplitude Error (AE-SumAmp)
Visual inspection suggests that TMS and sham TMS did not alter the retention of movement amplitude for the blocked practice groups (see Figure 4A, Block 13), but it deteriorated that of the random practice groups (see Figure 4B, Block 13). This differential effect, however, was not associated with a significant Stimulation Condition × Practice Order interaction, F(2, 54) = 0.38, p = .69.
The CI effect was not replicated on the learning of movement amplitude. Although visual inspection suggests that accuracy in movement amplitude of the random–no TMS group was better than that of the blocked–no TMS group, this difference was not significant (p = .19; see Figure 4, Block 13).
TMS and sham TMS increased Block 12–13 difference in amplitude errors for blocked and random practice groups. The Stimulation Condition × Practice Order interaction was not significant, F(2, 54) = 0.20, p = .82 (see Figure 5B). For blocked practice groups, there was no significant stimulation condition effect for Block 12–13 difference in amplitude errors, F(2, 27) = 0.10, p = .91. For random practice groups, there was no significant stimulation condition effect, F(2, 27) = 0.84, p = .44. This pattern of results was consistent with the results of the amplitude errors during recall phase (see Figure 4, Block 13).
Because AE-SumAmp is a measure derived from errors of three reversal points, researchers have only been able to find a situation where TMS disrupted amplitude learning for one of the three reversal points (i.e., Peak 1, Trough, Peak 2). To account for this issue, we examined amplitude error at three reversal points during retention phase with one-way ANOVAs separately for blocked and random practice groups. The three levels of the one-way ANOVAs are the three stimulation conditions. For blocked practice groups, there was no significant stimulation condition effect on amplitude errors of any of the reversal points: For Peak 1, F(2, 27) = 0.07, p = .93; for Trough, F(2, 27) = 0.08, p = .92; and for Peak 2, F(2, 27) = 2.00, p = 0.16. For random practice groups, there was no significant stimulation condition effect at any of the reversal points: For Peak 1, F(2, 27) = 3.01, p = .07; for Trough, F(2, 27) = 0.68, p = .56; and for Peak 2, F(2, 27) = 1.23, p = .308.
Discussion
Lin et al. (2008) demonstrated that the cortical motor regions are neural substrates for the CI effect in motor-skill learning. A TMS perturbation centered around M1 during practice selectively disrupted motor learning under random, but not blocked, practice conditions. This pattern of results suggested a difference in cortical motor processing between random and blocked practice conditions. In the present article, we further investigated the high CI effect on cortical motor processing by determining the association between specific kinematic parameters of the arm tasks and the different cortical motor activity. Single-pulse TMS was applied as an identical external perturbation over the cortical motor system during blocked and random practice. Because of the repetitive nature for blocked practice, the need for parameter specification is less in a blocked practice condition (T. D. Lee & Magill, 1983; T. D. Lee et al., 1997; Shea & Morgan, 1979; Shea & Zimny, 1983). TMS perturbation during blocked practice was not expected to deter learning of kinematic parameters of the movements. Conversely, if the cortical motor regions were neural substrates for processing kinematic details of the practiced tasks, learning of temporal (timing) or spatial (amplitude) parameters would be deterred by TMS for participants who practice under a random practice condition in which the need for parameter specification is high. The main finding of the present study was that TMS perturbation over the cortical motor regions selectively deterred learning of movement timing under random, but not blocked, practice. This pattern of results was not observed in learning movement amplitude. The following discussion focuses on (a) the functional role of the cortical motor regions in learning movement kinematics, (b) how this role is associated with the CI effect, and (c) how our results suggest experience-dependent neural plasticity.
Neuroimaging studies suggest that the primary motor, premotor, and supplementary motor areas are functional substrates for processing temporal aspects of goal-directed movements (Macar, Coull, & Vidal, 2006; Martin et al., 2006; Thickbroom et al., 2000). There is also evidence that greater cortical motor activity is elicited under conditions of interference when compared with repetitive, consistent motor practice (Lewis et al., 2004; Meister et al., 2005). We, therefore, presumed that during random practice, the greater cortical activity necessary to process movement timing was the target of the TMS disruptive effect (see Figure 3). Previous studies suggested that motor memory formation is susceptible to interference by a focal lesion or competing tasks until the memory traces have been consolidated (Krakauer & Shadmehr, 2006; Muellbacher et al., 2002). In the no–TMS condition, enhanced motor learning, reflecting relatively permanent plasticity in relevant brain regions, was induced following random practice conditions. However, when TMS was used to perturb the processing and consolidation of that training experience, motor learning was reduced, likely reflecting an inability for relevant brain regions to develop an adequate memory trace to consolidate into permanent plastic changes. In the present experiment, TMS during the intertrial interval created a virtual lesion that ultimately interfered with the consolidation of the temporal parameter, resulting in poor recall performance.
The sham TMS condition provided supporting evidence for the TMS virtual lesion effect. There was no difference in recall performance between the random–sham TMS and the random–No TMS groups, and both were better than the random–TMS group. This suggests that the effect occurred because of direct cortical perturbation. In contrast and more importantly, recall performance in the blocked–TMS and blocked–sham TMS groups were similar (see Figure 3A), suggesting that the cortical perturbation during blocked practice did not alter the neural processing of timing. This dissociation pattern (i.e., TMS interference with random practice, but not blocked, practice) confirms the brain–behavior relation between the cortical motor regions and the CI effect. Results of Block 12–13 difference were similar to the recall performance. Magnetic stimulation selectively increased Block 12–13 difference in timing errors for those who practiced in a random condition.
A novel finding of the present study was that the significant disruption in temporal-parameter learning occurred when TMS was delivered immediately following the presentation of feedback. This finding provides neurophysiologic evidence of a critical time window around the intertrial interval for the encoding of kinematic parameters to occur. This appears to be essential for random, but not blocked, practice. As such, our results supported the hypothesis that processing associated with random practice, such as action planning and parameter specification, was operative during this critical intertrial interval (T. D. Lee & Magill, 1983; T. D. Lee et al., 1997; Shea & Titzer, 1993; Shea & Wright, 1991; Shea & Zimny, 1983).
Cortical perturbation did not significantly interfere with amplitude learning for the blocked- or random-order conditions (see Figure 4). One interpretation was that the cortical motor regions do not process spatial parameters, and consequently TMS-induced perturbation would not deter learning of movement amplitude. However, this interpretation is unlikely given that M1, PMd, PMv, and SMA have been associated with planning, adjusting, and encoding task-specific spatial information (Chouinard & Paus, 2006; Coxon, Stinear, & Byblow, 2006; Crowe, Chafee, Averbeck, & Georgopolous, 2004; Diedrichsen, Hashambhoy, Rane, & Shadmehr, 2005; J. H. Lee & van Donkelaar, 2006; Liu, Denton, & Nelson, 2005; Monfils, Plautz, & Kleim, 2005; Naselaris, Merchant, Amirikian, & Georgopoulos, 2006). We speculated that the results associated with the movement amplitude may be due to the strategy that the participants used to improve task performance. Specifically, in this and previous studies conducted in our laboratory, the best strategy for participants to reduce error in the lever-arm task is to improve timing (Lin et al., 2008). It is conceivable that cortical motor regions are less tuned to processing movement amplitude because the behavioral requirement for this task seems to optimize movement timing. This interpretation is also supported by little improvement in amplitude errors observed during the practice phase and the lack of the CI effect in learning movement amplitude (see Figure 4). Further, the amplitude error greatly increased (almost returned to the level before practice) in the recall phase after blocked practice, regardless of whether the participant was stimulated (see Figure 4A). This may indicate that amplitude information cannot be remembered after blocked practice. In contrast, amplitude error increased after random practice only when the participant was stimulated (see Figure 4B). Though the group differences were not significant, this pattern of results suggested a likelihood that learning of movement amplitude could be improved by random practice and that this effect is deterred by TMS and sham stimulation.
Another important finding of the present study was that the traditional control condition for magnetic stimulation, the sham–TMS condition, is not necessarily neutral and showed potential influence to kinematics learning. The sham–TMS condition appeared to enhance performance in timing during early practice phase for the blocked practice groups (see Figure 3), but it deterred amplitude learning for the random practice groups (see Figure 4). Because there was no cortical effect from sham TMS, we interpreted that sham–TMS during practice may have necessitated a reallocation of attentional resources. This increase of attentional demands may lead to increased challenge during practice (Guadagnoli & Lee, 2004), resulting in enhanced motor learning for those who practiced in a blocked-order condition. Indeed, studies that have used interpolated dual tasks as external interference in a blocked practice condition have demonstrated enhanced motor learning (Shea & Wright, 1991). In contrast, increasing attentional demands may exceed the challenge point for those who practice in an originally challenging condition such as a random-order condition, resulting in decrement in learning.
Neural plasticity is the mechanism by which the brain encodes experience and learns new behaviors (Krakauer & Shadmehr, 2006). As stated previously, the best strategy for participants to reduce error in the lever-arm task is to improve timing and the TMS perturbation for timing but not to improve amplitude learning. These two results together provide support for a fundamental principle of the CNS: Plasticity is experience-dependent (Kleim & Jones, in press). This principle suggests that the activity of cortical brain regions associated with motor-skill learning depends on the current needs and experiences of the participant. In the present study, the current need of the lever-arm task was timing parameterization, whereas the experience of the participant was the condition of practice (i.e., blocked or random). Our experimental design with TMS allowed us to monitor the neural implementation of the motor-training experience. This, in turn, informed us about the contribution of the cortical motor regions in experience-dependent learning and plasticity.
One potential confound in the present study was that the three template trajectories differed in amplitude but not in movement time. Researchers could argue that participants primarily learn movement timing simply because the repetition of the movement time occurs more frequently than that of movement amplitude. To rule out this potential confound, we performed a post hoc analysis in which the data was grouped by each template trajectory. The participants showed no apparent transfer of movement time from one template to the next. Despite having reduced movement time errors from approximately 300 to less than 100 ms by the end of practice with one template, movement time errors for a different template jumped back to the 300 ms range, suggesting that the participants were not aware that the new template had the same duration as the previous one. Therefore, the findings of our study were not confounded by the more frequent repetition of movement time compared with amplitude across three template trajectories.
Unlike functional imaging studies that directly measured cortical activity using tools such as functional magnetic resonance (fMRI), in the present study, there was no direct evidence to show cortical activity. We can only conclude our findings by inferring the results from our data and previous studies. Cross, Schmitt, and Grafton (2007) used fMRI to demonstrate greater cortical motor activity while participants practiced motor sequences with a random compared with a blocked order. In addition, evidence has suggested that greater cortical activity is evoked while participants train in a nonrepetitive, random-like condition, compared with a repetitive, blocked-like condition (Gorbet et al., 2004). Though cortical motor activity was not directly measured in the present study, previous evidences suggest the likelihood that greater cortical activity is evoked in the random-order condition.
In summary, we have identified a brain–behavior relation between the CI effect and the cortical motor regions in learning movement kinematics. Our data provides further support that the cortical motor regions are putative substrates for processing and consolidating task-relevant kinematic information. This pilot project has localized the cortical motor regions as crucial substrates for the CI effect in learning movement parameters. Future studies include (a) the use of an alternative task that biases amplitude specification to further probe experience-dependent plasticity and (b) the application of a conventional focal figure-eight coil to probe the contribution of specific motor area, such as M1, PMd, or SMA to motor-skill learning.
Acknowledgments
This study was supported in part by the California Physical Therapy Association (CW, CL), a student research award (CL) from the North American Society for the Psychology of Sport and Physical Activity, and NINDS K23-NS045764 (AW). The authors thank Dr. James Gordon for comments regarding the manuscript.
REFERENCES
- Aoki T., Tsuda H., Takasawa M., Osaki Y., Oku N., Hatazawa J., et al. The effect of tapping finger and mode differences on cortical and subcortical activities: A PET study. Experimental Brain Research. 2005;160:375–383. doi: 10.1007/s00221-004-2008-9. [DOI] [PubMed] [Google Scholar]
- Battig W. F. Transfer and verbal pretaining to motor performance as a function of motor task complexity. Journal of Experimental Psychology. 1956;51:371–378. doi: 10.1037/h0039961. [DOI] [PubMed] [Google Scholar]
- Battig W. F. Facilitation and interference. In: Bilodeau E. A., editor. Acquisition of skill. New York: Academic Press; 1966. pp. 215–240. [Google Scholar]
- Battig W. F. The flexibility of human memory. In: Cermak L. S., Craik F. I. M., editors. Level of processing in human memory. Hillsdale, NJ: Erlbaum; 1979. pp. 23–44. [Google Scholar]
- Berardelli A., Inghilleri M., Polidori L., Priori A., Mercuri B., Manfredi M. Effects of transcranial magnetic stimulation on single and sequential arm movements. Experimental Brain Research. 1994;98:501–506. doi: 10.1007/BF00233987. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B., Phipps M., Kopylev L., Wharton C. M., Cohen L. G., Grafman J. Enhancing analogic reasoning with rTMS over the left prefrontal cortex. Neurology. 2001;56:526–528. doi: 10.1212/wnl.56.4.526. [DOI] [PubMed] [Google Scholar]
- Butefisch C. M., Khurana V., Kopylev L., Cohen L. G. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. Journal of Neurophysiology. 2004;91:2110–2116. doi: 10.1152/jn.01038.2003. [DOI] [PubMed] [Google Scholar]
- Cahill L., McGaugh J. L., Weinberger N. M. The neurobiology of learning and memory: Some reminders to remember. Trends in Neuroscience. 2001;24:578–581. doi: 10.1016/s0166-2236(00)01885-3. [DOI] [PubMed] [Google Scholar]
- Chouinard P. A., Paus T. The primary motor and premotor areas of the human cerebral cortex. Neuroscientist. 2006;12:143–152. doi: 10.1177/1073858405284255. [DOI] [PubMed] [Google Scholar]
- Chung S. C., Min B. C., Kim C. J., Cho Z. H. Total activation change of visual and motor area due to various disturbances. Journal of Physiological Anthropology and Applied Human Science. 2000;19(2):93–100. doi: 10.2114/jpa.19.93. [DOI] [PubMed] [Google Scholar]
- Coxon J. P., Stinear C. M., Byblow W. D. Intracortical inhibition during volitional inhibition of prepared action. Journal of Neurophysiology. 2006;95:3371–3383. doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- Cross E. S., Schmitt P. J., Grafton S. T. Neural substrates of contextual interference during motor learning support a model of active preparation. Journal of Cognitive Neuroscience. 2007;19:1854–1871. doi: 10.1162/jocn.2007.19.11.1854. [DOI] [PubMed] [Google Scholar]
- Crowe D. A., Chafee M. V., Averbeck B. B., Georgopoulos A. P. Participation of primary motor cortical neurons in a distributed network during maze solution: Representation of spatial parameters and time-course comparison with parietal area 7a. Experimental Brain Research. 2004;158:28–34. doi: 10.1007/s00221-004-1876-3. [DOI] [PubMed] [Google Scholar]
- Demiralp T., Karamürsel S., Karakullukçu Y. E., Gökhan N. Movement-related cortical potentials: Their relationship to the laterality, complexity and learning of a movement. International Journal of Neuroscience. 1990;51:153–162. doi: 10.3109/00207459009000518. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J., Hashambhoy Y., Rane T., Shadmehr R. Neural correlates of reach errors. Journal of Neuroscience. 2005;25:9919–9931. doi: 10.1523/JNEUROSCI.1874-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitman S. S., Grafman J., Wassermann E. M., Cooper V., O'Grady J., Pascual-Leone A., et al. Linguistic processing during repetitive transcranial magnetic stimulation. Neurology. 1998;50:175–181. doi: 10.1212/wnl.50.1.175. [DOI] [PubMed] [Google Scholar]
- Gabriele T. E., Hall G. R., Buckolz E. E. Practice schedule effects on the acquisition and retention of motor skill. Human Movement Science. 1987;6:1–16. [Google Scholar]
- Giuffrida C. G., Shea J. B., Fairbrother J. T. Differential transfer benefits of increased practice for constant, blocked, and serial practice schedules. Journal of Motor Behavior. 2002;34:353–365. doi: 10.1080/00222890209601953. [DOI] [PubMed] [Google Scholar]
- Goodwin J. E., Meeuwsen H. J. Investigation of the contextual interference effect in the manipulation of the motor parameter of over all force. Perceptual Motor Skills. 1996;83:735–743. doi: 10.2466/pms.1996.83.3.735. [DOI] [PubMed] [Google Scholar]
- Gorbet D. J., Staines W. R., Sergio L. E. Brain mechanisms for preparing increasingly complex sensory to motor transformations. Neuroimage. 2004;23:1100–1111. doi: 10.1016/j.neuroimage.2004.07.043. [DOI] [PubMed] [Google Scholar]
- Guadagnoli M. A., Lee T. D. Challenge point: A framework for conceptualizing the effects of various practice conditions in motor learning. Journal of Motor Behavior. 2004;36:212–224. doi: 10.3200/JMBR.36.2.212-224. [DOI] [PubMed] [Google Scholar]
- Hall K. G., Magill R. A. Variability of practice and contextual interference in motor skill learning. Journal of Motor Behavior. 1995;27:299–309. doi: 10.1080/00222895.1995.9941719. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000, July 13;406:147–150. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- Hatfield B. D., Haufler A. J., Hung T. M., Spalding T. W. Electroencephalographic studies of skilled psychomotor performance. Journal of Clinical Neurophysiology. 2004;21:144–156. doi: 10.1097/00004691-200405000-00003. [DOI] [PubMed] [Google Scholar]
- Kamen G. Reliability of motor-evoked potentials during resting and active contraction conditions. Medicine and Science in Sports and Exercise. 2004;36:1574–1579. doi: 10.1249/01.mss.0000139804.02576.6a. [DOI] [PubMed] [Google Scholar]
- Kaneko K., Kawai S., Fuchigami Y., Shiraishi G., Ito T. Effect of stimulus intensity and voluntary contraction on corticospinal potentials following transcranial magnetic stimulation. Journal of Neurological Science. 1996;139:131–136. [PubMed] [Google Scholar]
- Kleim J. A., Jones T. A. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. Journal of Speech Hearing and Language Research. doi: 10.1044/1092-4388(2008/018). in press. [DOI] [PubMed] [Google Scholar]
- Krakauer J. W., Mazzoni P., Ghazizadeh A., Ravindran R., Shadmehr R. Generalization of motor learning depends on the history of prior action. PLoS Biology. 2006;4(10):e316. doi: 10.1371/journal.pbio.0040316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer J. W., Shadmehr R. Consolidation of motor memory. Trends in Neuroscience. 2006;29(1):58–64. doi: 10.1016/j.tins.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., van Donkelaar P. The human dorsal premotor cortex generates online error corrections during sensorimotor adaptation. Journal of Neuroscience. 2006;26:3330–3334. doi: 10.1523/JNEUROSCI.3898-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. D., Magill R. A. The locus of contextual interference in motor-skill acquisition. Journal of Experimental Psychology: Human Learning and Memory. 1983;9:730–746. [Google Scholar]
- Lee T. D., Magill R. A. Can forgetting facilitate skill acquisition? In: Goodman D., Wilberg R. B., Franks I. M., editors. Differing perspectives in motor learning, memory, and control. Amsterdam: Elsevier Science; 1985. pp. 3–21. [Google Scholar]
- Lee T. D., Wishart L. R., Cunningham S., Carnahan H. Modeled timing information during random practice eliminates the contextual interference effect. Research Quarterly in Exercise and Sports. 1997;68:100–105. doi: 10.1080/02701367.1997.10608871. [DOI] [PubMed] [Google Scholar]
- Lewis P. A., Wing A. M., Pope P. A., Praamstra P., Miall R. C. Brain activity correlates differentially with increasing temporal complexity of rhythms during initialisation, synchronisation, and continuation phases of paced finger tapping. Neuropsychologia. 2004;42:1301–1312. doi: 10.1016/j.neuropsychologia.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Limons E., Shea J. B. Deficient processing in learning and performance. In: Colley A. M., Beech J. R., editors. Cognition and action in skilled behavior. Amsterdam: Elsevier Science; 1988. pp. 333–347. [Google Scholar]
- Lin C., Fisher B. E., Winstein C. J., Wu A. D., Gordon J. Contextual interference effect: Elaborative-processing or forgetting-reconstruction? A post hoc analysis of TMS-induced effects on motor learning. Journal of Motor Behavior. 2008;40:578–586. doi: 10.3200/JMBR.40.6.578-586. [DOI] [PubMed] [Google Scholar]
- Liu Y., Denton J. M., Nelson R. J. Neuronal activity in primary motor cortex differs when monkeys perform somato-sensory and visually guided wrist movements. Experimental Brain Research. 2005;167:571–586. doi: 10.1007/s00221-005-0052-8. [DOI] [PubMed] [Google Scholar]
- Macar F., Coull J., Vidal F. The supplementary motor area in motor and perceptual time processing: fMRI studies. Cognitive Process. 2006;7(2):89–94. doi: 10.1007/s10339-005-0025-7. [DOI] [PubMed] [Google Scholar]
- Martin T., Houck J. M., Bish J. P., Kicic D., Woodruff C. C., Moses S. N., et al. MEG reveals different contributions of somatomotor cortex and cerebellum to simple reaction time after temporally structured cues. Human Brain Mapping. 2006;27:552–561. doi: 10.1002/hbm.20200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meira C. M., Jr., Tani G. The contextual interference effect in acquisition of dart-throwing skill tested on a transfer test with extended trials. Perceptual Motor Skills. 2001;92:910–918. doi: 10.2466/pms.2001.92.3.910. [DOI] [PubMed] [Google Scholar]
- Meister I., Krings T., Foltys H., Boroojerdi B., Muller M., Topper R., et al. Effects of long-term practice and task complexity in musicians and nonmusicians performing simple and complex motor tasks: Implications for cortical motor organization. Human Brain Mapping. 2005;25:345–352. doi: 10.1002/hbm.20112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils M. H., Plautz E. J., Kleim J. A. In search of the motor engram: Motor map plasticity as a mechanism for encoding motor experience. Neuroscientist. 2005;11:471–483. doi: 10.1177/1073858405278015. [DOI] [PubMed] [Google Scholar]
- Muellbacher W., Ziemann U., Wissel J., Dang N., Kofler M., Facchini S., et al. Early consolidation in human primary motor cortex. Nature. 2002 Feb 7;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Naselaris T., Merchant H., Amirikian B., Georgopoulos A. P. Large-scale organization of preferred directions in the motor cortex. I. Motor cortical hyperacuity for forward reaching. Journal of Neurophysiology. 2006;96:3231–3236. doi: 10.1152/jn.00487.2006. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A., Houser C. M., Grafman J., Hallett M. Reaction time and transcranial magnetic stimulation. Lancet. 1992;339:1420. doi: 10.1016/0140-6736(92)91245-4. [DOI] [PubMed] [Google Scholar]
- Perez C. R., Meira C. M., Jr., Tani G. Does the contextual interference effect last over extended transfer trials? Perceptual Motor Skills. 2005;100(1):58–60. doi: 10.2466/pms.100.1.58-60. [DOI] [PubMed] [Google Scholar]
- Rossini P. M., Martino G., Narici L., Pasquarelli A., Peresson M., Pizzella V., et al. Short-term brain 'plasticity' in humans: Transient finger representation changes in sensory cortex somatotopy following ischemic anesthesia. Brain Research. 1994;642:169–177. doi: 10.1016/0006-8993(94)90919-9. [DOI] [PubMed] [Google Scholar]
- Sainburg R. L. Evidence for a dynamic-dominance hypothesis of handedness. Experimental Brain Research. 2002;142:241–258. doi: 10.1007/s00221-001-0913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. A., Lee T. D. Conditions of practice. In: Schmidt R. A., Lee T. D., editors. Motor control and learning: A behavior emphasis. Vol. 11. Champaign, IL: Human Kinetics; 2005. pp. 321–363. [Google Scholar]
- Schubotz R. I., von Cramon D. Y. A blueprint for target motion: fMRI reveals perceived sequential complexity to modulate premotor cortex. Neuroimage. 2002;16:920–935. doi: 10.1006/nimg.2002.1183. [DOI] [PubMed] [Google Scholar]
- Seidler R. D., Noll D. C., Thiers G. Feedforward and feedback processes in motor control. Neuroimage. 2004;22:1775–1783. doi: 10.1016/j.neuroimage.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Sergio L. E., Kalaska J. F. Changes in the temporal pattern of primary motor cortex activity in a directional isometric force versus limb movement task. Journal of Neurophysiology. 1998;80:1577–1583. doi: 10.1152/jn.1998.80.3.1577. [DOI] [PubMed] [Google Scholar]
- Shea J. B., Morgan R. Contextual interference effects on the acquisition, retention, and transfer of a motor skill. Journal of Experimental Psychology: Human Learning and Memory. 1979;5:179–187. [Google Scholar]
- Shea J. B., Titzer R. C. The influence of reminder trials on contextual interference effects. Journal of Motor Behavior. 1993;25:264–274. doi: 10.1080/00222895.1993.9941647. [DOI] [PubMed] [Google Scholar]
- Shea J. B., Wright D. L. When forgetting benefits motor retention. Research Quarterly in Exercise and Sports. 1991;62:293–301. doi: 10.1080/02701367.1991.10608726. [DOI] [PubMed] [Google Scholar]
- Shea J. B., Zimny S. T. Contextual effects in memory and learning movement information. In: Magill R. A., editor. Memory and control of action. Amsterdam: Elsevier; 1983. pp. 345–366. [Google Scholar]
- Taylor J. L., Wagener D. S., Colebatch J. G. Mapping of cortical sites where transcranial magnetic stimulation results in delay of voluntary movement. Electroencephalography Clinical Neurophysiology. 1995;97:341–348. doi: 10.1016/0924-980x(95)00123-3. [DOI] [PubMed] [Google Scholar]
- Thickbroom G. W., Byrnes M. L., Sacco P., Ghosh S., Morris I. T., Mastaglia F. L. The role of the supplementary motor area in externally timed movement: The influence of predictability of movement timing. Brain Research. 2000;874:233–241. doi: 10.1016/s0006-8993(00)02588-9. [DOI] [PubMed] [Google Scholar]
- Wassermann E. M. Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the international workshop on the safety of repetitive transcranial magnetic stimulation, June 5–7, 1996. Electroencephalography and Clinical Neurophysiology. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Weekley A. TEMPLATE. Garden Grove, CA: Weekley Consulting; 2004. [Google Scholar]
- Werhahn K. J., Fong J. K., Meyer B. U., Priori A., Rothwell J. C., Day B. L., et al. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroen-cephalography and Clinical Neurophysiology. 1994;93:138–146. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Wright D. L., Li Y., Coady W. Cognitive processes related to contextual interference and observational learning: A replication of Blandin, Proteau, and Alain (1994) Research Quarterly in Exercise and Sports. 1997;68:106–109. doi: 10.1080/02701367.1997.10608872. [DOI] [PubMed] [Google Scholar]
- Wright D. L., Li Y., Whitacre C. The contribution of elaborative processing to the contextual interference effect. Research Quarterly in Exercise and Sports. 1992;63:30–37. doi: 10.1080/02701367.1992.10607554. [DOI] [PubMed] [Google Scholar]
- Wright D. L., Magnuson C. E., Black C. B. Programming and reprogramming sequence timing following high and low contextual interference practice. Research Quarterly in Exercise and Sports. 2005;76:258–266. doi: 10.1080/02701367.2005.10599297. [DOI] [PubMed] [Google Scholar]
- Wu A. D. Los Angeles, CA: DataWizard Technologies; 2004. DataWizard [Computer software] [Google Scholar]
- Zhang J., Riehle A., Requin J. Analyzing neuronal processing locus in stimulus-response association tasks. Journal of Mathematical Psychology. 1997;41:219–236. doi: 10.1006/jmps.1997.1168. [DOI] [PubMed] [Google Scholar]
- Ziemann U. TMS-induced plasticity in human cortex. Reviews in the Neurosciences. 2004;15:253–266. doi: 10.1515/revneuro.2004.15.4.253. [DOI] [PubMed] [Google Scholar]
- Ziemann U., Ilic T. V., Pauli C., Meintzschel F., Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. Journal of Neuroscience. 2004;24:1666–1672. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]