Summary
Background
Incorporation of a taxane as adjuvant treatment for early breast cancer offers potential for further improvement of anthracycline-based treatment. The UK TACT study (CRUK01/001) investigated whether sequential docetaxel after anthracycline chemotherapy would improve patient outcome compared with standard chemotherapy of similar duration.
Methods
In this multicentre, open-label, phase III, randomised controlled trial, 4162 women (aged >18 years) with node-positive or high-risk node-negative operable early breast cancer were randomly assigned by computer-generated permuted block randomisation to receive FEC (fluorouracil 600 mg/m2, epirubicin 60 mg/m2, cyclophosphamide 600 mg/m2 at 3-weekly intervals) for four cycles followed by docetaxel (100 mg/m2 at 3-weekly intervals) for four cycles (n=2073) or control (n=2089). For the control regimen, centres chose either FEC for eight cycles (n=1265) or epirubicin (100 mg/m2 at 3-weekly intervals) for four cycles followed by CMF (cyclophosphamide 600 mg/m2, methotrexate 40 mg/m2, and fluorouracil 600 mg/m2 at 4-weekly intervals) for four cycles (n=824). The primary endpoint was disease-free survival. Analysis was by intention to treat (ITT). This study is registered as an International Standard Randomised Controlled Trial, number ISRCTN79718493.
Findings
All randomised patients were included in the ITT population. With a median follow-up of 62 months, disease-free survival events were seen in 517 of 2073 patients in the experimental group compared with 539 of 2089 controls (hazard ratio [HR] 0·95, 95% CI 0·85–1·08; p=0·44). 75·6% (95% CI 73·7–77·5) of patients in the experimental group and 74·3% (72·3–76·2) of controls were alive and disease-free at 5 years. The proportion of patients who reported any acute grade 3 or 4 adverse event was significantly greater in the experimental group than in the control group (p<0·0001); the most frequent events were neutropenia (937 events vs 797 events), leucopenia (507 vs 362), and lethargy (456 vs 272).
Interpretation
This study did not show any overall gain from the addition of docetaxel to standard anthracycline chemotherapy. Exploration of predictive biomarker-defined subgroups might have the potential to better target the use of taxane-based therapy.
Funding
Cancer Research UK (CRUK 01/001), Sanofi-Aventis, Pfizer, and Roche.
Introduction
Adjuvant chemotherapy has improved survival for women with early breast cancer over the past 30 years. During the 1990s, anthracycline chemotherapy was shown to be superior to CMF (cyclophosphamide, methotrexate, and fluorouracil).1,2 Incorporation of a taxane (paclitaxel or docetaxel) offered potential for further improvement of patient outcomes.
Initial reports from two trials of adjuvant taxanes were presented at the 2000 National Institutes of Health (NIH) Consensus Development Conference (Bethesda MD, USA). In these trials, four cycles of paclitaxel were added to four cycles of doxorubicin plus cyclophosphamide. Results from the CALBG 9344 trial showed a modest disease-free survival benefit for paclitaxel compared with control; the NSABP B-28 study showed no significant difference, although follow-up at that time was short (both trials have subsequently published results3,4). The NIH Consensus Panel concluded that there was insufficient evidence to support routine use of taxanes in early breast cancer and called for carefully designed studies to be undertaken, particularly trials that compared the incorporation of taxanes as adjuvant therapy with anthracycline treatment of similar duration.
TACT (Taxotere as Adjuvant Chemotherapy Trial) was developed in 2000 to investigate whether sequential docetaxel (Taxotere, Sanofi-Aventis, Bridgewater, NJ, USA) given at 3-weekly intervals after anthracycline chemotherapy would improve patient outcome compared with standard anthracycline chemotherapy of similar duration. With over 4000 patients, the study was powered to detect small but clinically worthwhile benefits in disease-free survival, to assess quality of life and, with a parallel translational research programme, to identify subgroups in which docetaxel might have specific benefit.
Methods
Patients
This multicentre, phase III, randomised controlled trial was undertaken in 103 centres in the UK and one centre in Belgium (including specialist cancer hospitals, university teaching hospitals, and smaller community general hospitals). Patients eligible for the trial were women aged more than 18 years with operable invasive breast cancer (International Union Against Cancer stage pT1-3a pN0-1 M0) who had undergone complete excision and were to be treated with adjuvant chemotherapy—ie, those with node-positive or high-risk node-negative disease (eg, grade 3, hormone-receptor-negative, or lymphovascular invasion). Additionally, patients needed to have normal haematological, hepatic, and renal function. Exclusion criteria included locally advanced or distant disease, bilateral breast cancer, pregnancy, and previous invasive malignancy within 10 years. Determination of oestrogen receptor (ER) was mandatory and randomisation was within 8 weeks of definitive surgery. Representative tumour blocks were requested prospectively for human epidermal growth factor receptor-2 (HER2) testing at central reference laboratories.5 Tissue from consenting patients was stored in microarrays for further translational research.
TACT (CRUK01/001) was approved by the national South East Multi-Research Ethics Committee (MREC 00/1/59) and the local ethics committees of all participating centres. All enrolled patients provided written informed consent. The Clinical Trials and Statistics Unit at the Institute of Cancer Research (Sutton, UK; ICR-CTSU) had overall responsibility for trial coordination with four collaborating clinical trials units responsible for regional data management and randomisation. The Trial Management Group was responsible for the day-to-day running of the trial. The trial was overseen by an Independent Trial Steering Committee. Emerging safety and efficacy data were reviewed regularly in confidence by the Independent Data Monitoring Committee. Data were collated at regular intervals at ICR-CTSU where all interim and final analyses were done. Central statistical monitoring was done by ICR-CTSU and was supplemented by selected on-site source document verification.
Randomisation
Patients were randomised in a 1:1 ratio to an experimental taxane regimen or control. Before activating the trial, centres declared which of two UK standard anthracycline regimens would be used as control. Independent randomisation was by telephone to the ICR-CTSU or one of four regional clinical trial units. Computer-generated permuted blocks were used; stratification was by centre, nodal status (none, one to three, or four or more), and ER status. This trial was open label.
Procedures
Control regimens were FEC (fluorouracil 600 mg/m2, epirubicin 60 mg/m2, and cyclophosphamide 600 mg/m2, all intravenously on day 1 every 21 days) for eight cycles or epirubicin (100 mg/m2 intravenously on day 1 every 21 days) for four cycles followed by CMF (cyclophosphamide 600 mg/m2, methotrexate 40 mg/m2, and fluorouracil 600 mg/m2, all intravenously on days 1 and 8 every 28 days; E-CMF) for four cycles. Centres could choose cyclophosphamide 100 mg/m2 given orally on days 1–14 as an alternative to intravenous administration on days 1 and 8. Patients allocated to the experimental group received FEC (doses as for control) for four cycles followed by docetaxel (100 mg/m2 intravenously on day 1 every 21 days; FEC-D) for four cycles. Docetaxel was infused over 1 h with dexamethasone premedication (8 mg orally twice a day for 3 days beginning the day before treatment). Patients who received docetaxel were also given prophylactic ciprofloxacin (500 mg orally twice a day on days 5–14). Granulocyte colony-stimulating factor was used according to local practice. Clinical, haematological, and biochemical assessments were done before every cycle. Chemotherapy was delivered when neutrophil counts were 1·5×109 cells per L or more and platelets were 100×109 per L or more. A 20% dose reduction was recommended for patients with grade 3 non-haematological toxicity with treatment delay allowed until resolution. Discontinuation of treatment was recommended if toxicity resulted in a delay of more than 3 weeks, or more than two dose reductions.
After chemotherapy, 5 years of tamoxifen was prescribed for patients whose tumours were positive for ER, progesterone receptor, or both. A protocol amendment in 2005 formalised use of aromatase inhibitors as an alternative to tamoxifen in accordance with local policy. Patients with HER2-positive tumours were allowed to enter clinical trials assessing trastuzumab. Radiotherapy, which started within 4 weeks of chemotherapy completion, was mandatory after breast conserving surgery and was used after mastectomy according to local guidelines.
Adverse events were assessed after every chemotherapy cycle and all patients were followed up every 3 months for 2 years post treatment. Subsequent clinical follow-up was as per local policy with relevant details being forwarded to the clinical trial units yearly or on occurrence of an event.
Selected centres invited all patients to participate in a questionnaire-based6–9 quality of life substudy.10,11 Detailed results and an economic analysis12 that examines costs incurred by the experimental regimen compared with the control regimen will be reported separately.
The primary endpoint of the study was invasive disease-free survival, defined as time from randomisation to first invasive relapse, new primary breast cancer (ipsilateral or contralateral), or death from any cause; patients who remained alive and disease-free at their date of last follow-up were censored. Secondary endpoints were metastasis-free survival (time from randomisation to first distant relapse or death from breast cancer), overall survival (time from randomisation to death from any cause) and tolerability of the regimens (including compliance and acute adverse events). Relative dose intensity was used as a measure of treatment compliance, as in previous large UK randomised trials.13 Adverse events were graded according to National Cancer Institute Common Toxicity Criteria14 and coded by use of the Medical Dictionary for Regulatory Activities (MedDRA version 10)15 with central clinical review (by PB-L and PE) when needed.
Statistical analysis
A 5-year disease-free survival of 70% after anthracycline chemotherapy was predicted, with 3342 patients needed to detect a 5% absolute improvement (5% two-sided significance; 90% power). In January, 2003, while recruitment was in progress, the trial steering committee recommended continuation of recruitment beyond that target to provide adequate power to detect a smaller yet clinically meaningful difference of 4%. This recommendation was based on external data that indicated improved outcome after anthracycline treatment.2,13 A revised sample size of 4000 patients (3848 plus a small allowance for dropouts) was agreed. 1038 disease-free survival events were needed to provide 80% power to detect an absolute difference in 5-year disease-free survival of 4% (from 71% to 75%, hazard ratio [HR] 0·84).
For survival-related endpoints, Kaplan-Meier curves were plotted and treatment groups compared by use of the log-rank test. HRs (with 95% CIs) were obtained from Cox proportional hazards regression models with HR less than 1 favouring the experimental regimen. The proportionality assumption of the Cox model was tested with Schoenfeld residuals and found to hold. Unless stated otherwise, all analyses were unadjusted and stratified by centre's choice of control regimen. The principal analysis includes all patients as randomised on an intention-to-treat (ITT) basis. Sensitivity analyses were done for patients receiving eight cycles of protocol treatment and for those receiving more than 85% relative dose intensity. A further sensitivity analysis excluding 16 unevaluable patients gave results consistent with the ITT analysis. A priori defined subgroup analyses by choice of control regimen, ER status, and nodal status were undertaken. Exploratory subgroup analyses were done by HER2 status and by combined ER and HER2 status in all patients, and in node-positive patients only (to replicate the analyses undertaken by Hayes and colleagues16). No adjustment was made for multiple testing in the efficacy analyses. Fixed-effects published data meta-analyses of relevant adjuvant taxane trials and of the subgroup of trials reporting results by ER/HER2 subgroups were done.
Relative dose intensities were compared between treatment groups using a Wilcoxon rank-sum test. Clinically important adverse events and those that differed significantly between treatment groups or which were reported with a frequency of more than 10% are presented. Analysis of late toxicity compared all signs and symptoms reported more than 2 months after treatment end. Since the toxicity profiles of FEC and E-CMF were known to differ, adverse event comparisons were also done by centre's choice of control regimen. Where appropriate, adverse event data were censored 3 months before relapse. The proportion of patients with grade 3 or 4 toxicity in each treatment group was compared by use of a χ2 test or exact test as appropriate. Quality of life data were analysed for patients with baseline forms completed before chemotherapy. Mean changes in scores from baseline were compared between groups by t tests. A significance level of p<0·01 allowed some adjustment for multiple testing of toxicity and quality of life endpoints. This analysis is based on a database snapshot frozen on May 1, 2008. All analyses were done in STATA 9.2. This study is registered as an International Standard Randomised Controlled Trial, number ISRCTN79718493.
Role of the funding source
The trial design was approved by Cancer Research UK's Clinical Trials Awards and Advisory Committee. Other than this, none of the funders had any role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
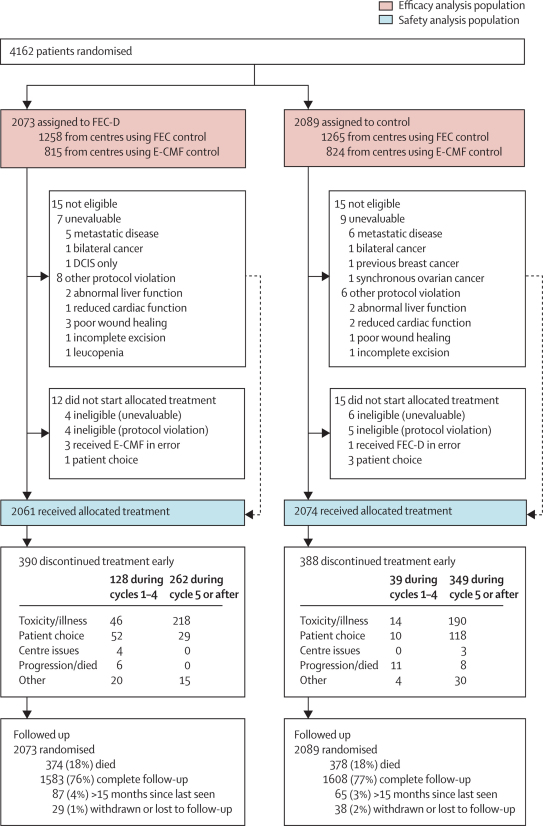
Figure 1 shows the trial profile. Between February, 2001, and July, 2003, 2073 women were randomly assigned to the FEC-D regimen and 2089 women were assigned to control. Median follow-up was 62·0 months (IQR 56·9–69·9) with complete follow-up data available for 3191 (94%) of 3410 alive patients.
Figure 1.
Trial profile
CMF=cyclophosphamide, methotrexate, and fluorouracil. DCIS=ductal carcinoma in situ. E-CMF=epirubicin followed by CMF. FEC=fluorouracil, epirubicin, and cyclophosphamide. FEC-D=FEC followed by docetaxel. Numbers of patients screened and assessed for eligibility were not routinely collected.
The patient population was representative of UK patients typically classified, in the early 2000s, as needing adjuvant chemotherapy with potential for taxane exposure (table 1); approximately a third of patients had ER-negative tumours and a quarter had HER2-positive tumours.
Table 1.
Baseline characteristics and details of other adjuvant treatment by randomised treatment group
| FEC-D (experimental) group (n=2073) | Control group (n=2089) | |||
|---|---|---|---|---|
| Age (years) | 48·9 (8·6) | 48·4 (8·5) | ||
| Age-group (years) | ||||
| <40 | 346 (16·7%) | 373 (17·9%) | ||
| 40–49 | 753 (36·3%) | 813 (38·9%) | ||
| 50–59 | 772 (37·2%) | 714 (34·2%) | ||
| ≥60 | 202 (9·7%) | 189 (9·0%) | ||
| Type of surgery and radiotherapy use | ||||
| Mastectomy | 1150 (55·5%) | 1116 (53·4%) | ||
| With radiotherapy | 929 (80·8%) | 885 (79·3%) | ||
| Wide local excision | 923 (44·5%) | 973 (46·6%) | ||
| With radiotherapy | 895 (97·0%) | 943 (96·9%) | ||
| Nodal status | ||||
| Node negative | 419 (20·2%) | 416 (19·9%) | ||
| 1–3 positive nodes | 917 (44·2%) | 922 (44·1%) | ||
| ≥4 positive nodes | 737 (35·6%) | 751 (36·0%) | ||
| Tumour grade* | ||||
| Grade 1 | 117 (5·7%) | 112 (5·4%) | ||
| Grade 2 | 763 (37·0%) | 773 (37·1%) | ||
| Grade 3 | 1182 (57·3%) | 1200 (57·6%) | ||
| Tumour size† | ||||
| <2 cm | 696 (33·6%) | 739 (35·4%) | ||
| 2–5 cm | 1164 (56·2%) | 1166 (55·9%) | ||
| >5 cm | 211 (10·2%) | 181 (8·7%) | ||
| ER status | ||||
| Positive | 1438 (69·4%) | 1438 (68·8%) | ||
| Negative | 635 (30·6%) | 651 (31·2%) | ||
| HER2 status‡ | ||||
| Positive | 420 (23·8%) | 427 (23·7%) | ||
| Negative | 1347 (76·2%) | 1377 (76·3%) | ||
| Endocrine treatment in ER-positive patients§ | ||||
| Tamoxifen monotherapy | 929 (64·6%) | 862 (59·9%) | ||
| Tamoxifen followed by aromatase inhibitor | 426 (29·6%) | 467 (32·5%) | ||
| Aromatase inhibitor monotherapy | 55 (3·8%) | 82 (5·7%) | ||
| No endocrine treatment/unknown | 28 (1·9%) | 27 (1·9%) | ||
| Trastuzumab in HER2-positive patients¶ | ||||
| Yes | 38 (9·0%) | 36 (8·4%) | ||
| No | 312 (74·3%) | 313 (73·3%) | ||
| Not known | 70 (16·7%) | 78 (18·3%) | ||
Data are n (%) or mean (SD). ER=oestrogen receptor. FEC-D=fluorouracil, epirubicin, and cyclophosphamide followed by docetaxel. HER2=human epidermal growth factor receptor 2.
FEC-D group, n=2062; control group, n=2085.
FEC-D group, n=2071; control group, n=2086.
FEC-D group, n=1767; control group, n=1804.
FEC-D group, n=1438; control group, n=1438.
FEC-D group, n=420; control group, n=427.
Eight cycles of randomised treatment were received by 3357 (81%) patients overall (table 2), and by similar proportions of patients in the experimental and control groups. The predominant reason for discontinuation of therapy was toxicity (figure 1). Median relative dose intensity per patient was 94·5% (IQR 85·6–99·2) for the experimental group and 95·9% (80·5–99·6) for the control group (p=0·06) in centres that used the FEC control regimen and 94·7% (87·1–99·4) for the experimental group and 96·8% (91·2–99·7) for controls (p<0·0001) in centres that used the E-CMF control regimen. 3209 patients (77%) had a relative dose intensity of more than 85%, which was achieved by similar proportions of patients in the experimental and control groups.
Table 2.
Compliance with randomised treatment
|
FEC-D (experimental) group (n=2073) |
Control group (n=2089) |
Total (N=4162) |
||||
|---|---|---|---|---|---|---|
| n (%) | Cumulative proportion (%) | n (%) | Cumulative proportion (%) | n (%) | Cumulative proportion (%) | |
| Number of cycles received | ||||||
| 0 | 12 (0·6%) | 0·6% | 15 (0·7%) | 0·7% | 27 (0·7%) | 0·7% |
| 1 | 9 (0·4%) | 1·0% | 8 (0·4%) | 1·1% | 17 (0·4%) | 1·1% |
| 2 | 5 (0·2%) | 1·3% | 9 (0·4%) | 1·5% | 14 (0·3%) | 1·4% |
| 3 | 7 (0·3%) | 1·6% | 10 (0·5%) | 2·0% | 17 (0·4%) | 1·8% |
| 4 | 107 (5·2%) | 6·8% | 12 (0·6%) | 2·6% | 119 (2·9%) | 4·7% |
| 5 | 115 (5·6%) | 12·3% | 30 (1·4%) | 4·0% | 145 (3·5%) | 8·2% |
| 6 | 73 (3·5%) | 15·8% | 243 (11·6%) | 15·7% | 316 (7·6%) | 15·7% |
| 7 | 74 (3·6%) | 19·4% | 76 (3·6%) | 19·3% | 150 (3·6%) | 19·3% |
| 8 | 1671 (80·6%) | 100·0% | 1686 (80·7%) | 100·0% | 3357 (80·7%) | 100·0% |
| Relative dose intensity*>85% | ||||||
| Overall | 1595 (77·3%) | .. | 1614 (77·4%) | .. | 3209 (77·3%) | .. |
| Cycles 1–4 | 1844 (89·3%) | .. | 1891 (90·7%) | .. | 3735 (90·0%) | .. |
| Cycles 5–8† | 1375 (66·6%) | .. | 1500 (71·9%) | .. | 2875 (69·3%) | .. |
FEC-D=fluorouracil, epirubicin, and cyclophosphamide followed by docetaxel.
Per patient relative dose intensity was calculated as a mean of the cycle-specific relative dose intensities over the planned number of cycles. 12 treated patients with no information on dose or dates of cycles are excluded (ie, FEC-D, n=2064; control, n=2086; total, n=4150).
χ2 (1 df)=13·6, p=0·0002 for control versus FEC-D.
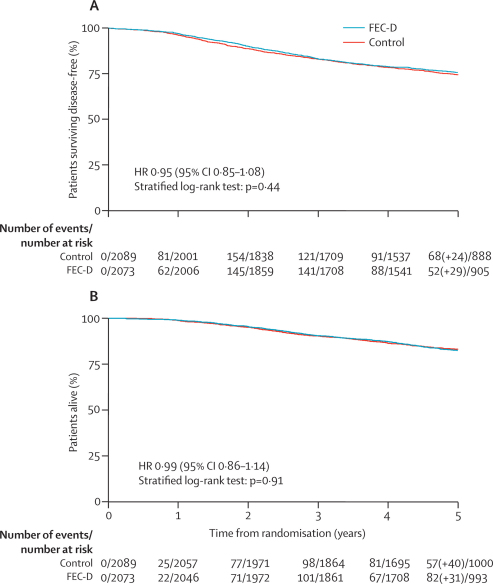
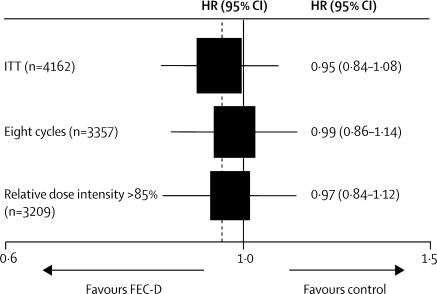
Events related to disease-free survival were reported for 1056 patients (table 3). Most events were related to distant recurrence, either as a first event or subsequent to further breast disease. No evidence was found of a difference in disease-free survival between the FEC-D group and the control group (overall HR 0·95, 95% CI 0·85–1·08; stratified log-rank test p=0·44; figure 2). 5-year disease-free survival rates were 75·6% (95% CI 73·7–77·5) for the experimental group and 74·3% (72·3–76·2) for controls (absolute difference [experimental–control] 1·3%, −2·2 to 4·8). Adjustment for factors known to affect prognosis (ER status, nodal status, HER2 status, age, tumour grade, and tumour size) gave an HR of 0·93 (0·82–1·05; p=0·25) and sensitivity analyses showed negligible effect of treatment non-adherence on the treatment group comparison (figure 3).
Table 3.
Events contributing to disease-free survival and numbers of distant relapses, second cancers, and deaths
| FEC-D (experimental) group (n=2073) | Control group (n=2089) | ||||
|---|---|---|---|---|---|
| Number of patients with event contributing to disease-free survival analysis | 517 | 539 | |||
| Local recurrence | 74 | 105 | |||
| Distant recurrence | 382 | 378 | |||
| New breast disease* | 32 | 34 | |||
| Death from other cause (no recurrence) | 29 | 22 | |||
| Distant relapse ever reported† | 446 | 465 | |||
| New breast disease ever reported* | 45 | 47 | |||
| All non-breast cancer second primary tumours | 44 | 41 | |||
| Gastrointestinal | 11 | 12 | |||
| Gynaecological | 9 | 10 | |||
| Skin | 10 | 6 | |||
| Genitourinary | 5 | 1 | |||
| Lung | 1 | 5 | |||
| Thyroid | 2 | 3 | |||
| Leukaemia‡ | 1 | 2 | |||
| Non-Hodgkin lymphoma | 1 | 0 | |||
| Pancreas | 1 | 1 | |||
| Meningioma | 2 | 0 | |||
| Head and neck | 1 | 1 | |||
| All deaths | 374 | 378 | |||
| Breast cancer§ | 345 | 356 | |||
| Deaths from other causes (without recurrence) | 29 | 22 | |||
| Cancer (non-breast) | 8 | 11 | |||
| Treatment toxicity¶ | 6 | 1 | |||
| Other | 15 | 10 | |||
| Vascular (cardiac) | 5 | 3 | |||
| Vascular (cerebral) | 2 | 1 | |||
| Vascular (thromboembolic) | 0 | 2 | |||
| Respiratory | 1 | 2 | |||
| Accident, suicide, alcoholism | 5 | 2 | |||
| Unknown | 2 | 0 | |||
FEC-D=fluorouracil, epirubicin, and cyclophosphamide followed by docetaxel.
Includes contralateral breast cancer recurrences and new contralateral and ipsilateral breast second primary tumours.
Includes 12 patients in the FEC-D group and 11 patients in the control group who died from breast cancer without separate report of metastatic relapse.
FEC-D group: acute myeloid leukaemia; control: chronic myleoid leukaemia, acute lymphoblastic leukaemia.
Includes deaths following breast cancer recurrence.
Deaths occurring during chemotherapy or within 30 days of chemotherapy completion and without disease relapse.
Figure 2.
Disease-free survival (A) and overall survival (B) by treatment group
FEC-D=fluorouracil, epirubicin, and cyclophosphamide followed by docetaxel. HR=hazard ratio. Number in parentheses indicates events occurring after year 5. Disease-free survival events were seen in 517 of 2073 patients in the FEC-D group and in 539 of 2089 patients in the control group.
Figure 3.
Sensitivity analysis hazard ratios for disease-free survival for all patients receiving eight cycles of chemotherapy and all patients with relative dose intensity more than 85%
FEC-D=fluorouracil, epirubicin, and cyclophosphamide followed by docetaxel. ITT=intention to treat.
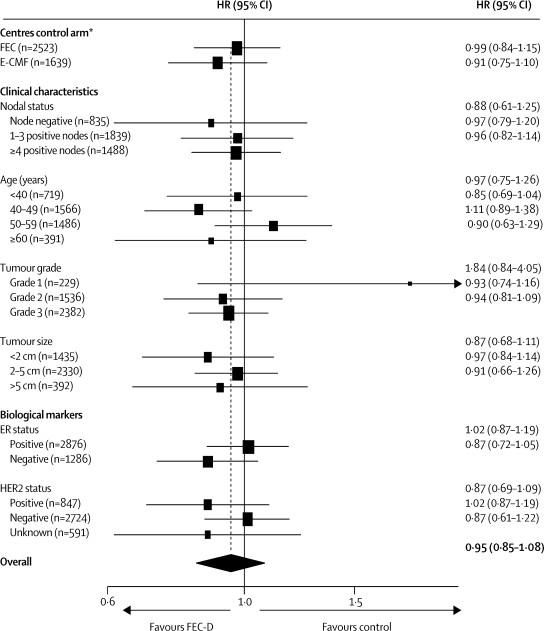
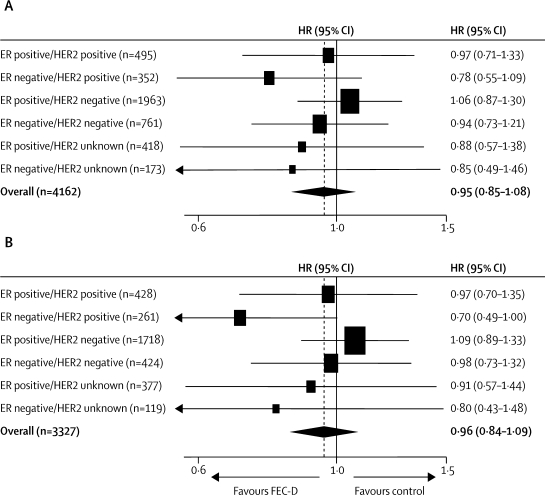
No evidence was found of a difference in treatment effect according to a centre's choice of control regimen (figure 4). The estimated difference in disease-free survival at 5 years (experimental–control) was 0·6% (−3·9 to 5·1) in centres that used the FEC control regimen and 2·5% (−3·0 to 8·0) in centres that used the E-CMF control regimen. No evidence of heterogeneity was seen between common clinical subgroups (figure 4). In relation to single biological makers, there was no strong evidence for a differential effect according to ER status (test for interaction [adjusted] p=0·10) or HER2 status (test for interaction [adjusted] p=0·13). An exploratory analysis, replicating the one undertaken in the CALGB 9344 trial,16 compared the putative treatment effect according to combined effects of ER and HER2 (figure 5); results for patients with ER-negative and HER2-positive tumours, especially those who had positive lymph nodes, were consistent with a clinically relevant improvement in disease-free survival with taxane-based treatment.
Figure 4.
Hazard ratios for disease-free survival by centre's choice of control regimen, and patient and tumour characteristics
E-CMF=epirubicin followed by cyclophosphamide, methotrexate, and fluorouracil. ER=oestrogen receptor. FEC=fluorouracil, epirubicin, and cyclophosphamide. FEC-D=FEC followed by docetaxel. HER2=human epidermal growth factor receptor 2. HR=hazard ratio. *p=0·55 for heterogeneity.
Figure 5.
Hazard ratios for disease-free survival by combined ER and HER2 status in (A) all patients and (B) node-positive patients
ER=oestrogen receptor. FEC-D=fluorouracil, epirubicin, and cyclophosphamide followed by docetaxel. HER2=human epidermal growth factor receptor 2. HR=hazard ratio.
Of 752 deaths, 374 were in the experimental group 378 were in the control group (table 3). Overall survival did not differ between groups (figure 2). 5-year overall survival rates were 82·5% (80·7–84·1) in the experimental group and 83·0% (81·3–84·6) in the control group. 51 patients (experimental, n=29; control, n=22) died with no relapse of breast cancer.
No evidence was found to suggest a difference in metastasis-free survival between experimental and control groups (446 vs 465 events; HR 0·96, 0·84–1·09; p=0·52). 5-year metastasis-free survival rates were 78·8% (95% CI 76·9–80·5) for the experimental group and 77·7% (75·8–79·5) for controls.
Seven patients died during or immediately after treatment (table 3); five of these deaths were caused by infection (FEC-D, n=4 [one patient died during the FEC phase and three patients died during the docetaxel phase of treatment]; E-CMF control, n=1 [the patient died during the CMF phase of treatment]) with the other two deaths (both in patients assigned to FEC-D) caused by cerebral haemorrhage.
The proportion of patients reporting any acute grade 3 or 4 toxicity, occurring during treatment and within 30 days of treatment end, was significantly greater in the experimental group than in the control group (table 4). As expected, the toxicity profile of the experimental and control groups differed. The higher frequency of grade 3 or 4 infection in the experimental group compared with the control group (293 [14%] patients vs 182 [9%] patients) was predominantly due to a higher infection rate in cycles five to eight in the FEC-D group in centres using FEC control (131 [11%] vs 41 [3%]). The higher frequency of grade 3 or 4 neutropenia after treatment with FEC-D compared with control (937 [45%] vs 797 [38%]) was mainly due to higher neutropenia rates in the FEC-D group than in the E-CMF control group (392 [49%] vs 297 [37%]). The following late adverse events (reported more than 2 months after treatment end) were more frequent in the experimental group than in the control group: musculoskeletal disorders (295 [14%] vs 211 [10%]; p<0·0001), CNS disorder (288 [14%] vs 93 [4%]; p<0·0001), myalgia/arthralgia (203 [10%] vs 131 [6%]; p<0·0001), skin disorders (99 [5%] vs 53 [3%]; p=0·0002), oedema (94 [5%] vs 60 [3%]; p=0·007), and alopecia (22 [1%] vs 7 [0·3%]; p=0·005). One case of acute myeloid leukaemia was reported in the experimental group.
Table 4.
Frequency of grade 3 or 4 adverse events during chemotherapy
|
Centres providing FEC control regimen |
Centres providing E-CMF control regimen |
||||||
|---|---|---|---|---|---|---|---|
| FEC-D (n=1254) | FEC control (n=1262) | p value | FEC-D (n=807) | E-CMF control (n=812) | p value | ||
| Any grade 3 or 4 toxic effect | 868 (69%) | 761 (60%) | <0·0001 | 592 (73%) | 513 (63%) | <0·0001 | |
| Haematological | |||||||
| Anaemia | 9 (0·7%) | 6 (0·5%) | 0·45* | 4 (0·5%) | 8 (1%) | 0·39* | |
| Febrile neutropenia | 87 (7%) | 25 (2%) | <0·0001 | 59 (7%) | 36 (4%) | 0·01 | |
| Leucopenia | 280 (22%) | 224 (18%) | 0·004 | 227 (28%) | 138 (17%) | <0·0001 | |
| Neutropenia | 545 (44%) | 500 (40%) | 0·05 | 392 (49%) | 297 (37%) | <0·0001 | |
| Thrombocytopenia | 8 (0·6%) | 11 (0·9%) | 0·65* | 4 (0·5%) | 16 (2%) | 0·01* | |
| Non-haematological | |||||||
| Alopecia | 113 (9%) | 117 (9%) | 0·82 | 97 (12%) | 96 (12%) | 0·90 | |
| Diarrhoea | 46 (4%) | 23 (2%) | 0·005 | 31 (4%) | 36 (4%) | 0·55 | |
| Infection | 183 (15%) | 86 (7%) | <0·0001 | 110 (14%) | 96 (12%) | 0·28 | |
| Lethargy | 275 (22%) | 158 (13%) | <0·0001 | 181 (22%) | 114 (14%) | <0·0001 | |
| Musculoskeletal—other† | 74 (6%) | 13 (1%) | <0·0001 | 70 (9%) | 18 (2%) | <0·0001 | |
| Myalgia/arthralgia | 60 (5%) | 1 (0·1%) | <0·0001* | 44 (5%) | 2 (0·2%) | <0·0001* | |
| Nausea/vomiting | 113 (9%) | 127 (10%) | 0·37 | 86 (11%) | 78 (10%) | 0·48 | |
| Neuropathy | 68 (5%) | 4 (0·3%) | <0·0001* | 30 (4%) | 7 (0·9%) | 0·0001* | |
| Oedema | 11 (0·9%) | 1 (0·1%) | 0·003* | 6 (0·7%) | 5 (0·6%) | 0·77* | |
| Pain | 8 (0·6%) | 1 (0·1%) | 0·02* | 50 (6%) | 2 (0·2%) | 0·004* | |
| Skin disorder (including nail changes) | 41 (3%) | 10 (0·8%) | <0·0001* | 26 (3%) | 14 (2%) | 0·05 | |
| Stomatitis (mucositis) | 87 (7%) | 28 (2%) | <0·0001 | 69 (9%) | 46 (6%) | 0·02 | |
E-CMF=epirubicin followed by cyclophosphamide, methotrexate, and fluorouracil. FEC=fluorouracil, epirubicin, and cyclophosphamide. FEC-D=FEC followed by docetaxel. Analysis of adverse events was undertaken on the safety population which included 4135 patients who received some randomised treatment. Events were coded by use of the Medical Dictionary for Regulatory Activities (MedDRA version 10).15 The proportion of patients with grade 3 or 4 toxicity in each treatment group was compared by use of a χ2 test or exact test (indicated by*) as appropriate.
Excluding arthralgia and myalgia.
In 829 patients who participated in the quality of life substudy, deterioration in quality of life during chemotherapy was clinically relevant. Significantly greater impairment was seen in the experimental group than in the control group with respect to physical (p<0·0001), role (p=0·002), emotional functioning (p=0·008), social functioning (p=0·003), pain (p=0·001), fatigue (p=0·006), and global quality of life (p=0·001); however, there was significantly more nausea and vomiting in the control group (p=0·01).10 Differences between groups diminished over 24 months and returned close to baseline levels.
Discussion
This report, from the largest of the primary adjuvant taxane trials, does not show any overall significant gain from the addition of docetaxel every 3 weeks to standard anthracycline chemotherapy of similar duration. Additionally, the anthracycline-docetaxel sequential schedule was associated with a higher frequency of adverse events and transiently poorer quality of life than the non-taxane control regimen. Recruitment for the TACT study was spread across specialist cancer hospitals, university teaching hospitals, and smaller community general hospitals, and at its peak accrual consisted of approximately a quarter of the UK eligible population, thus supporting the applicability of the results to general clinical practice. The size and maturity of this trial, together with its completeness of follow-up, suggest that the results should be considered in the balance of worldwide evidence on the effects of taxanes in early breast cancer.
The findings reported here suggest a smaller effect from the incorporation of docetaxel into adjuvant breast cancer treatment than might have been expected based on worldwide evidence to date.17,18 Several issues should be considered when interpreting a trial's results, including its contemporaneous setting. When this study began in 2001, two chemotherapy regimens, FEC60 (ie, epirubicin dose 60 mg/m2) and E-CMF, were commonly used for early breast cancer in the UK. Since this trial brought together a national community of clinical researchers around a specific trial question, it was crucial that both regimens were offered as choice of control. A key feature in the study's design was to ensure that the same number of cycles were delivered in each comparator group. Because the trial was powered for the overall anthracycline-taxane comparison, a choice of control regimen with unbiased allocation of treatment adds to the robustness of the results. By today's standards the epirubicin dose in FEC60 might be regarded as suboptimum;19,20 however, in 2001, eight cycles of FEC with an epirubicin dose above 60 mg/m2 were considered undeliverable to a general UK population. Although the dose of epirubicin has been subsequently increased,19 the optimum dose in adjuvant therapy remains controversial. The results reported here suggest that eight cycles of FEC60 were at least as effective as E-CMF (considered worldwide as a dose-intensive anthracycline standard regimen) when compared with FEC-D. Although there were early concerns about the number of women discontinuing therapy in both control and experimental groups after six cycles (the UK standard at the time), prompt measures, including information dissemination and advice, were introduced, which resulted in improved subsequent compliance.21 Sensitivity analyses including only those who received protocol-specified treatment showed that the overall result from the ITT population was unlikely to have been biased by non-compliance. Finally, aromatase inhibitor use was slightly higher in patients in the control group than in those in the experimental group, and although the trial pre-dated routine trastuzumab use, 74 patients are known to have received this drug via the HERA trial.22 The use of anti-HER2 or aromatase inhibitor therapy is highly unlikely to have affected the results.
Substantial heterogeneity exists between the designs of trials that test taxane use in early breast cancer. When the TACT study was designed, docetaxel given in 3-weekly intervals was the most widely used taxane regimen in Europe and was standard treatment for metastatic disease in the UK, which justified its use in this trial. Although no trial is exactly analogous to the TACT study, there are other trials of similar sequential taxane therapy in which total chemotherapy duration is similar in the comparator group. The PACS 01 trial23 included 1999 node-positive breast cancer patients and compared six cycles of FEC100 with three cycles of FEC100 followed by three cycles of docetaxel 100 mg/m2. The results of the study showed a small but significant improvement in both disease-free and overall survival in favour of sequential docetaxel. In a pre-planned subgroup analysis, no benefit was seen in women aged under 50 years, an anomaly that remains unexplained. The GEICAM 9906 study,24 which compared six cycles of FEC90 with four cycles of FEC90 followed by paclitaxel given once a week for 8 weeks, reported a benefit for both disease-free and overall survival for paclitaxel and did not find the speculative age effect seen in PACS 01.
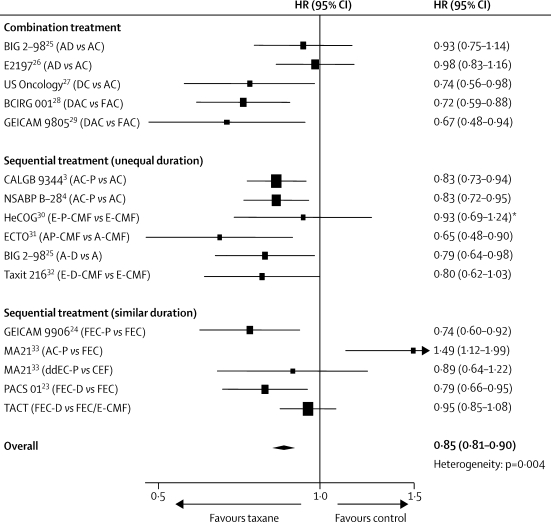
There is substantial variation between results from individual taxane trials.17,18 Figure 6 summarises an updated meta-analysis that is based on published trial results and those reported here. Generalisations about taxane benefit are difficult to make when individual trials are of different sizes, reported at different stages of maturity, have biologically heterogeneous populations, have used one or other taxane with different schedules, and are compared with different anthracycline control regimens of often unequal duration. Many patients seem to gain little benefit from additional taxanes, especially in regard to overall survival. An important role for adjuvant taxanes might be as a sequential alternative to anthracyclines, thus keeping both the overall anthracycline dose and subsequent exposure to the associated long-term adverse events (such as induction of leukaemia and cardiotoxicity) to a minimum.
Figure 6.
Meta-analysis of disease-free survival for trials of taxane-based versus anthracycline-based adjuvant chemotherapy
A=doxorubicin. C=cyclophosphamide. D=docetaxel. dd=dose dense. F=fluorouracil. P=paclitaxel. E=epirubicin. M=methotrexate. HR=hazard ratio. *HR from Cochrane review.17
The long-term benefits of taxanes need further investigation. The risk of relapse for patients with ER-positive disease continues for at least 15 years after diagnosis, partly as a consequence of effective endocrine therapy. Current estimates of the effect of adjuvant taxane therapy on disease outcome in patients with ER-positive tumours are affected by events in early years and further follow-up is needed before a possible longer term benefit can be identified or excluded in this patient population.
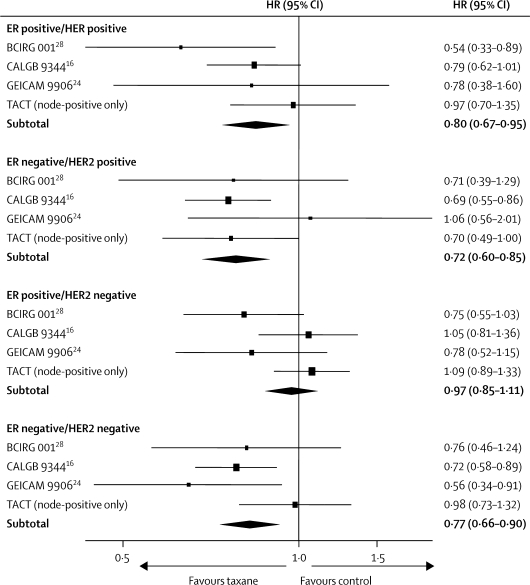
In view of the molecular diversity of breast cancer, adjuvant taxanes might provide different benefits for particular patient subgroups. Therefore, clinicians need to identify patients who would—and, just as importantly, who would not—obtain benefit from this treatment. In isolation, biological markers such as ER or HER234–36 seem to have little predictive value in determining taxane responsiveness; however, more complex biological groupings could prove more informative. A retrospective subgroup analysis of 1322 node-positive patients in the CALGB 9344 trial16 suggested that the benefits of paclitaxel occurred mainly in those with ER-negative or HER2-positive tumours with little or no gain in the largest subgroup of patients—namely, those with the ER-positive/HER2-negative phenotype. In a similar analysis in this trial, docetaxel benefit was most apparent in patients whose tumours were ER negative and HER2 positive, with other subgroups deriving lesser or no apparent benefit. Four trials have published results by ER and HER2 status (figure 7). We postulate that patients with ER-positive and HER2-negative cancers might not gain a clinically worthwhile benefit from taxanes; however, long-term follow-up is needed to confirm this hypothesis. The pending EBCTCG individual patient data meta-analysis of adjuvant taxanes in early breast cancer provides the best opportunity for clarification, especially if biological data from other trials can be incorporated into the analysis. The confirmation of such a hypothesis would have huge implications for treatment worldwide, through the reduction of treatment toxicity and improvement of resource use.
Figure 7.
Meta-analysis of disease-free survival for trials of taxane-based versus anthracycline-based adjuvant chemotherapy that reported combined ER/HER2 subgroup analyses
ER=oestrogen receptor. HER2=human epidermal growth factor receptor 2. p=0·03 heterogeneity test for comparison of the HRs across the four subgroups of ER/HER2 status.
Current drug development focuses on identifying subpopulations driven by tumour biology. The TACT study has a large translational project (TransTACT) investigating the concept of biologically driven chemotherapy selection, which will examine interactions between taxane benefit and the expression of tau protein,37 the AKT pathway,38 and the triple-negative phenotype.39 Other trials groups are also exploring innovative biological hypotheses in relation to individual drug sensitivity.40–42 We support the view that “one-size-fits-all” adjuvant chemotherapy trials in early breast cancer are too simple43 and we propose that future trials are designed around specific biological patient subgroups, with the aim of accelerating advances in tailored therapy.
Acknowledgments
Acknowledgments
The research costs of the TACT trial were met by Cancer Research UK (CRUK 01/001) and by educational grants from Sanofi-Aventis, Pfizer, and Roche. Trial recruitment was facilitated within centres by the NIHR-funded National Cancer Research Network. We thank all the patients who participated in this study, the doctors, nurses, radiographers, physicists, pathologists, and data managers at the participating centres, and the laboratory staff at Royal Marsden Hospital (London, UK), Nottingham City Hospital (Nottingham, UK), and Glasgow Royal Infirmary (Glasgow, UK). Recognition goes to all the trials unit staff at the Information & Statistics Division (ISD), Edinburgh (formerly SCTN), Cancer Trials & Research Unit (CTRU), Leeds, Wales Cancer Trials Network (WCTN), Cardiff, CRUK & UCL Cancer Trials Centre, London and The Institute of Cancer Research's Clinical Trials & Statistics Unit (ICR-CTSU), Sutton, who contributed to trial management and to data collection, with special thanks to M McLinden (ISD), J Copeland (CTRU Leeds), and A Yongue (ICR-CTSU). We would also like to thank the Independent Data Monitoring and Trial Steering Committees for their oversight of the trial and D Mills (ICR-CTSU) for helping to prepare the manuscript.
Contributors
With the exception of MD, JMSB, IE, PC, and SO'R, all authors are members of the TACT Trial Management Group. PE and PB-L were joint chief investigators and were jointly responsible for trial design and contributed to data interpretation and patient recruitment. JMB and EH were also jointly responsible for trial design, and oversaw trial management, data interpretation, and all statistical analyses. EH also conducted many of the statistical analyses. DC, RC, HE, and IS contributed to trial design, data interpretation, and patient recruitment. MV, JY, and CT contributed to trial design and patient recruitment. AW contributed to data interpretation and patient recruitment. PC and SO'R contributed to patient recruitment. PH and DC were responsible for the design of the quality of life substudy. DB was responsible for the health economics substudy, contributed to trial design, data interpretation, and patient recruitment. SJ was responsible for the translational substudy and contributed to trial design. CPe conducted statistical analyses. LJ contributed to trial design and data interpretation, and was responsible for trial management and data collection. MD, JMSB, and IE collected tissue samples and undertook central HER2 testing. All authors contributed to the writing or review of the manuscript.
TACT Trial Management Group
P Ellis; P Barrett-Lee; L Johnson; D Cameron; A Wardley; M Verrill; I Smith; J Yarnold; R Coleman; H Earl; C Twelves; C Poole; D Bloomfield; P Hopwood; S Johnston; C Peckitt; E Hall; J M Bliss; A Ashton (Cheltenham); J Banerji, D Coward (ICR-CTSU, Sutton); L Branston (WCTN, Cardiff); M Brunt, (WM Breast Group, Stoke); D Dodwell, T Perren (Yorkshire Breast Group, Leeds); I Devine (ISD); A Harnett (Norwich); V Hiley, S Pollard (CTRU, Leeds); S Houston (Guildford); A Jones (UCL/Royal Free Group, London); H Moore (Shrewsbury).
TACT Trialists
Principal and main co-investigators (centre, number of patients recruited): A Hutcheon (Aberdeen Royal Infirmary, 30); H Earl, K McAdam, C Wilson (Addenbrooke's Hospital, 59); M Crawford (Airedale General Hospital, 20); C Irwin (Alexandra Hospital, Redditch, 8); S Tinkler (Basingstoke & North Hampshire Hospital, 8); P Canney, J Evans, A Harnett, C Twelves (Beatson Oncology Centre, 150); J Clarke, J McAleer (Belfast City Hospital, 56); C Bradley, D Parker (Bradford Royal Infirmary, 35); C Price, E Whipp (Bristol Oncology Centre, 35); A Axford (Bronglais General Hospital, 9); N Davidson (Broomfield Hospital, 67); R Coombes, C Lowdell (Charing Cross Hospital, 65); K Benstead, S Elyan, R Owen (Cheltenham General Hospital, 97); A Stewart, A Wardley, R Welch, P Wilkinson (Christie Hospital, 146); A Harris (Churchill Hospital, Oxford, 17); D Spooner (City Hospital, Birmingham, 7); J Brock, P Clark, A Flavin, S O'Reilly, N Thorpe (Clatterbridge Centre for Oncology, 156); G Sadler (Conquest Hospital, Hastings, 3); D Dodwell, S Kumar (Cookridge Hospital, Leeds, 42); D Ritchie (Crosshouse Hospital, 31); D Otim-Oyet, P Woodings (Derbyshire Royal Infirmary, 34); N Bailey, S Kelly, C Tyrrell (Derriford Hospital, 34); P Mack (Diana Princess of Wales Hospital, 23); S Dean (Dorset County Hospital, 9); P Murray (Essex County Hospital, 52); P Canney (Falkirk and District Royal Infirmary, 27); J Bishop (Glan Clwyd, 20); M Soukop, S Troon (Glasgow Royal Infirmary, 40); P Ellis, D Miles (Guy's & St Thomas Hospital, 48); H Yosef (Hairmyres Hospital, 23); D Gilligan (Hinchingbrooke Hospital, 11); B Crosse, J Joffe (Huddersfield Royal Infirmary, 34); J Le Vay (Ipswich Hospital, 43); J Hardman, A Rathmell, N Storey, J Van der Voet (James Cook University Hospital, 81); A Harnett (James Paget Hospital, 4); C Abson, M O'Brien, D Pickering, A Saini (Kent Oncology Centre, 59); M Churn (Kidderminster Hospital, 9); E Sims (King Georges Hospital, Ilford, 18); P Ellis (King's College Hospital, 23); M McIlmurray (Lancaster Royal Infirmary, 13); S Khanna, I Peat (Leicester Royal Infirmary, 85); M Gaze, M McCormack, R Stein (Middlesex Hospital, 56); M Hall, M Harrison, A Makris, P Ostler (Mount Vernon Hospital, 94); T Branson, M Verrill (Newcastle General Hospital, 104); M Churn, R Mehra (New Cross Hospital, 13); D Adamson, J Dewar, M Highley (Ninewells Hospital, 42); C Macmillan, R Mathews, J Stewart (Northampton General Hospital, 78); M Napier (North Devon District Hospital, 44); A Jones (North Middlesex Hospital, 15); A Branson (North Tyneside Hospital, 3); M Quigley, E Sims (Oldchurch Hospital, 43); K McAdam (Peterborough District Hospital, 59); E Murray (Pilgrim Hospital, Boston, 5); S Dean, T Goode (Poole Hospital, 29); T Crosby (Princess of Wales Hospital, Bridgend, 14); A Chaturvedi (Princess Royal Hospital, Hull, 28); C Poole, D Rea (Queen Elizabeth Hospital, Birmingham, 29); H Lucraft (Queen Elizabeth Hospital, Gateshead, 11); A Ahmad (Queen Elizabeth Hospital, King's Lynn, 57); D Whillis (Raigmore Hospital, Inverness, 35); A Wardley (Royal Albert Edward Infirmary, Wigan, 30); J Barrett (Royal Berkshire Hospital, 12); T Hickish (Royal Bournemouth Hospital, 11); A Goodman, A Hong (Royal Devon & Exeter Hospital, 60); A Jones (Royal Free Hospital, 35); C Gaffney (Royal Gwent Hospital, 44); V Hall (Royal Hampshire County Hospital, 6); I Smith, J Yarnold (Royal Marsden Hospital, London & Sutton, 107); T Mughal, E Young (Royal Preston Hospital, 39); R Agrawal (Royal Shrewsbury Hospital, 66); A Last, N Murray, P Simmonds (Royal South Hants Hospital, 80); S Houston, R Laing, T Neal (Royal Surrey County Hospital, 70); D Bloomfield, G Deutsch (Royal Sussex County Hospital, 25); H Newman, G Rees (Royal United Hospital, Bath, 31); L Evans, A Hindley (Royal Victoria Hospital, Blackpool, 27); R Allerton (Russell's Hall Hospital, 12); K Gregory (Salisbury District Hospital, 31); D Spooner (Sandwell Hospital, 9); T Sreenivasan (Scunthorpe General Hospital, 9); T Joannides, R Leonard (Singleton Hospital, 54); J Bozzino (South Tyneside Hospital, 24); A Robinson, C Trask (Southend Hospital, 39); C Gallagher (St Bartholomew's Hospital, 32); J Mansi (St George's Hospital London, 51); T Perren (St James' University Hospital, 37); J Dubois, T Gulliford (St Mary's Hospital, Portsmouth, 18); C Alcock (Stoke Mandeville Hospital, 11); J Graham, P Riddle (Taunton and Somerset Hospital, 27); I Manifold (Thornbury, Sheffield, 8); N Bailey (Torbay Hospital, 28); I Vergote (University Hospital Leuven, 38); M Brunt (University Hospital of North Staffordshire, 42); P Barrett-Lee (Velindre Hospital, Cardiff, 90); R Grieve, D Jones, A Stockdale (Walsgrave Hospital, 85); M Moody (West Suffolk Hospital, 28); A Bowman, D Cameron, F Yuille (Western General Hospital, Edinburgh, 97); R Coleman, K Dunn, M Hatton, I Manifold, O Purohit, S Ramakrishnan (Weston Park Hospital, Sheffield, 153); A Jones (Whittington Hospital, 18); D Bloomfield (Worthing Hospital, 45); A Champion (Wrexham Maelor Hospital, 17); B Lavery (Wycombe General Hospital, 5); S Goodman (Yeovil District Hospital, 9); J Bishop (Ysbyty Gwynedd, 12).
Conflicts of interest
PB-L has received honoraria as a clinical advisory board member from Sanofi-Aventis and Pfizer. JMB has received educational research grants from Sanofi-Aventis, Pfizer, and Roche. PC has received consultancy fees and travel grants from Sanofi-Aventis and Pfizer. DC has received honoraria, travel grants, and departmental research funding from Sanofi-Aventis and Pfizer. RC has received consultancy fees and speaking honoraria from Sanofi-Aventis and Pfizer. HE has received honoraria and educational grants from Sanofi-Aventis. PE has received honoraria as a clinical advisory board member from Sanofi-Aventis, Pfizer, and Roche. EH has received educational research grants from Roche. SO'R has received honoraria from Sanofi-Aventis. IS has received honoraria (speaking and advisory boards) from Sanofi-Aventis and Roche. AW has received honoraria and travel grants from Sanofi-Aventis. MV has acted as a consultant to Roche and has received honoraria (speaking and advisory boards) and travel grants from Roche, Sanofi-Aventis, and Pfizer. JMSB, MD, and IE received educational grants from Roche to support HER2 testing within TACT. CPo has received consultancy fees and honoraria from Sanofi-Aventis and Roche. DB, PH, LJ, SJ, CT, CPe, and JY declare that they have no conflicts of interest.
References
- 1.Early Breast Cancer Trialists' Collaborative Group Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Henderson IC, Berry DA, Demetri GD. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 4.Mamounas EP, Bryant J, Lembersky B. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23:3686–3696. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 5.Dowsett M, Bartlett J, Ellis IO. Correlation between immunohistochemistry (HercepTest) and fluorescence in situ hybridization (FISH) for HER-2 in 426 breast carcinomas from 37 centres. J Pathol. 2003;199:418–423. doi: 10.1002/path.1313. [DOI] [PubMed] [Google Scholar]
- 6.Aaronson NK, Ahmedzai S, Bergman B. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 7.Sprangers MA, Groenvold M, Arraras JI. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14:2756–2768. doi: 10.1200/JCO.1996.14.10.2756. [DOI] [PubMed] [Google Scholar]
- 8.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 9.Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ. 1998;316:736–741. doi: 10.1136/bmj.316.7133.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopwood P, Ridolfi A, Russell S. Impact on quality of life (QoL) of FEC-T compared with FEC or E-CMF: UK Taxotere as Adjuvant chemotherapy Trial (TACT) 2-year follow-up. Proc Am Soc Clin Oncol. 2008;26(suppl 15) abstr 548. [Google Scholar]
- 11.Hopwood P, Ridolfi A, Peckitt C. Patients' views of distress and interference with daily activities due to side effects in the TACT (Taxotere as Adjuvant Chemotherapy) trial. Eur J Cancer. 2008;6 196 #490. [Google Scholar]
- 12.Campbell H, Epstein D, Griffin S. Modelling the cost-effectiveness of first, second and third generation polychemotherapy regimens in women with early breast cancer who have differing prognoses. Cancer Res. 2009;69(2_MeetingAbstracts):6106. [Google Scholar]
- 13.Poole CJ, Earl HM, Hiller L. Epirubicin and cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy for early breast cancer. N Engl J Med. 2006;355:1851–1862. doi: 10.1056/NEJMoa052084. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Cancer Therapy Evaluation Program: common toxicity criteria, version 2.0, DCTD, NCI, NIH, DHHS March, 1998.
- 15.Northrop Grumman Corporation MedDRA version 10.0. http://www.meddramsso.com/MSSOWeb/index.htm (accessed May 9, 2007).
- 16.Hayes DF, Thor AD, Dressler LG. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 17.Fergason T, Wilcken N, Vagg R, Ghersi D, Nowak AK. Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst Rev. 2007;4 doi: 10.1002/14651858.CD004421.pub2. CD004421. [DOI] [PubMed] [Google Scholar]
- 18.De Laurentiis M, Cancello G, D'Agostino D. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol. 2008;26:44–53. doi: 10.1200/JCO.2007.11.3787. [DOI] [PubMed] [Google Scholar]
- 19.Fumoleau P, Kerbrat P, Romestaing P. Randomized trial comparing six versus three cycles of epirubicin-based adjuvant chemotherapy in premenopausal, node-positive breast cancer patients: 10-year follow-up results of the French Adjuvant Study Group 01 Trial. J Clin Oncol. 2003;21:298–305. doi: 10.1200/JCO.2003.04.148. [DOI] [PubMed] [Google Scholar]
- 20.de Azambuja E, Paesmans M, Beauduin M. Long-term benefit of high-dose epirubicin in adjuvant chemotherapy for node-positive breast cancer: 15-year efficacy results of the Belgian Multicentre Study. J Clin Oncol. 2009;27:720–725. doi: 10.1200/JCO.2008.17.2155. [DOI] [PubMed] [Google Scholar]
- 21.Johnson L, Ellis P, Bliss JM. Fast recruiting trials—a Utopian dream or logistical nightmare? Br J Cancer. 2005;92:1697–1783. doi: 10.1038/sj.bjc.6602574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith I, Proctor M, Gelber RD. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 23.Roche H, Fumoleau P, Spielmann M. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol. 2006;24:5664–5671. doi: 10.1200/JCO.2006.07.3916. [DOI] [PubMed] [Google Scholar]
- 24.Martin M, Rodriguez-Lescure A, Ruiz A. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by paclitaxel for early breast cancer. J Natl Cancer Inst. 2008;100:805–814. doi: 10.1093/jnci/djn151. [DOI] [PubMed] [Google Scholar]
- 34.Martin M, Mackey J, Vogel C. Benefit from adjuvant taxanes and endocrine responsiveness in breast cancer. Breast. 2007;16(suppl 2):S127–S131. doi: 10.1016/j.breast.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Andre F, Broglio K, Roche H. Estrogen receptor expression and efficacy of docetaxel-containing adjuvant chemotherapy in patients with node-positive breast cancer: results from a pooled analysis. J Clin Oncol. 2008;26:2636–2643. doi: 10.1200/JCO.2007.14.9146. [DOI] [PubMed] [Google Scholar]
- 36.Hugh J, Hanson J, Cheang M. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 Trial. J Clin Oncol. 2009;27:1168–1176. doi: 10.1200/JCO.2008.18.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andre F, Hatzis C, Anderson K. Microtubule-associated protein-tau is a bifunctional predictor of endocrine sensitivity and chemotherapy resistance in estrogen receptor-positive breast cancer. Clin Cancer Res. 2007;13:2061–2067. doi: 10.1158/1078-0432.CCR-06-2078. [DOI] [PubMed] [Google Scholar]
- 38.McCubrey JA, Sokolosky ML, Lehmann BD. Alteration of AKT activity increases chemotherapeutic drug and hormonal resistance in breast cancer yet confers an achilles heel by sensitization to targeted therapy. Adv Enzyme Regul. 2008;48:113–135. doi: 10.1016/j.advenzreg.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 40.Bonnefoi H, Potti A, Delorenzi M. Validation of gene signatures that predict the response of breast cancer to neoadjuvant chemotherapy: a substudy of the EORTC 10994/BIG 00-01 clinical trial. Lancet Oncol. 2007;8:1071–1078. doi: 10.1016/S1470-2045(07)70345-5. [DOI] [PubMed] [Google Scholar]
- 41.Chang JC, Makris A, Gutierrez MC. Gene expression patterns in formalin-fixed, paraffin-embedded core biopsies predict docetaxel chemosensitivity in breast cancer patients. Breast Cancer Res Treat. 2008;108:233–240. doi: 10.1007/s10549-007-9590-z. [DOI] [PubMed] [Google Scholar]
- 42.Bartlett J, Munro A, Dunn J. Chromosome 17 polysomy (Ch17) as a predictor of anthracycline response: emerging evidence from the UK NEAT adjuvant breast cancer trial. Cancer Res. 2009;69(suppl 2):45. [Google Scholar]
- 43.Moore A. Breast-cancer therapy—looking back to the future. N Engl J Med. 2007;357:1547–1549. doi: 10.1056/NEJMe078153. [DOI] [PubMed] [Google Scholar]
Uncited reference
- 25.Francis P, Crown J, Di Leo A. Adjuvant chemotherapy with sequential or concurrent anthracycline and docetaxel: Breast International Group 02-98 randomized trial. J Natl Cancer Inst. 2008;100:121–133. doi: 10.1093/jnci/djm287. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein LJ, O'Neill A, Sparano JA. Concurrent doxorubicin plus docetaxel is not more effective than concurrent doxorubicin plus cyclophosphamide in operable breast cancer with 0 to 3 positive axillary nodes: North American Breast Cancer Intergroup Trial E 2197. J Clin Oncol. 2008;26:4092–4099. doi: 10.1200/JCO.2008.16.7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones S, Holmes FA, O'Shaughnessy J. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol. 2009;27:1177–1183. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 28.Martin M, Pienkowski T, Mackey J. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 29.Martin M, Lluch A, Segui MA. TAC versus FAC as adjuvant chemotherapy for high-risk node-negative breast cancer: results of the GEICAM 9805 trial. Ann Oncol. 2008;19(suppl 8):viii77–viii88. abstract 1830. [Google Scholar]
- 30.Fountzilas G, Skarlos D, Dafni U. Postoperative dose-dense sequential chemotherapy with epirubicin, followed by CMF with or without paclitaxel, in patients with high-risk operable breast cancer: a randomized phase III study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol. 2005;16:1762–1771. doi: 10.1093/annonc/mdi366. [DOI] [PubMed] [Google Scholar]
- 31.Gianni L, Baselga J, Eiermann W. European Cooperative Trial in Operable Breast Cancer (ECTO): improved freedom from progression (FFP) from adding paclitaxel (T) to doxorubicin (A) followed by cyclophosphamide methotrexate and fluorouracil (CMF) Proc Am Soc Clin Oncol. 1-6-2005;23(suppl 16):513. [Google Scholar]
- 32.Bianco AR, De Matteis A, Manzione L. Sequential epirubicin-docetaxel-CMF as adjuvant therapy of early breast cancer: Results of the Taxit216 multicenter phase III trial. Proc Am Soc Clin Oncol. 20-6-2006;24(suppl 18):LBA520. [Google Scholar]
- 33.Burnell MJ, Levine MN, Chapman JA. A phase III adjuvant trial of sequenced EC + filgrastim + epoetin-alpha followed by paclitaxel compared to sequenced AC followed by paclitaxel compared to CEF in women with node-positive or high-risk node-negative breast cancer (NCIC CTG MA.21) J Clin Oncol. 2007;25(suppl 18):550. [Google Scholar]