Abstract
Clear cell basal cell carcinoma (BCC) is a variant of BCC with a characteristic clear cell component that may occupy all or part of the tumor islands. Periodic acid-Schiff (PAS) staining for glycogen is variably positive, and mild deposition of sulfated mucin has been noted. However, to our knowledge, clear cell BCC with sialomucin deposition has not been reported. Here we report a case of clear cell BCC showing sialomucin deposition. The clear tumor cells stained with PAS and showed incomplete diastase-resistance. In addition, mucin staining with alcian blue was positive at pH 2.5 but not at pH 0.5.
Keywords: Basal cell carcinoma, sialomucins
INTRODUCTION
Basal cell carcinomas (BCCs) have several histological variants which may be due, in part, to the pluripotential capacity of the primary epithelial germ to differentiate in different directions, and to the different responses of the stroma to these tumors.1 Clear cell BCC is a variant of BCC with a characteristic clear cell component that may occupy all or part of the tumor islands; however, the basis for this unusual histological variant has not been elucidated.2 The affected cells are round to polyhedral in shape, with pale, eosinophilic, vacuolated, or finely granular cytoplasm.3 Periodic acid-Schiff (PAS) staining for glycogen is variably positive, and mild deposition of sulfated mucin has been noted.2,3 However, to our knowledge, clear cell BCC with sialomucin deposition has not been reported. Here, we report a case of clear cell BCC showing sialomucin deposition.
CASE REPORT
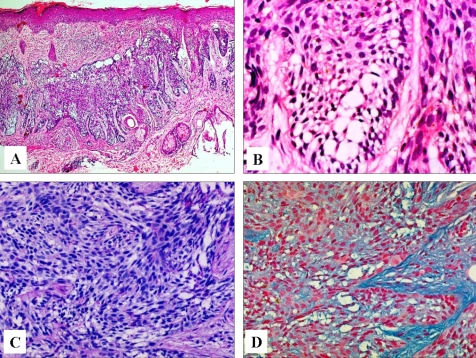
An 83-year-old Korean woman presented to our clinic with a slowly enlarging cutaneous lesion in the right infraorbital area that was first noticed three years earlier. The lesion was depressed with crusts and marginal infiltration and was about 2.0 cm in diameter (Fig. 1). A punch biopsy of the tumor was performed, and the pathologic examination showed that the tumor lobules were composed of irregularly arranged, atypical-appearing basaloid cells with portions of peripheral palisading. Vacuolated cells were distributed across a fairly large area within the lobules (Fig. 2A). The vacuoles varied in size and number, and often occupied the entire cytoplasm (Fig. 2B). The nuclei of the vacuolated cells were deformed and displaced to one side of the cells. Neither mitotic activity nor necrosis was observed. The clear tumor cells were stained with PAS, and showed incomplete diastase-resistance (Fig. 2C). Mucin staining with alcian blue of the tumor cells was positive at pH 2.5 but not at pH 0.5 (Fig. 2D). The stroma surrounding the tumor lobules stained with alcian blue at pH 0.5 and it stained more deeply than the tumor cells at pH 2.5. Immunohistochemically, the tumor cells were negative for both epithelial membrane antigen (EMA) and carcinoembryonic antigen (CEA). A pathologic diagnosis of clear cell BCC with sialomucin deposition was established, and the tumor was removed by Mohs micrographic surgery.
Fig. 1.
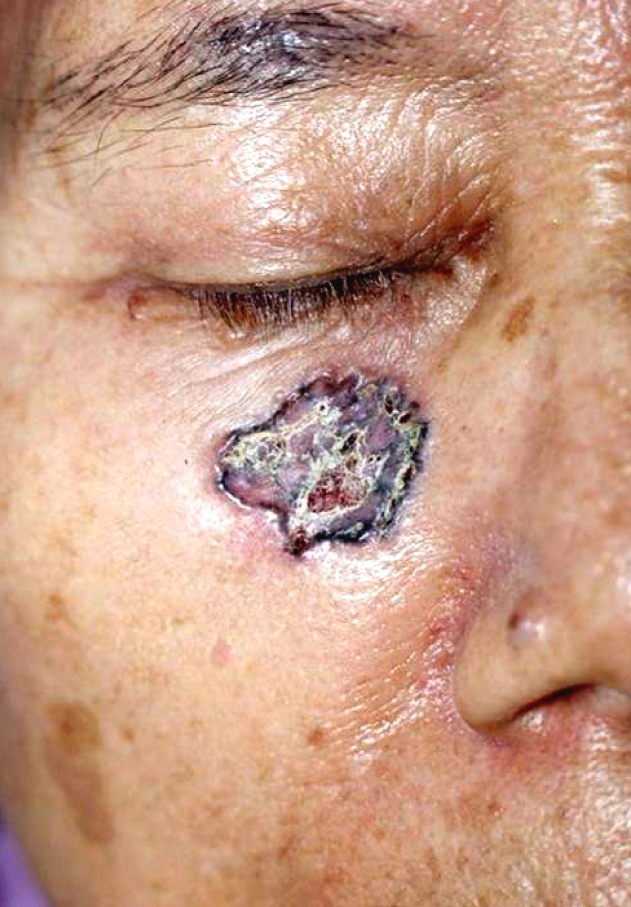
A solitary coin sized, irregularly pigmented, and depressed erosion with marginal elevation in the right infraorbital area.
Fig. 2.
Irregularly arranged basaloid cells and clear vacuolated cells with peripheral palisading of basaloid cells within infiltrating tumor lobules (hematoxylin and eosin, A, × 40; B, × 400). C. Tumor cells sparsely stained with PAS, and incomplete diastase-resistance (d-PAS, × 200). D. Tumor cells were stained with alcian blue at pH 2.5 (Alcian blue, × 200).
DISCUSSION
BCC is the most common type of skin cancer, and it is now widely accepted that BCC occurring later in life arises from pluripotential cells that form continuously during life. Like embryonic primary epithelial germ cells, pluripotential cells have the potential to form hair, sebaceous glands, and apocrine glands.4 This is why BCC shows a variety of histological variants and its microscopic diagnosis is not always obvious. In 1984, Barr and Williamson reported an unusual variant of BCC, which they named clear cell BCC.2 Because some clear cells contained glycogen, they suggested that the pathogenesis of clear cell BCC is mainly due to trichilemmal differentiation of the BCC. However, many investigators considered the large membrane-bound vacuoles, which occupied the cytoplasm on electron microscopy, as phagolysomes or end stage products of intracellular organelles. Clear cells are now generally thought to be the result of degeneration, and likely involve lysosomes or the mitochondria.3,5-7
Clear cell BCCs have shown variability in conventional histochemical findings. Most of the cases have shown deposition of glycogen, which was positive for PAS with a diastase-labile nature, and a few cases have also demonstrated the concomitant deposition of sulfated mucopolysaccharide.5-8 However, to our knowledge, sialomucin deposition in clear cell BCC has not yet been reported. According to histochemical findings, we considered our case as an unusual histological variant showing sialomucin deposition. Sialomucin contains nonsulfated acid mucopolysaccharides as well as PAS-positive neutral polysaccharides. Whereas nonsulfated acid mucopolysaccharides stain with alcian blue at pH 2.5 but not at pH 0.5, sulfated-acid mucopolysaccharides stain with alcian blue both at pH 2.5 and 0.5. Sialomucin has been observed in the dark cells in the eccrine glands, secretory cells of the apocrine glands, oral mucosal cyst and its lining cells, metastatic carcinoma of gastrointestinal cancer, some cases of Paget's disease, and almost all cases of extramammary Paget's disease (EMPD). Generally, EMPD is currently considered to arise as an intraepithelial neoplasm, and the origin of the Paget cell is thought to be from either intraepidermal cells of the apocrine gland ducts or from pluripotent keratinocyte stem cells.9 Based on the presence of sialomucin in sweat glands and the similar pathogenetic features of EMPD and the clear cell BCC in our case, we hypothesize that the sialomucin in clear cell BCC might be obtained as one of the products of tumor glandular differentiation, rather than from degenerated components of tumor cells. However, negative results with EMA and CEA immmunostaining in our case do not support glandular differentiation. Thus, further studies that include electron microscopy and involve a larger number of cases should be conducted to confirm our hypothesis.
Because the tumor cells of BCCs can differentiate toward the components of sweat gland structures, and EMPD originates from sweat gland structures in the epidermis, it was necessary to differentiate our case from BCC with sweat gland differentiation. BCC with sweat gland differentiation is usually classified as adenoid-type BCC. Adenoid-type BCC shows formations suggesting tubular or gland-like structures and cells are arranged in intertwining strands with a lace-like pattern.1 Immunohistochemical stainings for apocrine or eccrine differentiation are mostly negative in adenoid-type BCC, because it shows only low degree of differentiation.10 In addition, adenoid-type BCC generally produces sulfated mucopolysaccharide, but not sialomucin, from the tumor cells showing tubular or glandular structure. In our case, clear cell BCC did not show distinct histological findings suggestive of sweat gland differentiation and the tumor cells contained sialomucin, not sulfated mucopolysaccharide. Therefore, no pathogenetic similarities between our case and adenoid-type BCC were found.
BCC with sebaceous differentiation shows interspersed aggregates of sebaceous cells as well as basaloma cells. The sebaceous cells appeared to be similar to the clear cells in our case, but this entity could be excluded by the sialomucin deposition and negative staining of clear cells with EMA.11
In conclusion, we report a case of an unusual histological variant of clear cell BCC showing sialomucin deposition. However, the pathogenesis of the sialomucin deposition has not yet been elucidated.
References
- 1.Rippey JJ. Why classify basal cell carcinomas? Histopathology. 1998;32:393–398. doi: 10.1046/j.1365-2559.1998.00431.x. [DOI] [PubMed] [Google Scholar]
- 2.Barr RJ, Williamson C. Clear-cell basal cell carcinoma. Arch Dermatol. 1984;120:1086. [Google Scholar]
- 3.Starink TM, Blomjous CE, Stoof TJ, Van Der Linden JC. Clear cell basal cell carcinoma. Histopathology. 1990;17:401–405. doi: 10.1111/j.1365-2559.1990.tb00759.x. [DOI] [PubMed] [Google Scholar]
- 4.Pinkus H. Premalignant fibroepithelial tumors of skin. AMA Arch Derm Syphilol. 1953;67:598–615. doi: 10.1001/archderm.1953.01540060060009. [DOI] [PubMed] [Google Scholar]
- 5.Barnadas MA, Freeman RG. Clear cell basal cell epithelioma: light and electron microscopic study of an unusual variant. J Cutan Pathol. 1988;15:1–7. doi: 10.1111/j.1600-0560.1988.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 6.Oliver GF, Winkelmann RK. Clear-cell basal cell carcinoma: histopathological, histochemical, and electron microscopic findings. J Cutan Pathol. 1988;15:404–408. doi: 10.1111/j.1600-0560.1988.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 7.Barr RJ, Alpern KS, Santa Cruz DJ, Fretzin DF. Clear cell basal cell carcinoma: an usual degenerative variant. J Cutan Pathol. 1993;20:308–316. doi: 10.1111/j.1600-0560.1993.tb01267.x. [DOI] [PubMed] [Google Scholar]
- 8.Morioka D, Kinoshita Y, Tanabe T, Ueda Y. Clear-cell basal cell carcinoma of the upper eyelid: a case report. Ann Plast Surg. 1999;43:215–217. [PubMed] [Google Scholar]
- 9.Lloyd J, Flanagan AM. Mammary and extramammary Paget's disease. J Clin Pathol. 2000;53:742–749. doi: 10.1136/jcp.53.10.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood MG, Pranich K, Beerman H. Investigation of possible apocrine gland component in basal-cell epithelioma. J Invest Dermatol. 1958;30:273–279. [PubMed] [Google Scholar]
- 11.Misago N, Suse T, Uemura T, Narisawa Y. Basal cell carcinoma with sebaceous differentiation. Am J Dermatopathol. 2004;26:298–303. doi: 10.1097/00000372-200408000-00006. [DOI] [PubMed] [Google Scholar]