Summary
Background
Amputations in people with type 2 diabetes mellitus substantially impair their quality of life and impose high costs on health-care systems. Our aim was to assess the effect of fenofibrate on amputation events in a large cohort of patients with type 2 diabetes.
Methods
In the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study, 9795 patients aged 50–75 years with type 2 diabetes were randomly assigned by computer-generated randomisation sequence to receive fenofibrate 200 mg per day (n=4895) or matching placebo (n=4900) for 5 years' duration. Information about non-traumatic amputation—a prespecified tertiary endpoint of the study—was routinely gathered. Clinicians who were masked to treatment allocation adjudicated amputations as minor or major (below or above the ankle, respectively). Amputations were also classified on the basis of whether or not large-vessel disease was present in the limb, to distinguish those related to large-artery atherosclerosis from those predominantly related to microvascular disease. Analysis was by intention to treat (ITT). The FIELD study is registered as an International Standard Randomised Controlled Trial, number ISRCTN64783481.
Findings
All 9795 patients were included in the ITT population. 115 patients had one or more non-traumatic lower-limb amputations due to diabetes. Previous cardiovascular disease, microvascular disease, previous non-traumatic amputation or skin ulcer, smoking, and longer duration of diabetes were more frequent in patients who had amputations during the trial than in those who had other cardiovascular events or in those who had neither event (all p<0·001 for three-way comparison). Mean lipid concentrations differed between patients who had on-study amputations and those who had other cardiovascular events or neither event, but by no more than 0·2 mmol/L. The risks of first amputation (45 vs 70 events; hazard ratio [HR] 0·64, 95% CI 0·44–0·94; p=0·02) and minor amputation events without known large-vessel disease (18 vs 34 events; 0·53, 0·30–0·94; p=0·027) were lower for patients assigned to fenofibrate than for patients assigned to placebo, with no difference between groups in risk of major amputations (24 vs 26 events; 0·93, 0·53–1·62; p=0·79).
Interpretation
Classic markers of macrovascular and microvascular risk were associated with lower extremity amputations in patients with type 2 diabetes. Treatment with fenofibrate was associated with a lower risk of amputations, particularly minor amputations without known large-vessel disease, probably through non-lipid mechanisms. These findings could lead to a change in standard treatment for the prevention of diabetes-related lower-limb amputations.
Funding
Laboratoires Fournier SA (now part of Solvay Pharmaceuticals) and National Health and Medical Research Council of Australia.
Introduction
Diabetes mellitus is the leading cause of non-traumatic lower-extremity amputations in the developed world.1 In the USA in 2001, at least one amputation due to diabetes occurred every 2 h, with an annual cost exceeding US$1·6 billion.2,3 Despite rigorous management of reversible factors, probably around one in ten patients with diabetes will eventually need at least one amputation. Neither control of glycaemia or blood pressure nor lowering of cholesterol has prevented the risk of amputation, underscoring the importance of assessing the management of other potential risk factors. Any further therapeutic option to prevent the morbidity and mortality associated with amputation would be highly desirable.
The aim of the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study was to assess whether long-term lipid-lowering treatment with fenofibrate could reduce adverse macrovascular and microvascular outcomes in patients with type 2 diabetes, including amputations.4 Previously, we found that fenofibrate had a favourable effect on microvascular disease in terms of the need for laser therapy for diabetic retinopathy,5 beyond what could be expected from a moderate observed reduction in blood pressure. The effect of fenofibrate treatment was independent of haemoglobin A1c (HbA1c) and concomitant medications, and unlikely to be related to the drug's lipid-lowering effects.6 The study reported here analysed the effect of fenofibrate on lower-limb amputation events, and investigated the differential effects of this treatment on major and minor amputations with and without associated large-vessel disease.
Methods
Patients
The design and main results of the FIELD study, including safety profile, have been published elsewhere.4,5 Briefly, patients aged 50–75 years were eligible for inclusion if they had a diagnosis of type 2 diabetes according to WHO criteria, an initial plasma total cholesterol concentration between 3·0 mmol/L and 6·5 mmol/L plus a total cholesterol/HDL-cholesterol ratio of 4·0 or more, or a plasma triglyceride concentration between 1·0 mmol/L and 5·0 mmol/L, without needing lipid-modifying treatment at study entry. Individuals with renal impairment, chronic liver disease, symptomatic gallbladder disease, or those who had experienced a cardiovascular event within the 3 months before recruitment were excluded.
All patients provided written informed consent, and the study protocol was approved by local and national ethics committees in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Randomisation and masking
A central telephone computer randomisation service that used dynamic balancing7 to stratify patients according to important prognostic variables was used to randomly assign patients to intervention or control. All investigators and staff, except the authorised study statistician, were masked to treatment allocation both before and after randomisation. The success of masking was not formally assessed.
Procedures
9795 patients were enrolled and randomly assigned to receive once-daily micronised fenofibrate 200 mg (Laboratoires Fournier, Dijon, France; n=4895) or matching placebo (n=4900). Patients were followed up at 4–6-month intervals for a median follow-up of 5 years, and all study outcomes and serious adverse events were recorded. Non-traumatic amputation was a prespecified tertiary endpoint. All non-traumatic amputations that occurred during study follow-up (on-study amputations) were reviewed separately by two clinicians who were masked to treatment allocation (KR, LPL), and any discrepancies were resolved by mutual agreement. All available baseline and on-study lower-limb angiograms and duplex ultrasounds of patients who had an amputation were reviewed for this analysis, but vascular status was not routinely measured at baseline in this study, or obtained thereafter for those who did not have an amputation. Major amputations were defined as those above the ankle and minor amputations as those below the ankle.8 An additional classification based on the cause of amputation was also devised. The first group consisted of amputations of digits and forefoot without previous or concurrent large-vessel disease (including angioplasty and bypass surgery) in the same limb or evidence of causative embolism; these amputations were classified as minor amputations without known large-vessel disease and were judged to be related to microvascular disease. The second group consisted of major amputations as well as minor amputations with documented large-vessel disease in the affected limb or evidence of embolism. These amputations were judged to be related to atherosclerotic disease of the major peripheral arteries.
Statistical analysis
The study offered 80% power to detect an observed 22% reduction in cardiovascular events.4 All analyses were done on an intention-to-treat basis. Baseline characteristics were analysed with χ2 tests for categorical variables, t tests, or analysis of variance for continuous variables, or, if the distribution of the data was not normal, by Wilcoxon rank-sum tests (two-way analysis) or Kruskal-Wallis tests (three-way analysis). Proportional hazards regression was used to compute hazard ratios (HRs) and 95% CIs9,10 to assess the effect of fenofibrate treatment on the time to amputation (the proportional hazards assumption was assessed by use of the Harrell-Lee test11). Where appropriate, p values were computed with the log-rank test.10 Cumulative hazard curves of the time to the first amputation according to the amputation classification, and by treatment group, were calculated by use of the Kaplan-Meier method.10
For multiple event analysis, a Poisson model on the number of amputations was used and adjusted for months of observation and overdispersion,12 by use of the Pearson method.13 The Poisson analysis yielded an incidence rate ratio (analogous to the HR) that reflected the change in event rate per unit time for the fenofibrate group relative to the placebo group. A basic risk model was developed to identify the most important predictors of amputations. Variables in the model were initially determined by use of backwards selection of all baseline characteristics in table 1 plus fibrinogen and homocysteine in a proportional hazards model, and then confirmed by exhaustive search methods.14 The possibility of over-fitting because of the large number of potential predictors assessed and the small number of events was examined by calculating the heuristic shrinkage factor.11 The shrinkage factor of 0·9 indicates that the degree of over-fitting was negligible. Since only five patients, none of whom had undergone amputation, had any missing data, no statistical adjustment was made for this. All statistical inferences were drawn with a two-sided p value of 0·05. Results are presented unadjusted for multiple comparisons.15 SAS (version 9.1) and ACCORD (Analysis of Censored and Correlated Data; version 1.6.3, 2008) software was used for the analyses. This study is registered as an International Standard Randomised Controlled Trial, number ISRCTN64783481.
Table 1.
Baseline characteristics and medication
| Fenofibrate (n=4895) | Placebo (n=4900) | |
|---|---|---|
| General characteristics | ||
| Male | 3071 (63%) | 3067 (63%) |
| Age at visit 1 (years) | 62·2 (6·8) | 62·2 (6·9) |
| Diabetes duration (years)* | 5 (2–10) | 5 (2–10) |
| Body-mass index (kg/m2) | 29·8 (26·8–33·6) | 29·8 (26·7–33·4) |
| Systolic blood pressure (mm Hg) | 140 (15) | 141 (15) |
| Diastolic blood pressure (mm Hg) | 82 (9) | 82 (9) |
| Current or ex-smoker | 2916 (60%) | 2950 (60%) |
| Clinical history | ||
| Previous non-traumatic amputation or diabetic skin ulcer | 165 (3%) | 151 (3%) |
| Previous cardiovascular disease | 1068 (22%) | 1063 (22%) |
| History of hypertension* | 2776 (57%) | 2768 (56%) |
| Neuropathy | 707 (14%) | 687 (14%) |
| Laboratory data† | ||
| Total cholesterol (mmol/L) | 5·04 (0·69) | 5·03 (0·71) |
| LDL cholesterol (mmol/L) | 3·07 (0·64) | 3·07 (0·66) |
| HDL cholesterol (mmol/L) | 1·10 (0·26) | 1·10 (0·26) |
| Triglycerides (mmol/L) | 1·74 (1·34–2·34) | 1·73 (1·34–2·30) |
| Plasma haemoglobin A1c (%) | 7·08% (1·37) | 7·05% (1·33) |
| Plasma creatinine (μmol/L) | 77·7 (15·9) | 77·4 (15·7) |
| Microalbuminuria or macroalbuminuria‡ | 1268 (26%) | 1247 (25%) |
| Baseline medication | ||
| Aspirin | 1448 (30%) | 1455 (30%) |
| Angiotensin-converting enzyme inhibitor | 1716 (35%) | 1725 (35%) |
| Angiotensin-II receptor antagonist | 280 (6%) | 265 (5%) |
| Metformin alone | 828 (17%) | 823 (17%) |
| Sulphonylurea alone | 809 (17%) | 799 (16%) |
| Metformin and sulphonylurea | 1207 (25%) | 1196 (24%) |
| Insulin alone or with oral agent | 691 (14%) | 688 (14%) |
Data are number (%), mean (SD), or median (IQR). For further details see reference 5.
Reported at visit 1 (screening visit).
Mean of pre-randomisation visits for lipids, haemoglobin A1c, and creatinine.
Microalbuminuria defined as urine albumin/creatinine ratio ≥2·5 mg/mmoL and <25 mg/mmoL for men, and ≥3·5 mg/mmoL and <35 mg/mmoL for women; macroalbuminuria defined as urine albumin/creatinine ratio ≥25 mg/mmoL for men and ≥35 mg/mmoL for women.
Role of the funding source
Two non-voting representatives of the main sponsor attended meetings of the management committee. The sponsors of the study had no role in data collection or data analysis. The authors had full access to all the data in the study. The authors and study management committee had final responsibility for the decision to submit the manuscript for publication.
Results
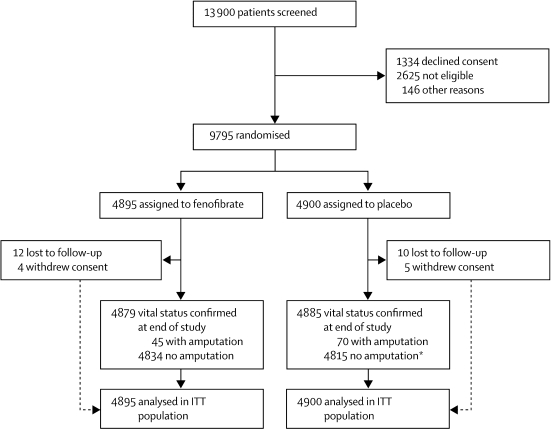
Figure 1 shows the trial profile, and table 1 the baseline characteristics of the study participants. 115 patients had lower-limb amputations due to diabetes, of whom 47 had more than one amputation (ranging from two to six). All patients who reported a non-traumatic amputation were followed up until the completion of the study. Additionally, there were three cases of amputation (all in patients assigned to placebo) that were not related to diabetes (a finger amputation secondary to known Raynaud's disease, and two small-toe amputations secondary to claw toe deformity and not related to diabetic neuropathy); these cases were therefore excluded from any further analysis.
Figure 1.
Trial profile of amputation study
ITT=intention to treat. *Three patients had non-traumatic amputation unrelated to diabetes.
Baseline characteristics differed between patients who had on-study amputations, those who had other cardiovascular events, and those who had neither event (table 2). Patients who had on-study amputations were more likely to be male, to be taller, or smoke, and had a longer median duration of diabetes than patients from the other two groups. They were also more likely to have reported previous cardiovascular disease (myocardial infarction, angina, coronary revascularisation, stroke, or peripheral vascular disease) or microvascular disease at baseline (including retinopathy, neuropathy, and nephropathy). Furthermore, occurrence of microalbuminuria and macroalbuminuria, and plasma HbA1c were all higher in patients who had amputations than in those who had other cardiovascular events or had neither event. Mean lipid concentrations differed, but by no more than 0·2 mmol/L (table 2). Patients with amputations had more prescriptions at baseline of angiotensin-converting enzyme inhibitors, and a higher proportion used insulin than patients in the other two groups, reflecting their longer diabetes duration, worse glycaemic control, and higher occurrence of microvascular and macrovascular complications.
Table 2.
Baseline characteristics by subsequent lower-limb amputation, other cardiovascular event, or neither
| Amputation due to diabetes during study follow-up*(n=115) | Other cardiovascular event (n=1251) | No amputation or cardiovascular event (n=8429) | p value† | |||
|---|---|---|---|---|---|---|
| Male | 93 (81%) | 944 (75%) | 5101 (61%) | <0·0001 | ||
| Age at visit 1 (years) | 65 (6) | 64 (7) | 62 (7) | <0·0001 | ||
| Diabetes duration (years) | 9 (4–15) | 7 (3–12) | 5 (2–9) | <0·0001 | ||
| Body-mass index (kg/m2) | 29·5 (26·6–33·4) | 29·7 (26·8–33·1) | 29·8 (26·8–33·6) | 0·594 | ||
| Height, men (cm) | 176 (171–180) | 174 (169–178) | 175 (170–179) | <0·0001 | ||
| Height, women (cm) | 165 (161–166) | 161 (156–164) | 161 (157–165) | 0·011 | ||
| Waist–hip ratio | 0·96 (0·91–1·00) | 0·95 (0·91–1·00) | 0·93 (0·88–0·98) | <0·0001 | ||
| Systolic blood pressure (mm Hg) | 144 (15) | 144 (16) | 140 (15) | <0·0001 | ||
| Diastolic blood pressure (mm Hg) | 82 (8) | 83 (9) | 82 (8) | 0·040 | ||
| Current smoker | 23 (20%) | 152 (12%) | 747 (9%) | <0·0001‡ | ||
| Ex-smoker | 61 (53%) | 688 (55%) | 4195 (50%) | |||
| Clinical history | ||||||
| Previous non-traumatic amputation or diabetic skin ulcer | 35 (30%) | 46 (4%) | 235 (3%) | <0·0001 | ||
| Previous cardiovascular disease§ | 67 (58%) | 512 (41%) | 1552 (18%) | <0·0001 | ||
| Previous MI, angina, CABG, or PTCA | 34 (30%) | 383 (31%) | 988 (12%) | <0·0001 | ||
| Previous stroke | 12 (10%) | 80 (6%) | 255 (3%) | <0·0001 | ||
| Previous peripheral vascular disease | 43 (37%) | 167 (13%) | 502 (6%) | <0·0001 | ||
| Microvascular disease | 65 (57%) | 380 (30%) | 1580 (19%) | <0·0001 | ||
| Retinopathy | 34 (30%) | 162 (13%) | 618 (7%) | <0·0001 | ||
| Neuropathy | 57 (50%) | 257 (21%) | 1081 (13%) | <0·0001 | ||
| Nephropathy | 8 (7%) | 42 (3%) | 229 (3%) | 0·013 | ||
| Laboratory data¶ | ||||||
| Total cholesterol (mmol/L) | 4·92 (0·64) | 5·07 (0·69) | 5·03 (0·71) | 0·026 | ||
| LDL cholesterol (mmol/L) | 3·00 (0·68) | 3·13 (0·63) | 3·06 (0·65) | 0·002 | ||
| HDL cholesterol (mmol/L) | 1·05 (0·27) | 1·04 (0·24) | 1·11 (0·26) | <0·0001 | ||
| Triglycerides (mmol/L) | 1·77 (1·30–2·31) | 1·82 (1·39–2·44) | 1·72 (1·34–2·31) | 0·0007 | ||
| Plasma haemoglobin A1c (%) | 7·5% (6·8–8·7) | 7·1% (6·4–8·1) | 6·8% (6·1–7·8) | <0·0001 | ||
| Plasma creatinine (μmol/L) | 85 (18) | 83 (17) | 77 (15) | <0·0001 | ||
| Homocysteine (μmol/L) | 11 (9–14) | 10 (9–13) | 9 (8–11) | <0·0001 | ||
| Dyslipidaemia‖ | 46 (40%) | 537 (43%) | 3127 (37%) | 0·0003 | ||
| Microalbuminuria** | 46 (40%) | 368 (29%) | 1697 (20%) | <0·0001 | ||
| Macroalbuminuria†† | 20 (17%) | 97 (8%) | 287 (3%) | <0·0001 | ||
| Baseline cardiovascular medication | ||||||
| Angiotensin-converting enzyme inhibitor | 56 (49%) | 476 (38%) | 2749 (33%) | <0·0001 | ||
| Aspirin | 43 (37%) | 523 (42%) | 2263 (27%) | <0·0001 | ||
| Angiotensin-II receptor agonist | 3 (3%) | 65 (5%) | 454 (5%) | 0·410 | ||
| Baseline blood-glucose-lowering medication | ||||||
| Diet alone | 8 (7%) | 234 (19%) | 2366 (28%) | <0·0001 | ||
| Metformin alone | 10 (9%) | 181 (14%) | 1530 (18%) | 0·0003 | ||
| Sulphonylurea alone | 12 (10%) | 208 (17%) | 1391 (17%) | 0·215 | ||
| Metformin and sulphonylurea | 40 (35%) | 362 (29%) | 1918 (23%) | <0·0001 | ||
| Other oral agent alone | 0 (0%) | 2 (0·2%) | 17 (0·2%) | 0·850 | ||
| Metformin or sulphonylurea or both and other agent | 5 (4%) | 27 (2%) | 138 (2%) | 0·041 | ||
| Insulin alone | 18 (16%) | 106 (8%) | 483 (6%) | <0·0001 | ||
| Insulin and oral agent | 22 (19%) | 131 (10%) | 586 (7%) | <0·0001 | ||
Data are number (%), mean (SD), or median (IQR). CABG=coronary artery bypass graft. MI=myocardial infarction. PTCA=percutaneous transluminal coronary angioplasty.
Patients who had both an amputation and another cardiovascular event were counted only in the amputations column.
p value for three-way comparison. p values are from χ2 tests for categorical variables, ANOVA for normally distributed continuous variables with homogeneous variance, or Kruskal–Wallis test for non-normally distributed continuous variables or those with non-homogeneous variance.
p value from three-way χ2 test of current smokers, ex-smokers, and non-smokers.
Previous cardiovascular disease comprises previous MI, angina, CABG, PTCA, stroke, peripheral vascular disease, and revascularisation.
Mean of pre-randomisation visits for lipids, haemoglobin A1c and creatinine.
Men: HDL cholesterol concentration <1·03 mmol/L and triglyceride concentration ≥1·7 mmol/L; women: HDL cholesterol concentration <1·29 mmol/L and triglyceride concentration ≥1·7 mmol/L.
Microalbuminuria defined as urine albumin/creatinine ratio ≥2·5 mg/mmoL and <25 mg/mmoL for men, and ≥3·5 mg/mmoL and <35 mg/mmoL for women.
Macroalbuminuria defined as urine albumin/creatinine ratio ≥25 mg/mmoL for men and ≥35 mg/mmoL for women.
Of the 115 patients who had an on-study lower-limb amputation due to diabetes, 65 patients had only minor amputations and 50 had major or both types of amputations. Patients with only minor amputations had a higher frequency of microvascular disease at baseline (42 [65%] patients vs 23 [46%] patients, p=0·046), in particular neuropathy (39 [60%] vs 18 [36%], p=0·011), than did those with major or both types of amputations. Patients with only minor amputations also had significantly lower mean systolic blood pressure (142 mm Hg [SD 12] vs 148 mm Hg [18], p=0·043), a lower occurrence of previous cardiovascular disease (31 [48%] vs 36 [72%], p=0·009), and higher mean concentration of plasma LDL cholesterol (3·1 mmol/L [0·6] vs 2·9 mmol/L [0·7], p=0·043) than did those with major or both types of amputations.
39 patients had only minor amputations without large-vessel disease in the affected limb and the remaining 76 had at least one major or minor amputation with large-vessel disease (table 3). Patients whose amputations were all minor without large-vessel disease were similar in most respects to others with amputations, except that they were a mean of 3 years younger, and had a higher body-mass index and a higher frequency of microvascular disease at baseline (in particular neuropathy). Analysis of the amputations according to the causative classification showed no significant difference in baseline medication between groups.
Table 3.
Baseline characteristics of patients who had a lower-limb amputation, by presence of large-vessel disease
| Minor amputation without large-vessel disease (n=39) | Major or minor amputation with large-vessel disease (n=76)* | p value† | |||
|---|---|---|---|---|---|
| Male | 30 (77%) | 63 (83%) | 0·441 | ||
| Age at visit 1 (years) | 63 (6) | 66 (6) | 0·019 | ||
| Diabetes duration (years) | 11 (7–22) | 9 (4–15) | 0·041 | ||
| Body-mass index (kg/m2) | 30·9 (28·7–36·4) | 28·6 (25·8–32·0) | 0·004 | ||
| Waist–hip ratio | 0·97 (0·90–1·00) | 0·96 (0·91–1·00) | 0·690 | ||
| Height (cm) | 174 (8) | 173 (8) | 0·278 | ||
| Systolic blood pressure (mm Hg) | 143 (12) | 145 (16) | 0·455 | ||
| Diastolic blood pressure (mm Hg) | 83 (7) | 81 (9) | 0·166 | ||
| Current smoker | 6 (15%) | 17 (22%) | 0·375‡ | ||
| Ex-smoker | 20 (51%) | 41 (54%) | |||
| Clinical history | |||||
| Previous amputation or diabetic skin ulcer | 15 (38%) | 20 (26%) | 0·180 | ||
| Previous cardiovascular disease§ | 17 (44%) | 50 (66%) | 0·022 | ||
| Previous MI, angina, CABG, or PTCA | 8 (21%) | 26 (34%) | 0·128 | ||
| Stroke | 2 (5%) | 10 (13%) | 0·182 | ||
| Peripheral vascular disease | 11 (28%) | 32 (42%) | 0·145 | ||
| Microvascular disease | 29 (74%) | 36 (47%) | 0·006 | ||
| Retinopathy | 14 (36%) | 20 (26%) | 0·286 | ||
| Neuropathy | 26 (67%) | 31 (41%) | 0·009 | ||
| Nephropathy | 2 (5%) | 6 (8%) | 0·581 | ||
| Laboratory data¶ | |||||
| Total cholesterol (mmol/L) | 5·03 (0·66) | 4·86 (0·63) | 0·187 | ||
| LDL cholesterol (mmol/L) | 3·09 (0·67) | 2·95 (0·68) | 0·294 | ||
| HDL cholesterol (mmol/L) | 1·00 (0·25) | 1·07 (0·27) | 0·179 | ||
| Triglycerides (mmol/L) | 1·95 (1·39–2·49) | 1·69 (1·28–2·23) | 0·225 | ||
| Plasma haemoglobin A1c (%) | 8·1% (7·0–9·2) | 7·4% (6·7–8·5) | 0·087 | ||
| Plasma creatinine (μmol/L) | 84 (18) | 86 (19) | 0·640 | ||
| Homocysteine (μmol/L) | 10·8 (8·8–12·8) | 11·6 (9·2–14·1) | 0·266 | ||
| Dyslipidaemia‖ | 18 (46%) | 28 (37%) | 0·335 | ||
| Microalbuminuria** | 14 (36%) | 32 (42%) | 0·520 | ||
| Macroalbuminuria†† | 10 (26%) | 10 (13%) | 0·095 | ||
| Baseline blood-glucose-lowering medication | |||||
| Diet alone | 1 (3%) | 7 (9%) | 0·185 | ||
| Metformin alone | 3 (8%) | 7 (9%) | 0·784 | ||
| Sulphonylurea alone | 2 (5%) | 10 (13%) | 0·182 | ||
| Metformin and sulphonylurea | 18 (46%) | 22 (29%) | 0·067 | ||
| Other oral agent alone | 0 (0%) | 0 (0%) | .. | ||
| Metformin or sulphonylurea or both and other agent | 3 (8%) | 2 (3%) | 0·208 | ||
| Insulin alone | 5 (13%) | 13 (17%) | 0·549 | ||
| Insulin and oral agent | 7 (18%) | 15 (20%) | 0·817 | ||
Data are number (%), mean (SD), or median (IQR). CABG=coronary artery bypass graft. MI=myocardial infarction. PTCA=percutaneous transluminal coronary angioplasty.
13 patients who had a minor amputation without large-vessel disease plus another type of amputation are included in this group.
p values are from χ2 tests for categorical variables, t test for normally distributed continuous variables, or Wilcoxon rank-sum test for non-normally distributed continuous variables.
p value from three-way χ2 test of current smokers, ex-smokers, and non-smokers.
Previous cardiovascular disease comprises previous MI, angina, CABG, PTCA, stroke, peripheral vascular disease, or revascularisation.
Mean of pre-randomisation visits for lipids, haemoglobin A1c, and creatinine.
Men: HDL cholesterol concentration <1·03 mmol/L and triglyceride concentration ≥1·7 mmol/L; women: HDL cholesterol concentration <1·29 mmol/L and triglyceride concentration ≥1·7 mmol/L.
Microalbuminuria defined as urine albumin/creatinine ratio ≥2·5 mg/mmoL and <25 mg/mmoL for men, and ≥3·5 mg/mmoL and <35 mg/mmoL for women.
Macroalbuminuria defined as urine albumin/creatinine ratio ≥25 mg/mmoL for men and ≥35 mg/mmoL for women.
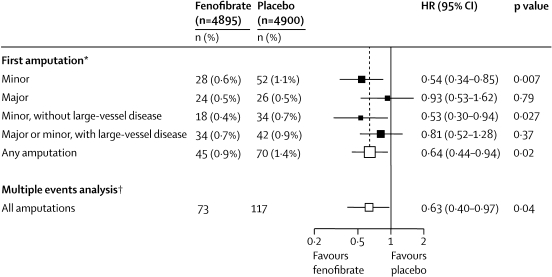
Of the 115 patients who had one or more non-traumatic amputations due to diabetes, 45 had been allocated to fenofibrate and 70 to placebo (figure 2). The risk of a first non-traumatic amputation was lower in the fenofibrate group than in the placebo group (HR 0·64, 95% CI 0·44–0·94; p=0·02; figure 2 and figure 3). This finding reflects the lower risks of minor amputations associated with fenofibrate compared with placebo (HR 0·54, 0·34–0·85; p=0·007). However, the risk of major amputations did not differ significantly between groups (HR 0·93, 0·53–1·62; p=0·79; figure 2 and figure 3).
Figure 2.
Effects of fenofibrate on first and all amputation events
HR=hazard ratio. Counts for each category of amputation are shown. *Patients were counted only once within each category but may be included in more than one category if they had more than one type of amputation. If a patient had more than one type of amputation, or more than one of the same type of amputation, only the first event of each type was analysed. †For all events, all amputations for each category are counted (Poisson method): the incidence rate ratio, analogous to the hazard ratio, is plotted.
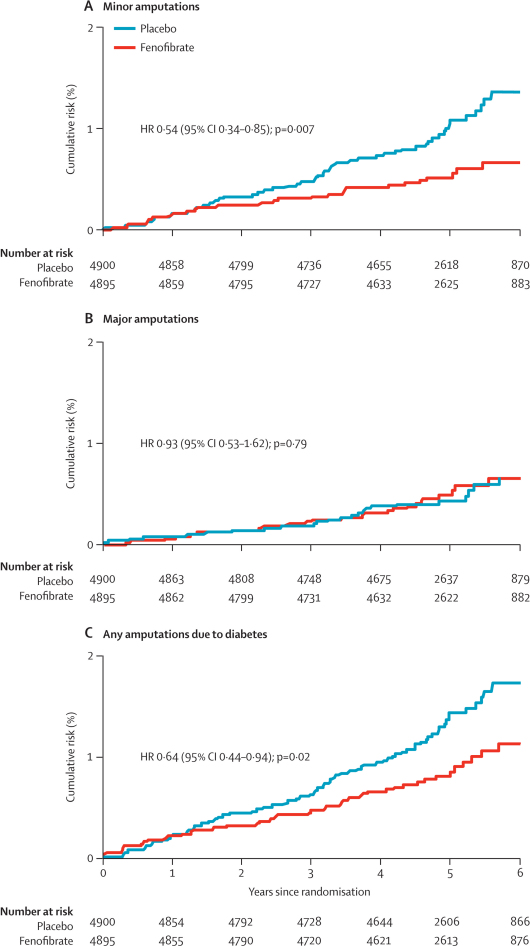
Figure 3.
Cumulative risk curves of time to first amputation (minor, major, any) event, by treatment group
Patients could be counted in more than one category, but only once per category.
Figure 2 shows that the risk of a first minor amputation without associated large-vessel disease was lower in the fenofibrate group than in the placebo group; however, the risk of a first major or minor amputation with large-vessel disease did not differ between treatment groups. The decrease in first diabetes-related amputations seemed to emerge after only 1·5 years of fenofibrate use, with continued divergence of the cumulative hazard curves over the mean 5-year follow-up period (figure 3).
The effect of fenofibrate on a first amputation was similar in those taking and not taking an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker at baseline (p=0·4 for heterogeneity of treatment effect). Similarly, the effect of fenofibrate did not differ between those with good (HbA1c <7·0%) or poor (HbA1c ≥7·0%) glycaemic control (p=0·6 for heterogeneity of treatment effect) or between those with or without dyslipidaemia (ie, men: HDL cholesterol concentration less than 1·03 mmol/L and triglyceride concentration 1·7 mmol/L or more; women: HDL cholesterol concentration less than 1·29 mmol/L and triglyceride concentration 1·7 mmol/L or more; p=0·5 for heterogeneity of treatment effect).
Of the 190 non-traumatic diabetes-related amputations in total, 73 occurred in patients assigned to fenofibrate and 117 in patients assigned to placebo. This result represents a further 28 amputations in 18 patients assigned to fenofibrate compared with 47 further amputations in 29 patients assigned to placebo. When multiple amputations per patient were considered, the HR for the effect of fenofibrate treatment on the number of amputations was 0·63 (0·40–0·97; p=0·040; figure 2).
The strongest predictors of a first on-study amputation in a multivariable model were a history of previous non-traumatic amputation or diabetic skin ulcer, neuropathy, or previous peripheral vascular disease. HRs were 5·6 (3·6–8·6) for previous amputation or skin ulcer, 2·7 (1·8–4·0) for neuropathy, and 2·5 (1·7–3·9) for peripheral vascular disease (all p<0·0001). Non-modifiable factors of height and age were also predictive: the HR for height (per 10 cm) was 1·6 (1·3–2·0) and for age (per each 5 additional years) was 1·3 (1·2–1·5; also both p<0·0001). Other predictors included current smoking, albuminuria, HbA1c (per 1% higher level), retinopathy, and percutaneous transluminal coronary angioplasty (data not shown). None of the lipid variables (total and HDL cholesterol concentrations, triglycerides) were significantly associated with amputation in multivariable analysis and therefore none remained in the final model. Adjusted for these significant risk factors, the HR for the effect of fenofibrate treatment on risk of a first diabetes-related amputation was 0·63 (0·43–0·92; p=0·016). This finding represents an absolute risk reduction of about 0·5% (95% CI 0·1–0·9). Serious adverse events associated with fenofibrate treatment have been reported elsewhere.5
Discussion
This study showed that amputation risk in patients with type 2 diabetes was lower in patients assigned to treatment with long-term fenofibrate than in those assigned to placebo. The cumulative hazard curves showed a reduction in amputation rates that seemed to emerge after just 1·5 years of fenofibrate use. The reduction in the risk of minor amputation where there was no known large-vessel disease was most striking, by contrast with a non-significant reduction for major or large-vessel amputations. This analysis substantiates the established macrovascular5 and microvascular (albuminuria5 and retinopathy6) benefits of fenofibrate use, which should also be considered in calculating the economic benefits of such treatment.
The number of patients needed to treat (NNT) with fenofibrate over 5 years to prevent at least one amputation in one patient is 197, but is 25 for someone with previous foot ulcer and albuminuria. These results compare with NNTs with fenofibrate of 17 and 90 to prevent laser treatment for retinopathy in patients with and without a history of retinopathy, respectively,6 68 to prevent progression of albuminuria,5 and 71 and 23 to prevent one or more cardiovascular events overall and in those with marked dyslipidaemia, respectively.16
These effects on amputation occurred in patients on optimum medical treatment (by current practice standards) to control risk factors that altered the progression of their diabetes. HbA1c did not change substantially from baseline in either treatment group over the 5-year study period (data not shown). Most importantly, the treatment effects of fenofibrate were irrespective of the level of glycaemic control and background use or not of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, strongly suggesting that the drug's effects are additive to other measures. In the UK Prospective Diabetes Study,17 a reduction in HbA1c of 0·9% over 10 years in patients treated with intensive blood glucose control compared with control patients did not result in significant changes in either amputation rate or death from peripheral vascular disease. Similarly, peripheral vascular disease was not significantly reduced by intensive glucose control in patients from the same study who were followed up for up to 20 years,18 or in patients from the ADVANCE study19 (the ACCORD study20 has not published on amputations to date). Neither the HOPE study,21 which assessed the angiotensin-converting enzyme inhibitor, ramipril, nor the ADVANCE study,22 which tested intensive blood pressure reduction with perindopril and indapamide, has reported any amputation data so far.
The most common pattern of dyslipidaemia in people with diabetes is hypertriglyceridaemia with or without reduced HDL cholesterol concentrations. However, the effects of treatment with fenofibrate on the risk of amputation might go beyond the improvements in controlling the lipid profile, since no lipid variables were associated with the risk of amputation in multivariable analyses. Additionally, the Heart Protection Study,23 which randomly assigned 5963 people with diabetes to either simvastatin or placebo, showed no difference in amputation rates between groups, despite substantial reductions in total cholesterol and LDL cholesterol concentrations and modest changes in triglyceride and HDL cholesterol concentrations in the intervention group compared with controls. This finding suggests that the effects of fenofibrate in reducing amputation risk are more likely to be non-lipid-mediated and, as such, unaffected by the different rates of statin use between treatment groups in the FIELD study.
Several theoretical mechanisms for the microvascular benefits of fenofibrate have been proposed. In a randomised placebo-controlled trial, treatment with fenofibrate was associated with improved endothelial-dependent vascular reactivity over 12 weeks,24 with reductions in markers of endothelial dysfunction and pro-inflammatory markers, including tumour necrosis factor α, interleukin 6, and interleukin 1β in plasma; another trial also showed that fenofibrate treatment was associated with reduced viscosity.25 These results need to be substantiated by longer-term studies. In patients with hypertriglyceridaemia or metabolic syndrome, fenofibrate improved flow-mediated dilator response to hyperaemia, with increased adiponectin concentrations and improved insulin sensitivity.26 The drug might exert its antiangiogenic effects directly,27 or by reducing tissue ischaemia through these actions.24–26 Fenofibrate also activates AMP kinase in endothelial cells via a peroxisome-proliferating receptor-α independent pathway, preventing retinal cell apoptosis,28 and possibly increasing nitric oxide synthesis.29 Fenofibrate could also be protective through the inhibition of oxidative stress30 (current FIELD studies of blood markers of inflammation and oxidation will help to investigate this mechanism further) and has been reported to have neuroprotective effects in rodents,31 which could be particularly important in view of the key role of neuropathy in risk of amputation.
An amputation due to diabetes occurs around every 30 s somewhere in the world.32 Amputations substantially impair quality of life33 and impose a major burden on health-care systems, with annual costs in the UK estimated at about £252 million34 and in the USA at about US$1648 million.3 Most of this expenditure is related to type 2 diabetes, with less than 10% accounted for by type 1 diabetes. Indirect costs would further increase these figures substantially.
Foot ulcers and infections are also a major source of morbidity, associated with neuropathy, abnormal foot biomechanics, peripheral arterial disease, and poor wound healing.35 Peripheral sensory neuropathy results in major or repeated minor trauma to the foot (often unnoticed), abnormal weight bearing, and subsequent callus formation and ulceration. Motor and sensory neuropathy leads to abnormal foot muscle mechanics and structural changes in the foot. Autonomic neuropathy results in anhidrosis and altered superficial blood flow that promotes drying of the skin and fissure formation. Peripheral arterial disease and poor wound healing impede the resolution of minor skin breaks, allowing them to enlarge and become infected.35
Prevention is, therefore, the most essential strategy for avoiding diabetic foot ulcers and amputations; this approach involves identifying patients at high risk, providing education about appropriate foot care, and implementing measures to prevent ulceration (such as protective footwear and podiatry). Such foot care has reduced amputation risk by 45%.36 Our study lends support to the following predictors of future lower-limb amputation that were previously suggested in a smaller cohort study of 1300 patients with a different study design:37 a history of previous foot ulcer, longer diabetes duration, presence of neuropathy or peripheral arterial disease, and poor glycaemic control.
Our study also identified height as a major predictor of amputations, with a 1·6-fold increase in risk for every 10 cm increase in height, which is similar to a previous observational report of an odds ratio of 1·8 for each 10 cm increase in height.38 Two previous studies have described, in a mixed cohort of patients with and without diabetes, that hypercholesterolaemia and hypertension result in worse peripheral arterial disease outcomes.39,40 Although in our study these conditions were not independent predictors of amputation risk exclusively, they contributed towards amputation risk indirectly, since peripheral vascular disease was found to be an independent major risk factor.
There are limitations to this study, including the fact that there was no standardised routine testing (for example, angiography or ankle-brachial index) at baseline to assess vascular status, because it was not required by the study protocol. It is therefore possible that some amputations could have been misclassified by the failure to detect large-vessel disease, because of either unreported angiograms, or vascular studies or comorbidities that could have precluded revascularisation. However, if such non-differential misclassification bias resulting from missed large-vessel disease had occurred, then the observed reduction with fenofibrate in those with minor amputation without large-vessel disease would likely be an underestimate of the true treatment effect in that group (because there was less effect seen in those with large-vessel disease).
FIELD is the largest randomised controlled trial of type 2 diabetes mellitus reporting data for amputations, with a very large set of baseline variables. The risks of overall amputation were substantially lower in the fenofibrate group than in the placebo group over 5 years, and the drug had a satisfactory safety profile (reported elsewhere).5 The proposed microvascular benefits of such treatment are suggested by the significant risk reduction for minor amputations without large-vessel disease. These benefits seem additive to any benefits arising from good glycaemic control and the use of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, and therefore represent an important breakthrough in the prevention of diabetic complications. These findings support the use of fenofibrate, irrespective of the presence of dyslipidaemia, for the treatment of patients with type 2 diabetes who are at high risk for amputation (including those with peripheral vascular disease, existing microvascular complications, and a long duration of diabetes). This approach could help to reduce the substantial morbidity, mortality, and economic burden associated with amputation due to diabetes.
Acknowledgments
Acknowledgments
This study was supported by a grant from Laboratoires Fournier SA, Dijon, France (now part of Solvay Pharmaceuticals), and by the National Health and Medical Research Council (NHMRC) of Australia and was coordinated independently by the NHMRC Clinical Trials Centre, University of Sydney, Australia, and overseen by the study Management Committee. We thank the National Heart Foundation of Australia, Diabetes Australia, Diabetes New Zealand, and the Finnish Diabetes Association for endorsing the study. We also thank R Pike, V Gebski, and D Tse for their assistance on this manuscript, and the many patients who participated in the FIELD study.
Contributors
KR, PGC, LPL, JDB, MCD'E, ML, JRB, and ACK contributed to the design of the study. KR, PGC, LPL, JDB, MCD'E, ML, and ACK participated in data collection. KR, PGC, LPL, MV, MCD'E, ML, and ACK participated in data analysis. All authors contributed to the writing or revision of the manuscript. All authors saw and approved the final version of the manuscript.
Conflicts of interest
PGC has received consultancy fees, speaker fees and/or travel grants from Eli Lilly, Novo Nordisk, and GlaxoSmithKline, and research support from Sanofi-Aventis, GlaxoSmithKline, Novo Nordisk, BayHill, Medtronic, and AstraZeneca. MCD'E has received consultancy fees, speaker fees, and/or travel grants from Eli Lilly, Novo Nordisk, Sanofi-Aventis, Merck Sharp & Dohme, Bristol-Myers Squibb, Pfizer, and AstraZeneca. ML has received honoraria from Merck Sharp & Dohme, support for attending meetings from Merck Sharp & Dohme, AstraZeneca and Eli Lilly, and research support from Novartis, Fournier, and Takeda. ACK has received consultancy fees, speaker fees and/or travel grants from Bristol-Myers Squibb, AstraZeneca, Solvay, Abbott, Novo Nordisk, Merck Sharp & Dohme, Eli Lilly, and Roche Diagnostics, has served as an expert witness for Medicines Australia, has been named in a patent application related to fenofibrate and diabetic retinopathy and has received research support from Bristol-Myers Squibb, Solvay, Abbott, and Roche Diagnostics. The other authors declare that they have no conflicts of interest.
References
- 1.Bild DE, Selby JV, Sinnock P. Low-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care. 1980;12:24–31. doi: 10.2337/diacare.12.1.24. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–932. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 3.Gordois A, Scuffham P, Shearer A. The health care cost of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26:1790–1795. doi: 10.2337/diacare.26.6.1790. [DOI] [PubMed] [Google Scholar]
- 4.The FIELD Study Investigators The need for a large-scale trial of fibrate therapy in diabetes: the rationale and design of the Fenofibrate Interventional and Event Lowering in Diabetes (FIELD) study. Cardiovasc Diabetol. 2004;3:9. doi: 10.1186/1475-2840-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The FIELD study investigators Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 6.Keech AC, Mitchell P, Summanen PA. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 7.Signorini DF, Leung O, Simes RJ, Beller E, Gebski VJ. Dynamic balanced randomisation for clinical trials. Stat Med. 1993;12:2343–2350. doi: 10.1002/sim.4780122410. [DOI] [PubMed] [Google Scholar]
- 8.Sheahan MG, Hamdan AD, Veraldi JR. Lower extremity minor amputations: the roles of diabetes mellitus and timing of revascularization. J Vasc Surg. 2005;42:476–480. doi: 10.1016/j.jvs.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Peto R, Pike MC, Armitage P. Design and analysis of randomised clinical trials requiring prolonged observation of each patient. Br J Cancer. 1976;34:585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collett D. Modelling survival data in medical research. 2nd edn. Chapman & Hall; London: 2003. [Google Scholar]
- 11.Harrell F, Lee KL. Proceedings of the 11th Annual SAS User's Group International Conference. SAS Institute; Cary, NC: 1986. Verifying assumptions of the Cox proportional hazards model; pp. 823–828. [Google Scholar]
- 12.Lawless JF. Negative binomial and mixed Poisson regression. Can J Stat. 1987;15:209–225. [Google Scholar]
- 13.McCulloch CE, Searle SR. Generalized, linear, and mixed models. Wiley; New York: 2001. [Google Scholar]
- 14.Kuk AYC. All subsets in proportional hazards models. Biometrika. 1984;71:587–592. [Google Scholar]
- 15.Rothman KJ. No adjustments are needed for multiple testing. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 16.Scott R, O'Brien R, Fulcher G. Effects of fenofibrate treatment on cardiovascular disease risk in 9795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32:493–498. doi: 10.2337/dc08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 18.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 19.The ADVANCE Collaborative group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 20.The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 22.Patel A, ADVANCE Collaborative Group Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 23.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 24.Ryan KE, McCance DR, Powell L, McMahon R, Trimble ER. Fenofibrate and pioglitazone improve endothelial function and reduce arterial stiffness in obese glucose tolerant men. Atherosclerosis. 2007;194:e123–e130. doi: 10.1016/j.atherosclerosis.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Rosenson RS, Helenowski IB. Fenofibrate abrogates postprandial blood viscosity among hypertriglyceridemia subjects with the metabolic syndrome. Diab Met Syndr Clin Res Rev. 2009;3:17–23. [Google Scholar]
- 26.Koh K, Han S, Quon M. Beneficial effects of fenofibrate to improve endothelial dysfunction and raise adiponectin levels in patients with primary hypertriglyceridemia. Diabetes Care. 2005;28:1419–1424. doi: 10.2337/diacare.28.6.1419. [DOI] [PubMed] [Google Scholar]
- 27.Panigrahy D, Kaipainen A, Huang S. PPAR alpha agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc Natl Acad Sci USA. 2008;105:985–990. doi: 10.1073/pnas.0711281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Ahn JH, Kim JH. Fenofibrate regulates retinal endothelial cell survival through the AMPK signal transduction pathway. Exp Eye Res. 2007;84:886–893. doi: 10.1016/j.exer.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Murakami H, Murakami R, Kambe F. Fenofibrate activates AMPK and increases eNOS phosphorylation in HUVEC. Biochem Biophys Res Commun. 2006;341:973–978. doi: 10.1016/j.bbrc.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 30.Losada M, Alio JL. Malondialdehyde serum concentration in type 1 diabetic with and without retinopathy. Doc Ophthalmol. 1997;93:223–229. doi: 10.1007/BF02569062. [DOI] [PubMed] [Google Scholar]
- 31.Deplanque D, Gelé P, Pétrault O. Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J Neurosci. 2003;23:6264–6271. doi: 10.1523/JNEUROSCI.23-15-06264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.International Diabetes Federation . Time to act: diabetes and foot care. International Diabetes Federation; Brussels: 2005. [Google Scholar]
- 33.Tenvall GR, Apelqvist J. Health-related quality of life in patients with diabetes mellitus and foot ulcers. J Diabetes Complications. 2000;14:235–241. doi: 10.1016/s1056-8727(00)00133-1. [DOI] [PubMed] [Google Scholar]
- 34.Gordois A, Scuffham P, Shearer A, Oglesby A. The health care costs of diabetic peripheral neuropathy in the UK. The Diabetic Foot. 2003;6:62–73. doi: 10.2337/diacare.26.6.1790. http://findarticles.com/p/articles/mi_m0MDQ/is_2_6/ai_107836467/?tag=content;col1 (accessed April 30, 2009). [DOI] [PubMed] [Google Scholar]
- 35.Pecoraro RE, Reiber GE, Burgess E. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13:513–521. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- 36.Edmonds ME, Blundell MP, Morris ME, Thomas EM, Cotton LT, Watkins PJ. Improved survival of the diabetic foot: the role of a specialised foot clinic. Q J Med. 1986;60:763–771. [PubMed] [Google Scholar]
- 37.Davis WA, Norman PE, Bruce DG, Davis TM. Predictors, consequences and costs of diabetes-related lower extremity amputation complicating type 2 diabetes: the Fremantle Diabetes Study. Diabetologia. 2006;49:2634–2641. doi: 10.1007/s00125-006-0431-0. [DOI] [PubMed] [Google Scholar]
- 38.Tseng C. Prevalence of lower-extremity amputation among patients with diabetes mellitus: is height a factor? Can Med Assoc J. 2006;174:319–323. doi: 10.1503/cmaj.050680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murabito JM, D'Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication. A risk profile from the Framingham Heart Study. Circulation. 1997;96:44–49. doi: 10.1161/01.cir.96.1.44. [DOI] [PubMed] [Google Scholar]
- 40.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]